Abstract
The testosterone decline is one of the potential causes of oxidative stress-induced anxiety and depressive behaviors, and cognitive impairment induces irreversible neuronal damage, which is not clearly understood. The orchidectomized rat model was used; the hippocampal neurons and anxiety behavior were analyzed. Adult male albino rats were divided into control and orchidectomy (ORX) groups, orchidectomy (ORX + T), and normal (Cont + T) groups. Testosterone propionate was used as a testosterone supplement. The anxiety and depressive-like behavior observed in ORX animals in the open field (OF) and elevated plus-maze experiments were effectively overturned in the ORX + T group. Studies on isolated hippocampus showed reduced antioxidant enzymes (SOD, CAT, and glutathione (GSH) compounds), increased lipid peroxidation (LPO), elevated caspase3, and reduced anti-apoptotic protein Bcl-2, and increased apoptotic nuclei in TUNEL staining of the hippocampus in the ORX rats. These observations indicate free radical-mediated neural damage. Testosterone presence promoted the antioxidant defense system and restored normal pyramidal neuron morphology in ORX + T. This study confirms that testosterone is indispensable in the normal adult hippocampus and deficiency seems to be a potential risk factor for neurodegenerative disorders. Besides, androgen appears to be a possible therapeutic strategy for treating depression/neurodegenerative diseases in aging men.
1. Introduction
Neurodegenerative disorders affect billions of people worldwide [Citation1]. Though numerous complicated mechanisms are involved in the sequence of chronic and acute neurodegenerative disorders, many disorders split up mutual pathogenic features like oxidative stress, mitochondrial dysfunction, protein misfolding, neuroinflammation, excitotoxicity, neuronal damage, and aging. These pathogenic features initiate disability in the cellular system like neurons and neuroglial cells that progressively undergo dysfunction until cell death. The apoptosis seems to play a key role in advancing several neurologic disorders like Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) [Citation2,Citation3]. In the physiological process, the imbalance between pro-and antioxidant species leads to oxidative stress, causing cellular and molecular damage, thus playing a crucial role in age-related disease development. Emerging research evidence suggests that inhibiting the formation of free radicals or interrupting the propagation of free radicals can control the autoxidation and subsequently reduce oxidative stress. The oxidative damage is highly dependent on the enzyme’s acquired defects in the redox-mediated signaling pathways [Citation4]. Normally brain cells effectively regulate oxygen consumption and reactive oxygen species (ROS) production, with its limited antioxidant resource. Any disturbance in this mechanism makes it highly vulnerable to oxidative stress. Oxidative damage has been related to various neurodegenerative conditions like AD, PD, amyotrophic lateral sclerosis, demyelinating diseases, cerebrovascular disorders, and psychiatric disorders [Citation5,Citation6]. With aging, the balance between ROS production and available antioxidants diminishes, increasing the chance of age-related neurological disorders.
Enzymatic antioxidants like superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) compounds (GPx, GSH, GST, and GR), and other non-enzymatic antioxidants play a key role in terminating the chain reaction of free radicals. Maintaining the balance between cellular reduction and oxidation (redox) is mandatory for ROS-mediated signaling and mitochondrial function [Citation7]. Interruptions in the antioxidant system like GSH markedly instigate the mitochondrial ROS production, initiate the membrane depolarization, and play a pivotal role in scavenging ROS through the enzymatic and non-enzymatic pathways. The free thiol group of GSH contributes to non-enzymatic antioxidant activity, whereas GSH reductase, glutathione peroxidase (GPx), and GSH-S transferase provide enzymatic reactions [Citation8].
Testosterone is a neuroactive steroid hormone, having an influential role over the nervous system through androgen receptors [Citation9]. Men with higher testosterone exhibited a reduced incidence of depression and mood disorders. The age-dependent decline in testosterone level could cause increased anxiety, mood disorder and stressed psychological behavior and these facts indicate the organizational or activational effect of testosterone[Citation10]. The testosterone decline is one of the potential causes of neuronal oxidative stress that could induce cognitive impairment [Citation11].
The hippocampus is one of the brain’s main regions attributed to cognitive functions. Hippocampal neurons seem to undergo major biochemical changes during learning and memory and exhibit adult neurogenesis to establish neuronal connections and function. The hippocampal neurons are influenced by testosterone, and its depletion leads to reduced synaptic density and firing, increased cell death, cognitive decline, and anxiety [Citation12]. The testosterone’s neuroprotective effect could be due to the prevention of ROS formation [Citation13]. However, apoptosis in hippocampal neurons induced by oxidative stress under testosterone depletion is still not elucidated. Using an orchidectomized rat model, the impact of testosterone-depletion induced oxidative damage in hippocampal neurons, and anxiety-like behavior were investigated in this study.
2. Materials and methods
2.1. Reagents and chemicals
The following essential reagents & chemicals were obtained from Hi-Media, Merck, SRL, Qualigens, and Sigma: Hematoxylin, Eosin, DNase, Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) biotin nick-end labeling (TUNEL), 1,1,3,3-tetra ethoxy propane (malondialdehyde [MDA]), Sodium Dodecyl sulphate (SDS), Thiobarbituric acid (TBA), Pyrogallol, Ethylene diamine tetraacetic acid (EDTA), Diethylene triamine penta acetic acid (DTPA), Potassium dichromate, Hydrogen peroxide, Sodium azide, GSH, Trichloroacetic acid (TCA), 5-5′′dithiobis (2-nitrobenzoic acid) substrate (DTNB), Reduced GSH, 1-chloro-2,4-dinitrobenzene (CDNB). The primary antibodies β-actin, caspase 3, and Bcl-2, were purchased from Biorbyt and Santa Cruz Biotechnology Inc., Dallas, TX and secondary antibodies from Biorbyt, Santa Ana, CA and GeNei, Bangalore, India. Protease Inhibitor was acquired from Sigma-Aldrich, Kenilworth, NJ and enhanced chemiluminescence kit from Thermo Scientific – Pierce Biotechnologies, Rockford, IL.
2.2. Animals and study groups
The experiment was carried using adult male Wistar albino rats weighing 250–300 g. Thirty-six rats were procured from the central animal house facility, University of Madras. Animals were housed three per cage, fed with standard rodent pellet and water ad libitum. The animals were maintained on a 12 h light/dark cycle under a controlled environment with the temperature at 22–24 °C and relative humidity of 50–60%. Quarantine procedures and animal maintenance were followed according to the recommendations of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (India) guidelines for laboratory animal facility [Citation14]. The study protocol was approved by the institutional animal ethics committee, Dr. ALM PGIBMS, University of Madras, Chennai, India. The rats were randomly allocated for the following groups, i.e. Control (Normal), Orchidectomy (ORX), ORX supplemented with testosterone (ORX + T), and Control (Normal) supplemented Testosterone (Cont + T). The testosterone propionate was dissolved in propylene glycol and administered at a dosage of 5 mg/kg (i.p) as a testosterone supplement.
The ORX and ORX + T groups underwent bilateral ORX. The procedure was done under ketamine and xylazine anesthesia. After the onset of anesthesia, the scrotal hairs were clipped, and the surgery area was cleaned with an antiseptic solution. The testicles were approached through a ventral scrotal incision. The spermatic cord structures were cauterized with bipolar diathermy, and both the testes were removed. The wound was closed in layers using an absorbable suture thread. The rats were allowed to recover in separate cages, and the postoperative period was uneventful. The testosterone supplementation to the Cont + T group was given for 14 d. Testosterone administration was started in the ORX + T rats after two weeks of recovery until the end of the study, i.e. 14 d. The observation/data collection was done during this period for all the groups.
2.3. Open field (OF)
An OF test was conducted to determine the exploratory activity of the rats. The OF apparatus was constructed using plywood (100 × 100 × 45 cm) consist of sixteen squares drawn with white lines on its floor and open at the top. Each rat was placed at the center of the arena (floor) and allowed to explore for 300 s. The floor was disinfected, thoroughly cleaned and allowed to dry between each subjects’ explorations/trails. The scoring was given to an animal for its exploratory behavior in the OF. The time spent in the peripheral squares indicates increased anxiety, and that in the central squares indicates lesser anxiety or normal [Citation15].
2.4. Elevated plus maze (EPM)
The elevated plus-maze is a widely used technique to analyze rats’ anxiety response, based on their response to the novel environment and avoiding conflict. The wooden made apparatus consists of four narrow arms (two open and two enclosed arms) of equal length, oriented along a single plane, and elevated 60 cm from the ground. The enclosed arms have walls (30 cm), while the open arms are without walls. The open arms lie perpendicular to the enclosed arms across its center that forms a plus-maze. The anxiety behavior was studied by measuring the animals’ relative exploration between the two different environments. During the experiment, the rats were placed in the central platform facing an open arm, allowing them to explore the new environment for 300 s freely. The apparatus was cleaned between each animal’s trial with disinfectant and dried. The rodent’s innate curiosity to explore novel environments and the fear for the open arms are used in this experiment to determine the animal’s anxiety-like behavior [Citation16]. The total number of entries in these arms and the time spent in the open and closed arms relative to overall trial duration (300 s) were extrapolated for anxiety behavior.
2.5. Euthanasia and tissue harvesting
At the end of an experimental period, the animals were euthanized. The hippocampus was isolated from the brain and was processed for biochemical, immunoblotting, and histopathological evaluations.
2.6. Biochemical analysis
For the biochemical analysis, the hippocampus tissue was weighed and homogenized in 0.1 M Tris–HCL (pH 7.4) for 15 min at 3500 rpm in a cooling centrifuge (−4 °C). The supernatant and used to assess lipid peroxidation (LPO), enzymatic, and non-enzymatic activities.
2.6.1. Estimation of lipid peroxidation (LPO)
The LPO status in the tissue was estimated using the method previously described for the hippocampus [Citation17]. In brief, the reaction was performed by adding the tissue homogenate with SDS, acetic acid and TBA. After keeping the tubes in a boiling water bath for 50 min, the solution was processed to measure the intensity of pink chromogen at 532 nm using a spectrophotometer (Epoch, Biotek, Winooski, VT). The reactive substances thiobarbituric acid (TBARS) were measured. The extent of LPO was expressed as µmol of MDA/mg protein.
2.6.2. Estimation of superoxide dismutase (SOD)
The SOD (EC.1.15.1.1) activity in the tissue homogenate was assayed by following the pyrogallol auto-oxidation at 420 nm using an ELISA spectrophotometer. The activity was expressed in units/milligram protein, where a unit is equivalent to the amount of SOD required to inhibit the 50% of pyrogallol auto-oxidation per minute. Pyrogallol auto-oxidizes rapidly at a faster rate to produce several intermediate products. The standard reagents like Tris–HCL buffer, EDTA, and DTPA were added with the sample. The pyrogallol was added then to determine its auto-oxidation rate. The inhibition rate of pyrogallol’s auto-oxidation brought about by the enzyme present in the tissue homogenate was measured [Citation18].
2.6.3. Estimation of catalase (CAT)
The CAT (EC.1.11.1.6.) activity was measured as described in the earlier study [Citation19]. The CAT activity assessment was expressed in μmol of hydrogen peroxide consumed per minute per milligram of protein. This method was based on reducing dichromate into chromic acetate with an unstable intermediate formation of per chromic acid while hydrogen peroxide was heated. In brief, the reaction mixture was made by adding the homogenate and the phosphate buffer to hydrogen peroxide; the reaction was arrested after 60 s with dichromate-acetic acid. The reaction mixture was kept in a boiling water bath for 10 min, then allowed to cool down. The optical density was measured at 570 nm using an ELISA spectrophotometer against the blank.
2.6.4. Estimation of glutathione peroxidase (GPx)
The GPx (EC. 1.11.1.9.) activity was estimated by converting reduced GSH into oxidized glutathione (GSSG) in the presence of the enzyme GPx [Citation20]. The tissue homogenate was taken and mixed with phosphate buffer, sodium azide, EDTA, H2O2, and GSH. The reaction was arrested at 0, 1.5, and 3 min with 10% TCA and centrifuged at 5000 rpm for 10 min. Phosphate solution was added to the supernatant, followed by DTNB, and the color developed was read at 412 nm in the ELISA spectrophotometer against the blank. GSH peroxidase activity in the tissues was expressed as μg/mg protein. One unit of enzyme activity is the amount of the enzyme that converts one nmol of GSH to GSSG in the presence of H2O2/min.
2.6.5. Estimation of reduced glutathione (GSH)
The GSH in the tissue homogenates was estimated using the method of Hasegawa & Mochizuk [Citation21]. The 5,5′-Dithiobis (DTNB) reacts with aliphatic thiol compounds to produce 1 mole of p-nitrothiophenol anion/mole of thiol. An intense yellow color was produced at the end of the reaction and it was used to measure the thiol concentration of GSH.
The tissue homogenate was added with 5% TCA and centrifuged at 5000 rpm for 10 min. The supernatant was mixed with 0.6 mM DTNB, and the final volume was made up with phosphate buffer. The optical density was measured at 412 nm in the ELISA spectrophotometer within 60 s, against the blank. The amount of GSH in the tissues was expressed as nmol/min/mg protein.
2.6.6. Estimation of glutathione-S-transferase (GST)
The activity of GST (EC. 2.5.1.18.) was assessed. The reaction mixture was prepared by adding tissue homogenate with phosphate buffer, CDNB followed by GSH and incubated at 37 °C for 15 min. The reaction mixture was cooled and transferred into 96 well plates, and the optical density was read at 340 nm using an ELISA spectrophotometer. The rate of change in enzyme activity per minute was calculated. The GST activity was expressed as μmol of CDNB conjugated/min/mg of protein [Citation22].
2.7. Histological examination (hematoxylin and eosin stain)
The brain tissue was fixed in 4% paraformaldehyde and processed for paraffin embedding. The sections (coronal plane) of 5 μm thickness were taken at the hippocampus level using a rotatory microtome (Leica biosystem, Nußloch, Germany). The sections were dewaxed in xylene, rehydrated in graded alcohol, brought to water, and stained with hematoxylin and eosin. The hippocampus’s sub-regions were analyzed, and images were documented using a light microscope (Accu-scope, Commack, NY).
2.8. TUNEL assay
In-situ cell death detection kit, fluorescein (Roche Applied Sciences, Mannheim, Germany) was used for TUNEL assay. The formalin-fixed paraffin sections were used as per the manufacture’s protocol. Briefly, the hydrated section was permeabilized and incubated with TUNEL (terminal deoxynucleotidyl transferase and labeled nucleotide mixture) reaction mixture for 60 min at 37 °C. The sections were then washed thrice with PBS and counter-stained with DAPI. The sections were observed under a fluorescent microscope (Nikon Corporation, Tokyo, Japan) at an excitation of 450–580 nm and emission of 515–565 and the images were documented.
2.9. Western blot analysis
The apoptotic (Caspase 3) and anti-apoptotic protein (Bcl-2) expression levels in the hippocampal tissues were quantified using the immunoblotting assay. The hippocampus isolated from the brain was homogenized in radioimmunoprecipitation assay (RIPA) buffer (pH7.4) and centrifuged at 12,000 rpm for 15 min. The protein concentration was determined using a Bradford [Citation23] method. The known protein concentration was subjected to SDS PAGE, and resolved proteins were electrotransferred into the PVDF membrane and blocked with blocking buffer (5%) at room temperature for 3 h. The membrane was incubated with primary antibody at 4 °C overnight and for 45 min with HRP conjugated secondary antibody. The protein expression was revealed by exposing the membrane to the enhanced chemiluminescence (ECL) substrate – Super SignalTM West Femto Maximum Sensitivity substrate kit (Thermo Scientific Pierce). The protein bands were quantified using Quantity One Software (Bio-Rod, Hercules, CA) and normalized with the internal control (β-actin). The relative protein level is expressed in arbitrary units.
2.10. Statistical analysis
GraphPad Prism version 6.01 (Graphpad Software Inc., San Diego, CA) software was used to analyze the results obtained from all the quantitative analysis. The data were subjected to one-way ANOVA with Tukey’s post-hoc test (p < .05).
3. Results
3.1. Open field
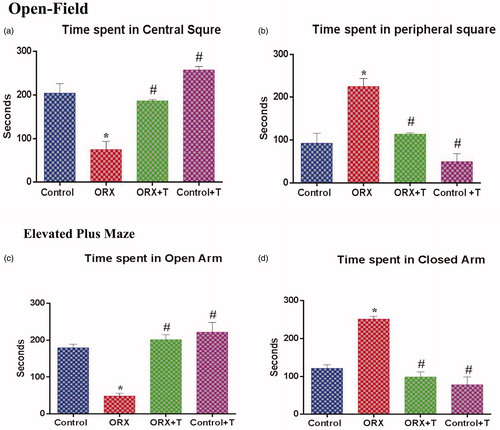
The data from the OF test were interpreted to the animals’ anxiety status as shown in . The ORX rats spent significant time [F (3, 8) = 22.50, p < .0003 & η2 = 0.8940] in the peripheral square compared with control. The ORX + T, control, and Cont + T animals spent significantly more time [F (3, 8) = 26.45, p < .0002 & η2 = 0.9084] in the central square.
Figure 1. The anxiety level of the animal was assessed via Open field and Elevated Plus Maze by comparing the time spent in the center square, peripheral square, open and closed arm, respectively. a) Time spent in the center square, b) Time spent in the peripheral square, c) Time spent in the open arm, and d) Time spent in the closed arm. *p ≤ .05 versus control group, #p ≤ .05 versus ORX group. Values represent mean ± SEM (n = 6/Group).
3.2. Elevated plus-maze
The subjects’ time in the open arm was considered non-anxiety or normal and that in the closed arm as anxiety or stress/depression. The ORX + T and Cont + T rats spent more time in the open arm [F (3, 8) = 43.29, p < .0001 & η2 = 0.9420] whereas, ORX animals spent significantly more time in the closed arm [F (3, 8) = 32.96, p < .0001 & η2 = 0.9251] ().
3.3. Biochemical assessment of hippocampus
The hippocampus analysis revealed a significantly increased LPO in the ORX group with an increased MDA level [F (3, 20) = 5.455, p < .0066 & η2 = 0.4500] () compared to the control and ORX + T groups.
Figure 2. (a) Illustrates the lipid peroxidation level in the hippocampus of control and various experimental groups. The values are expressed in μmol of MDA formed/mg protein. (b) Graphs show the superoxide dismutase (SOD) level in control and other experimental groups. The values are expressed in units/mg of protein. (c) Depicts the catalase level in the hippocampus from various experimental groups. The values are expressed in μmol of H2O2 consumed/min/mg protein. (d) The glutathione peroxidase (GPx) level in control and other groups. The values are expressed in nmol of GSH consumed/min/mg protein. (e) Represents reduced glutathione (GSH), and values are expressed in nm/min/mg protein. f) Illustrates the glutathione-S-transferase (GST) level in the hippocampus of various experimental groups. The values are expressed in μmol of CDNB-NADPH complex formed/min/mg protein. *p ≤ .05 versus Control group, #p ≤ .05 versus ORX group. Values represent mean ± SEM (n = 6/Group).
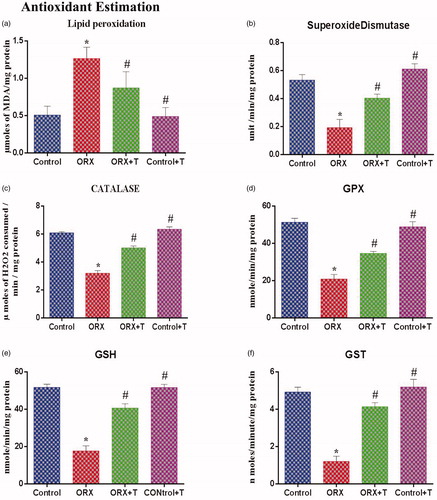
The SOD activity [F (3, 20) = 18.60, p < .0001 & η2 = 0.7361], CAT [F (3, 20) = 92.78, p < .0001 & η2 = 0.9330] and glutathione compounds (GPX [F (3, 20) = 42.55, p < .0001 & η2 = 0.8646], GSH [F (3, 20) = 57.28, p < .0001 & η2 = 0.8957] & GST [F (3, 20) = 37.21, p < .0001 & η2 = 0.8481]) were significantly improved in ORX + T when compared with ORX group ().
3.4. Histological assessment of hippocampus (H & E stain)
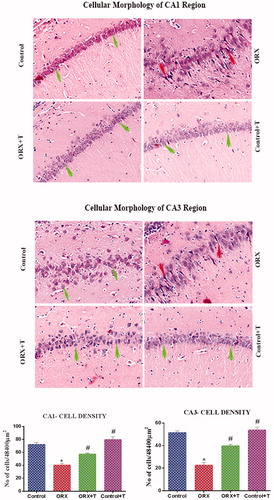
The H & E stained hippocampus sections in the CA1 and CA3 regions in control rats showed round shape neuronal soma with a bright nucleus, indicating normal morphology (). Compared to control and ORX + T groups, the CA1 and CA3 cells in ORX rat showed a significantly increased darkly stained, shrunken, and pyknotic nucleus presenting cells [F (3, 20) = 39.24 and 59.66, p < .0001 & η2 = 0.8548 and 0.8995]. The Cont + T group showed normal morphology.
Figure 3. Image depicts H & E stained photomicrograph of CA1 (top) and CA3 (middle) subregion of the hippocampus. The green arrow shows viable cells with bright nucleus staining, and the red arrow represents the darkly stained shrunken dead cells. Magnification: 200 X. The histogram (bottom) shows the number of cells in various CA1 and CA3 subregions of the hippocampus. *p ≤ .05 versus control group, #p ≤ .05 versus ORX group. The values are expressed as no cells per cubic millimeter and represented as mean ± SEM (n = 6/Group).
3.5. In-situ localization of apoptotic nuclei in the hippocampus
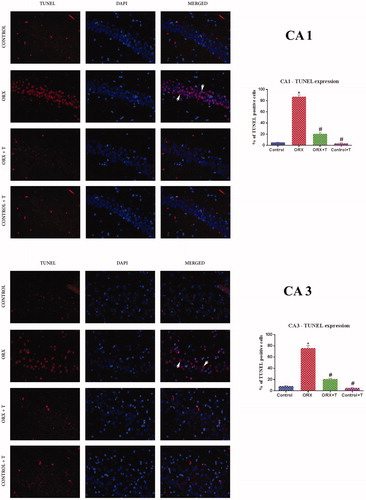
The TUNEL positive or apoptotic nuclei were predominant in the CA1 and CA3 regions of the ORX group. However, in ORX + T rats, the apoptotic nuclei were reduced. There was no noticeable change observed in the control and Cont + T groups. The TUNEL positive nuclei in CA1 and CA3 subregions of the hippocampus were quantified using ImageJ software (NIH, Bethesda, MD). The TUNEL positive cells versus viable or normal cells were represented in percentage [F (3, 24) = 379.9 and 196.4, p < .0001 & η2 = 0.9794 and 0.9672] ().
Figure 4. Photomicrograph demonstrating in situ detection of apoptotic cells by TUNEL stain in a) CA1 subregion b) CA3 sub-region of various experimental groups’ hippocampus. White arrowhead shows viable cells merged with TUNEL positive bright red cells. Magnification: 400X cells. Corresponding graphs represent the quantification of TUNEL positive cell expression and its relative percentage against DAPI expression. Data are presented as mean ± SEM (n = 6/Group).
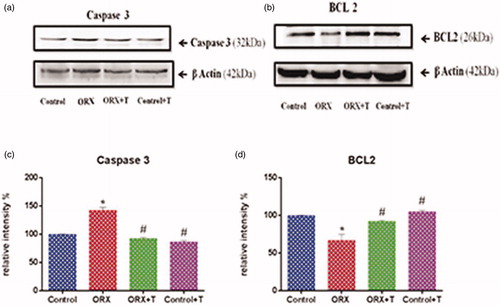
3.6. Quantitative assessment of apoptotic proteins
The apoptotic related protein, caspase 3 expression was significantly up-regulated in ORX rats [F (3, 8) = 69.11, p < .0001 & η2 = 0.9628] than the control rat () and a concomitant reduction in Bcl-2 (). However, there was a significant increase in the level of Bcl-2 in ORX + T [F (3, 8) = 16.27, p < .0009 & η2 = 0.8592] and the Cont + T rat hippocampus.
Figure 5. Shows western blot analysis of apoptotic and anti-apoptotic proteins of the hippocampus in experimental groups. Images represent western blot bands (a & b) and graphs (c & d) represents the relative intensity of protein expression. *p ≤ .05 versus control group, #p ≤ .05 versus ORX group. Values are represented as mean ± SEM (n = 6/Group).
4. Discussion
To study the anxiety behavior influenced by testosterone depletion affected by oxidative injury to hippocampal neurons, we used the orchidectomized model. After two weeks from ORX, the testosterone showed complete depletion or became undetectable in all the orchiectomized animals. The increased anxiety and depressive-like behavior that were observed in ORX rats in the OF and elevated plus-maze, were effectively overturned after testosterone supplementation in the ORX + T group. Testosterone affects various brain functions, especially regions associated with cognition and sexual activity [Citation24]. Literature indicates that anxiety is mediated via testosterone metabolites activating the AR in neuronal cells [Citation25]. The AR is a ligand-dependent nuclear transcription factor and member of the steroid hormone nuclear receptor family [Citation26]. The AR inhibited animal model showed an increased corticosterone and ACTH level, indicating that AR inhibition reflected as stress stimuli in these animals and led to an alteration in the hypothalamic-pituitary-adrenal axis [Citation27].
The testosterone depletion after castration in pubertal and adult rats showed an anxiety-like behavior in the OF and elevated plus-maze compared to peri-pubertal castrated rats [Citation28], indicating the increased dependency for testosterone in the adult brain function. Testosterone’s anxiolytic effect and its metabolites seem to vary with the dose and duration of exposure [Citation26]. Understanding the association between testosterone and anxiety could present potential therapeutic targets. It has been indicated that the anxiolytic effect of testosterone is more than its metabolites like 3α-diol and androsterone, which are binds to the GABAA/benzodiazepine receptor [Citation29]. Literature indicates that testosterone replacement and the outcome depend on the age, dose, and duration of treatment. In this study, the castrated adult rats supplemented with testosterone showed an anxiolytic effect.
The antioxidant complexes play a crucial role in preventing cellular oxidative stress and the ensuing LPO. MDA is the most abundant LPO-specific aldehyde in the biological system [Citation30]. A significantly increased MDA level was observed in the hippocampal tissue taken from ORX rats. Concomitantly, SOD, CAT, GPx, GSH, and GST enzymes activities in the ORX rat hippocampal tissue were significantly reduced compared to the control group. It indicates that the antioxidant defense mechanism in the hippocampal neurons gets disturbed in the absence of testosterone. In the case of testosterone supplementation, ORX + T rats showed antioxidant enzyme levels similar to the control rats. It shows that testosterone can influence stabilizing the antioxidant system in hippocampal neurons and thereby culminate free radicals and propagation of LPO.
The brain tissue is more vulnerable to oxidative stress, and LPO due to its high oxygen consumption, abundant polyunsaturated fatty acids, ions, and low antioxidant competence [Citation4], and elevated LPO is a critical player in neurodegenerative diseases like AD, PD, etc. [Citation6]. Initiated LPO propagates continuously like a chain reaction. During the process, MDA is one of the end-products, a highly toxic aldehyde molecule with the ability to exacerbate oxidative injuries [Citation31]. The prevention of chain initiation and propagation of free radical-mediated LPO can be achieved by an antioxidants mechanism that inhibits oxidative damage to various cell structures and the toxicity process [Citation32] and could otherwise push the cell to the death pathway. Studies have shown that testosterone supplementation reduces MDA levels in the brain and has effectively prevented LPO by promoting antioxidant expressions [Citation33].
Oxidative stress plays a key role in aging-related neurodegenerative disorders [Citation11]. The superoxide anion or hydrogen peroxide from the mitochondria is also produced from several cellular sites that include plasma membrane, cytosol, and peroxisomes [Citation34]. These species can attack nucleic acids, enzymes, proteins, and cellular layers that result in severe neuronal damage. Under normal conditions, these radicals were neutralized by the counteraction of antioxidant defense systems. Antioxidant deficiency aggravates mitochondrial damage and increases the imbalance in the mitochondrial redox mechanism in the neural tissue leading to neurodegenerative disorders [Citation35]. Further, the absence of testosterone exacerbates the mitochondrial defects by affecting fission–fusion action in the mitochondrial protein and reducing the enzyme complex I activity of the mitochondrial respiratory chain and progresses mitochondrial dysfunction.
This cascade of events leads to a diverse range of mitochondrial disorders and ultimately reduces ATP production and triggers apoptosis [Citation36]. The defects of mitochondria and the overproduced ROS are detected in the brain of subjects with aging-related neurodegenerative disorders. This study results indicate that testosterone exerts a protective effect against apoptosis by controlling ROS production and preserving the mitochondrial respiratory chain.
The primary line of defense mechanism in a neuronal cell against ROS is offered by ubiquitous metalloenzyme SOD, detoxifying the oxygen-free radical and producing H2O2. The H2O2 is further catalyzed into H2O and O2 by CAT and GPx [Citation37]. GSH inhibits LPO through free radical scavenging, retains the membrane integrity, elevates non-enzymatic detoxification of hydroxyl radicals and acts as a principal mitochondrial antioxidant defense system. The increased level of GSH observed in the ORX + T group indicates its protective role against oxidation via the GSH redox cycle and it might also directly detoxify the oxygen species [Citation38].
In the hippocampus, maintaining the balance of antioxidant enzymes is crucial to set out the superoxide anion and peroxides. GST works synergistically with GSH and GPx to detoxify the ROS and LPO. The GST is widely distributed in mitochondria, other intracellular organelles and also bound with cell membranes [Citation39]. The GSH is the chief thiol and forms a primary antioxidant defense against ROS when reacting with ROS; it could be oxidized to form GSSG and recycled back to GSH by GSH reductase [Citation40]. The detoxification of H2O2 maintains the redox balance in the cell’s cytosol, plasma membrane, and mitochondria through GSH [Citation41]. The concentration of GSH depends on the equilibrium between its consumption and biosynthesis, and a decrease in GSH adversely affects the cellular thiol balance. The ORX rat hippocampus showed a reduced level of these antioxidant systems and indicated a severe imbalance, ensuing castration. Increased or uncontrolled H2O2 molecules affect cell viability and elevate oxidative stress, forcing the neurons for an apoptotic pathway [Citation42]. They can react with various molecules like nitric oxide to yield peroxynitrate anions that react to CO2, causing damage to nitrotyrosine protein. Likewise, it can react to LPO breakdown molecules through reduced transition metals and produce hydroxyl radicals [Citation38]. The pathophysiology can cause neuronal damage in the ORX rats and consequent alteration in behavioral tasks.
The testosterone depletion by gonadectomy resulted in neuronal damage or apoptosis through oxidative stress. Though the neuronal cell death mechanism is poorly understood, mitochondrial involvement seems to be a key player [Citation43]. Understanding the link between androgen and neuroprotection is indispensable to delineate therapeutic targets for neurodegenerative diseases. It has been recognized that testosterone plays an influential role in the neuronal process, such as proliferation, differentiation, and cell death through its receptors [Citation44]. In ORX + T rat hippocampal tissue, the apoptosis has been prevented. Evidence shows that the AR‐dependent androgen-activated MAPK/ERK signaling in neurons can prevent apoptosis [Citation45].
The histological observation indicated cells with pyknotic nuclei in the CA1 and CA2 subregions of the ORX rat hippocampus. With TUNEL staining, there was a clear indication of positive apoptotic nuclei in these subregions of the castrated hippocampus. Concomitantly, there was a significant increase in caspase3 activation and a significant reduction in Bcl-2 indicates triggered apoptotic pathway in the neurons of ORX rat hippocampus. In the ORX + T rat hippocampus showed significantly increased Bcl-2 and reduced caspase3. Thus, prevented the cells from the apoptotic pathway in ORX + T rats and demonstrated the neuroprotective effect of testosterone.
Studies indicate that androgen’s usefulness as a therapeutic strategy under pre-existing neuro-pathology or neurodegenerative conditions needs systematic analysis and its limitations in the context of aging [Citation38]. The analysis is required for a greater understanding of the putative membrane-associated AR pathway. The age of the subject, duration of administration and nature of pathology are very important factors in the therapeutic usage of testosterone [Citation46].
In this study, the normal adult rats treated with testosterone (Cont + T) showed no obvious behavioral change. Histology and biochemical analysis of the hippocampus showed normal cell morphology and no indication of oxidative toxicity due to the elevated testosterone in the system. The testosterone administration to normal and orchiectomized adult rats did not produce any undesirable side effects and indicated a good tolerance range in the short-term. These animals did not show aggressive or anxiety-like behavior.
Understanding the androgen role in neurons’ antioxidant expression under normal and diseased conditions is vital for an effective therapeutic implication. It would efficiently address neuronal oxidative damage and apoptotic pathway in aging and aging-related neurodegenerative disorders associated with hypogonadism. This study confirms that testosterone is indispensable in the normal adult hippocampus and deficiency seems to be a potential risk factor for neurodegenerative disorders.
Ethics approval
The study was approved by the institutional animal ethical committee, Dr. ALM PGIBMS, University of Madras, Taramani (IAEC Approval NO: 01/06/2015). The quarantine procedures and animal maintenance were according to the recommendations of “The committee for the purpose of control and supervision of experiments on animals” (CPCSEA), India, guidelines for laboratory animal facility in India.
Consent for publication
Not applicable, as the manuscript does not contain data from any person’s.
Author contributions
SJM: Performed the experiments, data collection, and statistical analysis; PS: Conceived the study idea, methodology design, acquiring funding for the research, consolidating data, and writing the manuscript.
Disclosure statement
The author’s confirmed that no conflicts of interest exist. All authors participated in interpreting the results, reviewing drafts of the manuscript, and approved the manuscript’s final version for publication. All authors read and approved the final manuscript.
Data availability statement
We declare that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes.
Additional information
Funding
References
- Uddin MS, Al Mamun A, Jakaria M, et al. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci Total Environ. 2020;707:135624.
- Rahman S, Archana A, Jan AT, et al. Dissecting endoplasmic reticulum unfolded protein response (UPRER) in managing clandestine modus operandi of Alzheimer’s disease. Front Aging Neurosci. 2018;10:30.
- Abushouk AI, Negida A, Ahmed H, et al. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: future applications in Parkinson’s disease. Biomed Pharmacother. 2017;85:635–645.
- Tan BL, Norhaizan ME, Liew WPP, et al. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. 2018;9:1162.
- Uddin MS, Kabir MT, Al Mamun A, et al. APOE and Alzheimer’s disease: evidence mounts that targeting APOE4 may combat Alzheimer’s pathogenesis. Mol Neurobiol. 2019;56(4):2450–2l465.
- Uttara B, Singh AV, Zamboni P, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462.
- Farhat Z, Browne RW, Bonner MR, et al. How do glutathione antioxidant enzymes and total antioxidant status respond to air pollution exposure? Environ Int. 2018;112:287–293.
- Seppan P, Muhammed I, Mohammad ZIK, et al. Pathobiology of ischiocavernosus and bulbospongiosus muscles in long-term diabetic male rats and its implication on erectile dysfunction. Aging Male. 2019;1:1–12.
- Celec P, Ostatníková D, Hodosy J. On the effects of testosterone on brain behavioral functions. Front Neurosci. 2015;9:12.
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12.
- Pan W, Han S, Kang L, et al. Effects of dihydrotestosterone on synaptic plasticity of the hippocampus in mild cognitive impairment male SAMP8 mice. Exp Ther Med. 2016;12(3):1455–1463.
- Lei Y, Renyuan Z. Effects of androgens on the amyloid-β protein in Alzheimer’s disease. Endocrinology. 2018;159(12):3885–3894. [cited 2020 Feb 11]Available from: https://academic.oup.com/endo/article/159/12/3885/5094961.
- CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257–274.
- Sestakova N, Puzserova A, Kluknavsky M, et al. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol. 2013;6(3):126–135.
- Baldo B, Petersén Å. Analysis of nonmotor features in murine models of Huntington disease. Movement disorders. 2nd ed. Amsterdam, Netherlands: Elsevier; 2015. p. 583–602.
- Abhijit S, Tripathi SJ, Bhagya V, et al. Antioxidant action of grape seed polyphenols and aerobic exercise in improving neuronal number in the hippocampus is associated with decrease in lipid peroxidation and hydrogen peroxide in adult and middle-aged rats. Exp Gerontol. 2018;101:101–112.
- Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006;27(4):451–457.
- Doungue HT, Kengne APN, Kuate D. Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract, and juice in aluminum chloride-induced Alzheimer’s disease rats. Nutrire. 2018;43(1):23.
- Popović N, Stojiljković V, Pejić S, et al. Modulation of hippocampal antioxidant defense system in chronically stressed rats lithium. Oxid Med Cell Longev. 2019;2019:8745376.
- Hasegawa N, Mochizuki M. Improved effect of Pycnogenol on impaired spatial memory function in partial androgen deficiency rat model. Phytother Res. 2009;23(6):840–843.
- Nagashima R, Sano S, Huong NQ, et al. Enhanced expression of glutathione S-transferase in the hippocampus following acute treatment with trimethyltin in vivo. J Pharmacol Sci. 2010;113(3):267–270.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Hartmans C, Comijs H, Jonker C. Cognitive functioning and its influence on sexual behavior in normal aging and dementia. Int J Geriatr Psychiatry. 2014;29(5):441–446.
- Hodosy J, Zelmanová D, Majzúnová M, et al. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol Biochem Behav. 2012;102(2):191–195.
- Wagner BA, Braddick VC, Batson CG, et al. Effects of testosterone dose on spatial memory among castrated adult male rats. Psychoneuroendocrinology. 2018;89:120–130.
- Cunningham RL, Lumia AR, McGinnis MY. Androgen receptors, sex behavior, and aggression. Neuroendocrinology. 2012;96(2):131–140.
- Carrier N, Saland SK, Duclot F, et al. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol Psychiatry. 2015;78(4):259–269. [cited 2020 Dec 16]Available from: https://www.sciencedirect.com/science/article/pii/S0006322315000402.
- Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr). 2009;31(2):119–126.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438.
- Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28(12):1685–1696.
- Petrou AL, Petrou PL, Ntanos T, et al. A possible role for singlet oxygen in the degradation of various antioxidants. A meta-analysis and review of literature data. Antioxidants. 2018;7(3):35.
- Meydan S, Kus I, Tas U, et al. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J Chem Neuroanat. 2010;40(4):281–285.
- Cruz-Topete D, Dominic P, Stokes KY. Uncovering sex-specific mechanisms of action of testosterone and redox balance. Redox Biol. 2020;31:101490.
- Ahmed H, Abushouk AI, Gabr M, et al. Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed Pharmacother. 2017;90:638–649.
- Wang F, Yang J, Sun J, et al. Testosterone replacement attenuates mitochondrial damage in a rat model of myocardial infarction. J Endocrinol. 2015;225(2):101–111.
- Lee KH, Cha M, Lee BH. Neuroprotective effect of antioxidants in the brain. Int J Mol Sci. 2020;21(19):7152.
- Holmes S, Singh M, Su C, et al. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology. 2016;157(7):2824–2835.
- Marí M, Morales A, Colell A, et al. Mitochondrial glutathione: features, regulation and role in disease. Biochim Biophys Acta. 2013;1830(5):3317–3328.
- Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homeostasis network. Free Radic. Biol. Med. 2016;95:27–42.
- Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289(13):8735–8741.
- Carvour M, Song C, Kaul S, et al. Chronic low-dose oxidative stress induces caspase-3-dependent PKCdelta proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann NY Acad Sci. 2008;1139:197–205.
- Hu K, Li S, Xiao B, et al. Protective effects of quercetin against status epilepticus induced hippocampal neuronal injury in rats: involvement of X-linked inhibitor of apoptosis protein. Acta Neurol Belg. 2011;111(3):205–212.
- Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol Metab. 2001;12(10):460–468.
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94(6):1639–1651.
- Matsumoto AM. Is high dosage testosterone an effective male contraceptive agent? Fertil Steril. 1988;50(2):324–328.
