Abstract
Aims: Subdermal implantable buprenorphine (BSI) was recently approved to treat opioid use disorder (OUD) in clinically-stable adults. In the pivotal clinical trial, BSI was associated with a higher proportion of completely-abstinent patients (85.7% vs 71.9%; p = .03) vs sublingual buprenorphine (SL-BPN). Elsewhere, relapse to illicit drug use is associated with diminished treatment outcomes and increased costs. This study evaluated the cost-effectiveness of BSI vs SL-BPN from a US societal perspective.
Methods: A Markov model simulated BSI and SL-BPN cohorts (clinically-stable adults) transiting through four mutually-exclusive health states for 12 months. Cohorts accumulated direct medical costs from drug acquisition/administration; treatment-diversion/abuse; newly-acquired hepatitis-C; emergency room, hospital, and rehabilitation services; and pediatric poisonings. Non-medical costs of criminality, lost wages/work-productivity, and out-of-pocket expenses were also included. Transition probabilities to a relapsed state were derived from the aforementioned trial. Other transition probabilities, costs, and health-state utilities were derived from observational studies and adjusted for trial characteristics. Outcomes included incremental cost per quality-adjusted-life-year (QALY) gained and incremental net-monetary-benefit (INMB). Uncertainty was assessed by univariate and probabilistic sensitivity analysis (PSA).
Results: BSI was associated with lower total costs (−$4,386), more QALYs (+0.031), and favorable INMB at all willingness-to-pay (WTP) thresholds considered. Higher drug acquisition costs for BSI (+$6,492) were outpaced, primarily by reductions in emergency room/hospital utilization (−$8,040) and criminality (−$1,212). BSI was cost-effective in 89% of PSA model replicates, and had a significantly higher NMB at $50,000/QALY ($20,783 vs $15,007; p < .05).
Conclusions: BSI was preferred over SL-BPN from a health-economic perspective for treatment of OUD in clinically-stable adults. These findings should be interpreted carefully, due to some relationships having been modeled from inputs derived from multiple sources, and would benefit from comparison with outcomes from studies that employ administrative claims data or a naturalistic comparative design.
Introduction
Over 2.4 million people in the United States (US) suffer from opioid use disorder (OUD)Citation1, which is characterized as compulsive use of heroin or prescription pain medications, despite adverse social, psychological, or physical consequencesCitation2. These consequences manifest as increased risks of severe sequela such as HIV, hepatitis, skin and blood infections, overdose, and opioid-induced death: the latter of which has increased 400% since the year 2000, and now accounts for 91 deaths every day in the USCitation3,Citation4. Other burdens of opioid abuse and dependence have been incurred by society via associated increased crime, lost wages and work productivity, and health risks to non-abusers (e.g. accidental pediatric and perinatal exposure)Citation5,Citation6.
Economically, opioid abuse has become a national concern, with related healthcare costs levied primarily on managed care payors, and associated societal costs on employers and local governments. Direct medical costs of opioid abusers are nearly nine-times those of non-abusersCitation7, while the total estimated annual medical and societal costs attributable to opioid abuse total $62B (adjusted to 2016 from 2009)Citation5. These annual OUD-attributable costs are driven largely by workplace ($28B), healthcare ($28B), and criminal justice ($6B) expenditures. Thus, there is a strong economic incentive to stabilize opioid abusers and prevent relapse to illicit opioid use.
Persons with OUD can reduce their risk of relapse to illicit opioid use by remaining engaged in treatment programs that include a combination of psychosocial counseling and pharmacological maintenance treatment, which include sublingual buprenorphine (SL-BPN), methadone, and injectable naltrexone. The effectiveness of these medications notwithstanding, each has characteristics that may exacerbate relapse risk and perpetuate the opioid epidemic. For example, SL-BPN and methadone can be diverted for non-medical useCitation8,Citation9, and the latter in particular is associated with social stigma and workplace discriminationCitation10,Citation11. Injectable and implantable naltrexone formulations cannot be diverted, but their application is limited by a required 7–10 day pre-treatment abstinence periodCitation12, and potentially increased risk of drug overdose death among relapsed patientsCitation13.
Subdermal implantable buprenorphine (BSI) has recently been approved in the US, based on the results of a Phase 3 clinical trial in which it was non-inferior to SL-BPN for the proportion of patients remaining abstinent for ≥4 months over the course of a 6 month trialCitation14,Citation15. As an implantable formulation of buprenorphine, it is theorized that BSI will offer the agonist benefits of SL-BPN, but with lower risks of diversion, misuse, and non-adherenceCitation16, and subsequent reductions in associated medical costsCitation17. Anecdotally, however, concerns have been raised over the higher acquisition and administration costs of BSI relative to SL-BPN, given that the latter is available in generic formsCitation18.
Given the aforementioned clinical and economic implications of BSI coupled with the currently uncertain sociopolitical milieu in healthcare fundingCitation19, clinicians, payors, and policy-makers may be interested in the health-economic implications of implementing OUD treatment with BSI. This analysis was developed as a resource for these interested parties to assess whether BSI offers acceptable value for its cost.
Methods
Overview
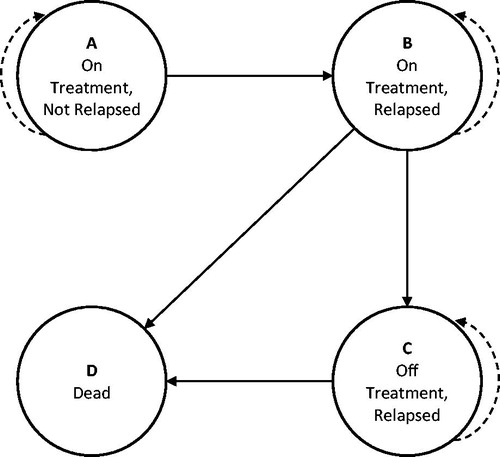
Consistent with health technology assessment guidelinesCitation20 and recent similar analysesCitation2,Citation9,Citation21, a Markov model was developed to assess the cost-effectiveness of BSI vs SL-BPN among opioid-dependent, clinically-stable adults from a US societal perspective. The model structure () was comprised of four mutually exclusive health states through which simulated cohorts passed monthly for 12 months. The 12-month time horizon was chosen because the only clinical evidence comparing BSI and SL-BPN has a follow-up of 6-months, and extrapolation of that data beyond 12 months would have been unreliable. Furthermore, the 12-month modeled-time horizon is consistent with previous similar analysesCitation21,Citation22, as well as with guidelines from the National Institute on Drug Abuse that recommend a minimum of 12 months of OUD substitution therapyCitation21,Citation23.
Differences in component costs and effectiveness parameters were summarized and the primary outcomes were the incremental cost-effectiveness ratio (ICER) for BSI cost per quality-adjusted life-year (QALY) gained (Equationequation 1(1) ) and the incremental net monetary benefit (INMB) of BSI vs SL-BPN (Equationequation 2
(2) ).
(1)
(2)
A willingness-to-pay (WTP) of $50,000 per QALY was applied as the cost-effectiveness threshold. Outcomes at or below that value were considered cost-effective. No discounting was applied, due to the short time-horizon.
State transition probabilities
The model structure consisted of four mutually-exclusive health states: (a) on treatment, not relapsed; (b) on treatment, relapsed; (c) off treatment, relapsed; and (d) dead (). This composition is similar to previous US-based cost-effectiveness analyses of maintenance treatments reported by Jackson et al.Citation9 and Schackman et al.Citation24. A key difference was an additional health state in the previous analyses wherein one could be off treatment and not relapsed. This state was not included in the present model due to an absence of evidence on off-treatment abstinence, which was also encountered in the aforementioned two previous analysesCitation9,Citation24. Those analyses modeled the health state with indirect evidence, but the same approach applied here would have unduly biased the outcomes in favor of BSI. Had this state been included, it would have been reasonable to assume that BSI patients would transition from “off treatment without relapse” to “off treatment with relapse” less frequently than SL-BPN patients, because discontinued BSI patients would continue to receive buprenorphine for up to 6 months via their implants, depending on the time of discontinuation relative to the most recent administration. Again, the lack of evidence for this state coupled with an emphasis on a conservative analysis led to this state not being included.
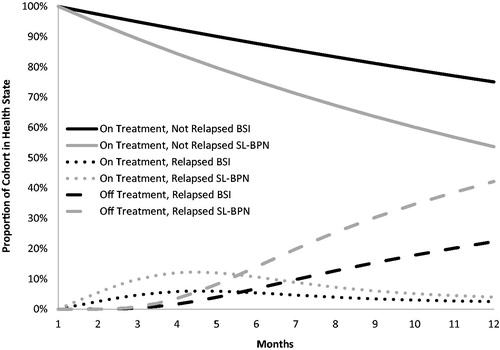
The monthly probability of transition from State A to State B was derived from the pivotal Phase 3 clinical trial comparing BSI vs SL-BPNCitation15. This was based on the hazard ratio (HR) of time to first evidence of on-treatment illicit opioid use by urine toxicology: HR = 0.49 (0.25–0.97); p = .03715, which was extrapolated to 12 months with an exponential function. An exponential function was chosen over other options (e.g. Weibull), based on its closer approximation of the clinical trial outcomes up to 24 weeks (). The remaining transitions were modeled with inputs drawn from the peer-reviewed literature (Appendix). These were preferred over clinical trial-based evidence for two reasons: (1) the health states modeled here are often not included in clinical trials, and (2) clinical trial-based evidence for these states would not have reflected the real world perspective sought in this analysis due to the artificial nature of follow-up and application of retention methods employed in clinical trials.
Other rates and probabilities
Modeled treatment cohorts were subjected to state-dependent penalties associated with relapse and continued illicit opioid use. These penalties were identified from published observational and cost-effectiveness analyses for OUD treatmentsCitation9,Citation21 in which the patient populations were similar to that considered here (i.e. treatment-experienced, stable at onset, and/or receiving low dose maintenance therapy). The state-dependent penalties included events incurring direct medical costs (i.e. emergency room, hospitalization, out/inpatient rehabilitation, accidental pediatric poisoning by oral buprenorphine, and new hepatitis C virus [HCV] seroconversion), as well as events incurring non-medical costs (i.e. criminal justice and work-time loss). Criminal justice costs pertained only to the economic consequences of crimes committed during the modeled time-horizon. It was assumed that the probabilities of arrest and relapse-related healthcare utilization increased among those discontinuing treatment (i.e. State C)Citation25,Citation26. New HCV seroconversions arose from intravenous heroin use and intravenous misuse of oral buprenorphine. The proportion of the cohorts relapsing to intravenous heroin (as opposed to oral prescription opioids) was based on the proportion of patients in the pivotal clinical trialCitation15 who reported being primarily users of heroin. Intravenous misuse and accidental pediatric poisoning with buprenorphine was also possible in the BSI cohort, because a proportion of that cohort received supplemental oral buprenorphine according to supplemental utilization reported in the pivotal clinical trial.
Consistent with its 6-month dosing, it was assumed that the BSI cohort was implanted in Cycle 1 and explanted/implanted (for those retained in treatment) or explanted (for those discontinuing treatment) in Cycle 7. The SL-BPN cohort received medication monthly on a schedule of 8 mg per patient per day, but a small proportion of those modeled to have diverted their SL-BPN were also modeled to receive replacement medication (Appendix). Both treatment cohorts received monthly psychosocial counseling as long as they were retained in treatment. The frequencies of other in-treatment healthcare utilization related to the provision of either treatment were determined by dosing frequency (i.e. monthly for SL-BPN and every 6 months for BSI). These treatment components were extracted by Jones et al.Citation27 in their cost analysis of office-based treatment for clinically-stable OUD patients.
Costs and utilities
Costs associated with clinical and societal penalties of relapse and illicit opioid use were drawn from observational studies and administrative claims analyses of the economic burden of OUD (Appendix). These studies were identified though a targeted review of the peer-reviewed literature. All costs where adjusted to 2016 USD using the medical component and the all items component of the seasonally-adjusted Consumer Price Index for all urban consumersCitation28. The exceptions to this were the costs of BSI implantation and explantation. Because BSI has only recently been available in the US market, and because reimbursement codes for those procedures were not available at the time of this analysis, we applied procedure costs based on reimbursement amounts for single subdermal depot medication implants and explants multiplied to account for multiple units (i.e. four buprenorphine rods).
Health state utilities were drawn from a cost-effectiveness analysis of office-based buprenorphine treatment in the US24. This source was chosen because it was a single source for all utilities needed, and the utilities differentiated between those relapsing to illicit use of prescription medications and those relapsing to intravenous heroin use.
Sensitivity analysis
The impact of underlying model uncertainty was assessed with univariate and probabilistic sensitivity analysis and probabilistic sensitivity analysis (PSA). In the univariate sensitivity analysis, the model was run using the minimum and maximum acceptable values for the inputs (Appendix). The influence of a given input is represented by the difference between the cost-effectiveness when using the minimum vs the maximum value. In the PSA, inputs were varied simultaneously according to pre-defined distributions and ranges (Appendix) for 1,000 simulated iterations of the model; 95% credible intervals were derived from these bootstrapped iterations to assess the uncertainty around the summary outcomes.
Results
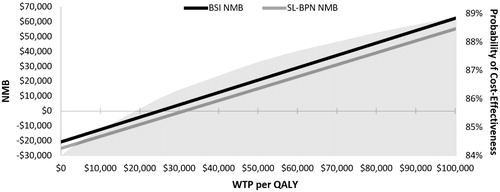
Over a 12-month modeled time-horizon, the BSI cohort achieved higher rates of complete abstinence (75% vs 54%) and retention in treatment (78% vs 58%) compared to SL-BPN (), and, as a result, incurred lower total costs ($20,733 vs $25,119) and more QALYs (0.832 vs 0.801). Thus, BSI was the dominant treatment option. The term “dominant” is reported instead of the cost-effectiveness ratio, because the negative numerator associated with cost-savings renders the ratio ambiguousCitation29. BSI was also cost-effective from the perspective of INMB, whereby it had a larger net monetary benefit than SL-BPN at all WTP considered (). At the base case WTP ($50,000 per QALY), BSI was associated with an INMB of $5,953, which conveys that, for each patient treated with BSI instead of SL-BPN, society gained $5,953 in benefits.
Figure 3. Net monetary benefit and probability of cost effectiveness at various WTP. BSI, buprenorphine subdermal implant; NMB, net monetary benefit; QALY, quality-adjusted life-year; SL-BPN, sublingual buprenorphine; WTP, willingness-to-pay.
Direct medical costs comprised 93% and 88%, respectively, of total costs for BSI and SL-BPN cohorts (). BSI was associated with higher costs for drug acquisition ($9,414 vs $2,922) and supplemental use ($54 vs $37), but lower costs related to emergency room and hospitalization utilization ($8,444 vs $16,484) and lower costs from treatment administration, diversion, new HCV infection, rehabilitation service utilization, and pediatric poisonings. All combined, BSI-associated direct medical costs were lower than in the SL-BPN cohort ($19,367 vs $22,031). The same trend was observed for non-medical costs where BSI was associated with savings of $1,721 ($1,367 vs $3,088). Non-medical cost savings were mostly attributable to lower criminal justice costs in the BSI cohort ($1,265 vs $2,476) ().
Table 1. Modeled outcomes at 12 months.
In univariate sensitivity analysis, the relative monthly probability of relapse while on treatment had the largest impact on outcomes. At its lowest possible value (i.e. smallest difference in relapse probability between treatments) the INMB was −$3,498. At its highest possible value the INMB was $15,263. This is a range of ±157% around the base case INMB of $5,953. The probability of arrest and the utility of being on treatment without relapse were the second and third most influential variables, yielding ranges of ±12% and ±9% around the base case, respectively.
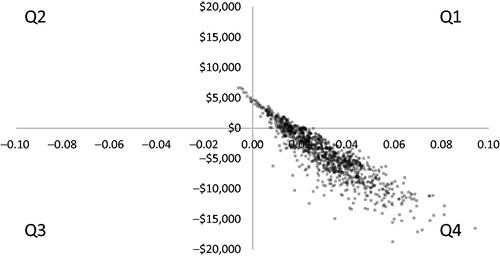
A PSA with 1,000 iterations was conducted to assess model uncertainty, as represented by the 95% credible intervals (CI) around the cost-effectiveness outcomes. This was first applied to the cost per QALY outcome, whereby BSI was cost-effective in 89% of the iterations and dominant in 84% (). Informative CIs could not be calculated from this perspective, due to the majority of the ratios having a negative, and, therefore, ambiguous, value. This limitation was overcome by using the same iterations to calculate the CI around net monetary benefit. In doing so, we observed that the median net monetary benefit of BSI was significantly higher than that of SL-BPN at a WTP of $50,000 per QALY: BSI = $20,812 (95% CI = $20,689–$20,935) vs SL-BPN = $15,099 (95% CI = $14,778–$15,420); p < .05. The shaded region in depicts the cumulative probability of BSI having a positive INMB (i.e. that it is cost-effective). This probability across the 1,000 bootstrapped simulations reached 88% at $50,000 per QALY.
Figure 4. Scatterplot of PSA cost (y-axis) per QALY (x-axis) outcomes (n = 1,000 iterations). Q1 BSI is more costly and more effective than SL-BPN, >$50,000 per QALY (10%); Q1 BSI is more costly and more effective than SL-BPN, ≤$50,000 per QALY (5%); Q2 BSI is more costly and less effective than SL-BPN (1%); Q3 BSI is less costly and less effective than SL-BPN (0%); Q4 BSI is less costly and more effective than SL-BPN (84%). BSI, buprenorphine subdermal implant; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; SL-BPN, sublingual buprenorphine.
Discussion
Using a Markov model conducted from a US societal perspective and populated with the outcomes of a pivotal Phase 3 clinical trial and relevant inputs from the peer-reviewed literature, BSI was found to be cost-effective vs SL-BPN for office-based OUD treatment among clinically-stable adults. This determination is predicated on lower total costs and better outcomes associated with BSI. The relative rates of on-treatment relapse for the two treatment cohorts had the largest impact on modeled outcomes; however, the model was robust to underlying input variability and uncertainty, as evidenced by: (a) significantly higher net monetary benefit for BSI, (b) non-overlapping credible intervals derived from 1,000 iterations of that measure, and (c) 90% of model replicates in the PSA having resulted in BSI being cost-effective.
Medication diversion cost was not a major factor in this analysis, despite being noted as a driver of the OUD economic burdenCitation5. This could have been due to multiple factors. First, SL-BPN is inexpensive compared to BSI, and replacing a month’s supply—which was the only explicit economic impact of diversion in the present analysis—would have also been quite inexpensive. Second, a large component of diversion’s economic impact may be found in the increased risk of relapse related to the diversion of one’s oral therapyCitation3. The economic impact of diversion may, therefore, be indirectly subsumed under the direct medical and societal costs associated with the transition from being on therapy with illicit opioid use to being off therapy with illicit opioid use.
That drug acquisition costs favored SL-BPN (BSI = $9,414 vs SL-BPN = $2,922) was unsurprising, given that BSI is a branded product, while SL-BPN is generic. In addition, the modeled SL-BPN cohort received 8 mg per patient per day, which has anecdotally been described as a relatively low dose that is appropriate for a clinically-stable population. This assumption was based on the dosing from the pivotal clinical trialCitation15 and allowed us to model an appropriate comparator cohort relative to BSI, which is indicated for OUD patients who are clinically-stable on ≤8 mg of transmucosal buprenorphine dailyCitation14. Nevertheless, interpreting higher monthly price in a cost-effectiveness context is informative. For example, we applied a WTP of $50,000 per QALY, and the maximum justifiable BSI acquisition cost can theoretically be considered the value at which the ICER equals $50,000. If one adjusted BSI acquisition cost until the ICER reached that threshold, the cost per 6 month administration would increase from $4,950 to $8,080. The current price of BSI is justifiable by this standard, but perhaps payors might disagree given that it includes non-medical costs and assumes a WTP measure (i.e. cost per QALY) that is more relevant to a societal perspective. In this case, we might instead include direct medical costs only and apply a more restrictive definition of cost effectiveness: $14,000 per patient retained in treatment. This has been used as a measure of cost-effectiveness elsewhere in the OUD literatureCitation21, and the threshold value is equal to the excess annual direct medical costs borne by third-party payors for patients in the US with OUDCitation30–32. Under these circumstances, BSI acquisition cost could be increased to $7,817 and still remain cost-effective.
These findings are based on an economic model that represents reality imperfectly, but its external validity can be approximated by comparing the modeled outcomes for the SL-BPN cohort to outcomes reported in analyses of real-world data. In a systematic review of the economic burden of OUD in the US, Oderda et al.Citation33 described administrative claims analyses with similar features to the present study. Five of the analyses assessed annual direct medical costs among commercially-insured persons treated for OUD, and the reported range across the studies was $13,007–$99,536 (adjusted to 2016 US$)Citation30–32,Citation34,Citation35. One of the five studies was an analysis of high- vs low-cost OUD patientsCitation34, without which the range was $22,706–$30,293. At $22,031, our modeled total direct medical cost for the SL-BPN cohort is consistent with this range. Our outcomes are at the lower end, owing to two factors. First, our model included a limited number of medical cost components in order to produce conservative outcomes that could be reliably estimated based on available data. Commercial claims analyses on the other hand generally include all possible direct medical costs, and may inherently report higher observed costs vs modeled costsCitation36. Second, the patient population assumed in our model included only clinically stabilized adults (i.e. those who achieved prolonged clinical stability on ≤8 MG daily of transmucosal buprenorphine), which is consistent with the approved indication for BSICitation14. The claims analyses reported in Oderda et al.Citation33 included these patients, but also un-stabilized patients and new entrants to OUD treatment for whom treatment success is less likely and direct medical costs are higherCitation34,Citation37. Taking these factors into consideration, the present model calculates direct medical costs that are consistent with real-world analyses, and provides a reasonable estimation of the cost-effectiveness of BSI vs SL-BPN.
This model also included non-medical costs attributable to criminal justice (e.g. arrests, economic impact on victims, incarceration, etc.), lost wages/productivity, and out of pocket costs, which together comprised 6% vs 12%, respectively, of the total costs in the BSI and SL-BPN groups, and 40% of the total cost differential between them. Real-world economic analyses of persons with OUD have reported that non-medical costs may contribute a larger economic burden than direct medical costsCitation5,Citation38. The disparity is primarily due to a single factor. The real-world economic burden studies included both clinically stable and unstable persons with OUD; whereas the present model included only clinically stable persons. Clinical instability is associated with higher societal burden (i.e. non-medical costs), particularly criminalityCitation39, and so it follows that the real-world data studies would report proportionally higher non-medical costs.
Although this model was developed according to established practices in health-economic modelingCitation20 with clinical and economic inputs chosen to be consistent with the opioid dependent, clinically stable population, some of the relationships and outcomes simulated here have not been observed in real-world settings because BSI has only recently been approved in the US. Thus, there is some risk of misestimation due to differences in underlying sample characteristics of the contributing studies. Again, this risk was mitigated by deriving inputs from studies with sample characteristics similar to the cohort assumed here. As the number of patients treated with BSI increases, electronic health records and administrative claims databases will offer opportunities to further understand the health-economic implications of BSI.
The outcomes of this model support BSI as a pharmacoeconomically preferable treatment option for opioid dependent, clinically-stable adults. This stable patient sub-group comprises only a portion of the treated OUD population, but the benefits of BSI in this sub-group might also translate into a re-distribution of resources to more effectively treat other sub-groups (e.g. less stable, new entrants to treatment). While no health-economic model should circumvent clinical judgment, providers, payors, and policy-makers should be aware that BSI offers an opportunity to improve outcomes and reduce costs.
Transparency
Declaration of funding
This work was funded by Braeburn Pharmaceuticals, holder of the US rights to Probuphine.
Declaration of financial/other relationships
RD is a former employee of Braeburn Pharmaceuticals. JC is an employee of EPI-Q Inc., which received consulting fees related to the development and execution of this work. MF was not compensated for his role in this work, but he served as a clinical investigator for subdermal implantable buprenorphine clinical trials and he previously received consulting fees from Braeburn Pharmaceuticals related to subdermal implantable buprenorphine. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16-4984, NSDUH Series H-51); 2016. https://www.samhsa.gov/data/. Accessed November, 15 2016
- Asche CV, Clay E, Kharitonova E, et al. Budgetary impact of the utilization of buprenorphine/naloxone sublingual film and tablet for Medicaid in the United States. J Med Econ 2015;18:600-11
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016;374:154-63
- Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65:1445-52
- Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med 2011;12:657-67
- Inocencio TJ, Carroll NV, Read EJ, et al. The economic burden of opioid-related poisoning in the United States. Pain Med 2013;14:1534-47
- White AG, Birnbaum HG, Mareva MN, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm 2005;11:469-79
- Cicero TJ, Ellis MS, Surratt HL, et al. Factors contributing to the rise of buprenorphine misuse: 2008-2013. Drug Alcohol Depend 2014;142:98-104
- Jackson H, Mandell K, Johnson K, et al. Cost-effectiveness of injectable extended-release naltrexone compared with methadone maintenance and buprenorphine maintenance treatment for opioid dependence. Subst Abus 2015;36:226-31
- Earnshaw V, Smith L, Copenhaver M. Drug addiction stigma in the context of methadone maintenance therapy: an investigation into understudied sources of stigma. Int J Ment Health Addict 2013;11:110-22
- Tran BX, Vu PB, Nguyen LH, et al. Drug addiction stigma in relation to methadone maintenance treatment by different service delivery models in Vietnam. BMC Public Health 2016;16:238
- SAMHSA Advisory. An introduction to extended-release injectable naltrexone for the treatment of people with opioid dependence; 2012. http://store.samhsa.gov/shin/content//SMA12-4682/SMA12-4682.pdf. Accessed November 15, 2016
- Gibson AE, Degenhardt LJ, Hall WD. Opioid overdose deaths can occur in patients with naltrexone implants. Med J Aust 2007;186:152-3
- FDA News Release. FDA approves first buprenorphine implant for treatment of opioid dependence. Silver Spring, MD: FDA. 2016. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm503719.htm. Accessed May 26, 2016
- Rosenthal RN, Lofwall MR, Kim S, et al. Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA 2016;316:282-90
- White J, Bell J, Saunders JB, et al. Open-label dose-finding trial of buprenorphine implants (Probuphine) for treatment of heroin dependence. Drug Alcohol Depend 2009;103:37-43
- Bebinger M. The promise and price of new addiction treatment implant. WBUR; 2016. http://www.wbur.org/commonhealth/2016/05/19/probuphine-opioid-treatment-implant. Accessed May 19, 2016
- Bebinger M. FDA considering pricey implant as treatment for opioid addiction. National Public Radio (NPR); 2016. http://www.npr.org/sections/health-shots/2016/05/20/478577515/fda-considering-pricey-implant-as-treatment-for-opioid-addiction. Accessed May 20, 2016
- Cartwright WS. Economic costs of drug abuse: financial, cost of illness, and services. J Subst Abuse Treat 2008;34:224-33
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Heal J Int Soc Pharmacoeconomics Outcomes Res 2013;16:231-50
- King JB, Sainski-Nguyen AM, Bellows BK. Office-based buprenorphine versus clinic-based methadone: a cost-effectiveness analysis. J Pain Palliat Care Pharmacother 2016;30:55-65
- Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess 2007;11:1-171
- NIDA. Principles of drug addiction treatment: A research-based guide, 3rd edition. NIDA; 2012. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/podat_1.pdf. Accessed April 6, 2017
- Schackman BR, Leff JA, Polsky D, et al. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med 2012;27:669-76
- Kopak AM, Haugh S, Hoffmann NG. The entanglement between relapse and posttreatment criminal justice involvement. Am J Drug Alcohol Abuse 2016;42:606-13
- Clark RE, Baxter JD, Barton BA, et al. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence. Health Serv Res 2014;49:1964-79
- Jones ES, Moore BA, Sindelar JL, et al. Cost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend 2009;99:132-40
- United States Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers. 2016. https://data.bls.gov/cgi-bin/dsrv?cu. Accessed February 1, 2017
- Koerkamp BG. Uncertainty in medical decision making. Rotterdam, The Netherlands: Erasmus University; 2009. https://repub.eur.nl/pub/17360/091201_GrootKoerkamp,Bas.pdf. Accessed November 15, 2016
- Rice JB, Kirson NY, Shei A, et al. Estimating the costs of opioid abuse and dependence from an employer perspective: a retrospective analysis using administrative claims data. Appl Health Econ Health Policy 2014;12:435-46
- Rice JB, Kirson NY, Shei A, et al. The economic burden of diagnosed opioid abuse among commercially insured individuals. Postgrad Med 2014;126:53-8
- Roland CL, Joshi AV, Mardekian J,. Prevalence and cost of diagnosed opioid abuse in a privately insured population in the United States. J Opioid Manag 2013;9:161-75
- Oderda GM, Lake J, Rudell K, et al. Economic burden of prescription opioid misuse and abuse: a systematic review. J Pain Palliat Care Pharmacother 2015;29:388-400
- Rice J, Bodnar K, Shei A, et al. Differences between high cost and low-cost patients diagnosed with opioid abuse. Value Health 2014;17:A214-15
- Pasquale MK, Joshi AV, Dufour R, et al. Cost drivers of prescription opioid abuse in commercial and Medicare populations. Pain Pract 2014;14:E116-25
- McCue MJ, Palazzolo JR. Analysis of actual versus projected medical claims under the first year of ACA-mandated coverage. Inquiry 2016;53:1-5
- Tkacz J, Pesa J, Vo L, et al. Opioid analgesic-treated chronic pain patients at risk for problematic use. Am J Manag Care 2013;19:871-80
- McCollister KE, French MT. The relative contribution of outcome domains in the total economic benefit of addiction interventions: a review of first findings. Addiction 2003;98:1647-59
- Marel C, Mills KL, Darke S, et al. Static and dynamic predictors of criminal involvement among people with heroin dependence: findings from a 3-year longitudinal study. Drug Alcohol Depend 2013;133:600-6
- Campbell MD, Kolodner G, Spencer RA, et al. Drug test results as a predictor of retention among patients using buprenorphine in a comprehensive outpatient treatment program. J Addict Dis 2016;35:315-24
- Degenhardt L, Randall D, Hall W, et al. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009;105:9-15
- Lavonas EJ, Banner W, Bradt P, et al. Root causes, clinical effects, and outcomes of unintentional exposures to buprenorphine by young children. J Pediatr 2013;163:1373-7
- US Food and Drug Administration. Briefing document for the FDA Advisory Committee Meeting held December 11, 2015. Silver Spring, MD:US FDA; 2015. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM480733.pdf. Accessed November 15, 2016
- Larance B, Degenhardt L, O’Brien S, et al. Prescribers’ perceptions of the diversion and injection of medication by opioid substitution treatment patients. Drug Alcohol Rev 2011;30:613-20
- Lofwall MR, Wunsch MJ, Nuzzo PA, et al. Efficacy of continuing medical education to reduce the risk of buprenorphine diversion. J Subst Abuse Treat 2011;41:321-9
- Jordan AE, Des Jarlais DC, Arasteh K, et al. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend 2015;152:194-200
- Eiden C, Nogue E, Diot C, et al. Three complementary approaches to characterize buprenorphine misuse. Subst Use Misuse 2016;51:1912-19
- Braeburn Pharmaceuticals. Braeburn Pharmaceuticals announces commercialization plans for Probuphine (buprenorphine) implant, six-month treatment for opioid dependence. Braeburn Pharmaceuticals; 2016. https://braeburnpharmaceuticals.com/category/press-release/. Accessed February 1, 2017
- Massachusetts Executive Office of Health and Human Services. List A: Opioids without abuse-deterrent properties. 2016. http://www.mass.gov/eohhs/docs/dph/quality/drugcontrol/drug-formulary/2016-meetings/cost-info-long-lasting-opioids-040716.pdf. Accessed February 15, 2017
- Le TK, Kalsekar A, Macaulay D, et al. Treatment patterns, health care resource utilization, and costs in U.S. patients diagnosed with chronic hepatitis C infection who received telaprevir or boceprevir. J Manag Care Spec Pharm 2015;21:308-18
- Chandwani HS, Strassels SA, Rascati KL,. Estimates of charges associated with emergency department and hospital inpatient care for opioid abuse-related events. J Pain Palliat Care Pharmacother 2013;27:206-13
- Lee J. The costs of drug rehab. Drug Rehab 2013. http://www.choosehelp.com/topics/drug-rehab/the-costs-of-drug-rehab. Accessed February 1, 2016
- Institute for Clinical and Economic Review. Management of patients with opioid dependence: A review of clinical, delivery system, and policy options; 2014. http://icer-review.org/wp-content/uploads/2016/01/CEPAC-Opioid-Dependence-Final-Report-For-Posting-July-211.pdf. Accessed November 15, 2016
- US Census Bureau. Income Data Tables; 2016. https://www.census.gov/topics/income-poverty/income.html. Accessed June 1, 2017
- Lengyel T, Brown M. Everyone pays: A social cost analysis of incarcerating parents for drug offenses in Hawai’i; 2009. https://caphawaii.files.wordpress.com/2012/07/everyonepays_full.pdf. Accessed November 15, 2016
- Centers for Medicare and Medicaid Services. CY 2017 Physician Fee Schedule Final Rule. 2017. https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeesched/. Accessed February 1, 2017