Abstract
Aims: Prophylaxis with standard-acting recombinant factor IX (rFIX) in hemophilia B patients requires frequent injections. Extended half-life (EHL) products allow for prolonged dosing intervals, and so reduce this treatment burden. Three technologies are employed to extend the half-life of FIX; glycopegylation, Fc-fusion, and albumin fusion. rIX-FP is a novel albumin fusion protein, which allows for a prolonged dosing interval of up to 14 days. A systematic review and indirect statistical comparison was performed to evaluate the efficacy of both EHL and standard-acting rFIX products compared with rIX-FP in Phase III trials for prophylaxis in adult hemophilia B patients.
Materials and methods: A systematic search was conducted in both EMBASE and PubMed to identify Phase III trials of prophylactic rFIX treatment in previously treated hemophilia B patients aged ≥12 years (FIX ≤2%). Annualized bleeding rate (ABR), spontaneous ABR (AsBR), and joint ABR (AjBR) data were extracted from each study. A z-test was performed using the mean of each parameter, and the mean difference in outcome between studies was calculated.
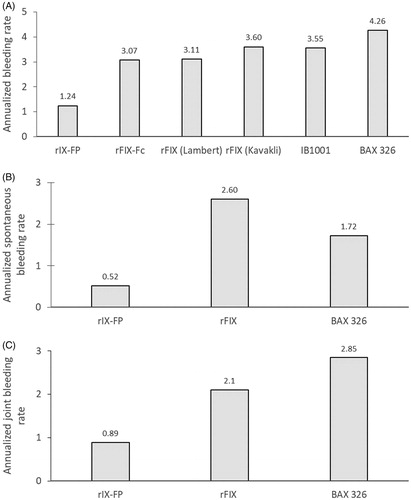
Results: Seven articles investigating six rFIX products were identified. Median ABR, AsBR, and AjBR ranged from 0–3.0, 0–1.0, and 0–1.1 (means = 0.8–4.26, 0.13–2.6, and 0.34–2.85), respectively. rIX-FP achieved the lowest median and mean values in all three parameters. Z-tests showed that mean ABR was significantly lower for rIX-FP 7-day prophylaxis compared with the majority of standard-acting and other EHL rFIX products.
Limitations: The low number of appropriate trials available for comparison limits the quantity of data available for comparison, and restricts the use of methods of adjustment for variance in study design or patient characteristics. However, these limitations are shared with similar analyses published in this field.
Conclusion: This indirect comparison of Phase III trials indicates that rIX-FP efficacy compares favorably vs other rFIX products for prophylaxis in hemophilia B.
Introduction
Hemophilia B is a recessive X-linked bleeding disorder in which inherited or spontaneous F9 gene mutations cause a deficiency or the complete absence of coagulation factor IX (FIX)Citation1,Citation2. A deficiency of FIX can lead to spontaneous bleeding events and bleeding following an injury, the frequency and severity of which varies according to residual clotting factor levelsCitation1. The primary aim of hemophilia B treatment is to prevent and treat bleeding events with FIX replacement therapy, given either on-demand or by scheduled prophylaxisCitation1,Citation3.
Response to treatment can be measured in several different ways. Annualized bleeding rate (ABR) and spontaneous ABR (AsBR) are the most commonly used objective measurements of prophylaxis efficacy. ABR captures the full range of any bleeding event, and is often used as the primary endpoint in prophylaxis studies. In contrast, AsBR only captures bleeding events with no apparent or known cause, which are typical of patients with severe disease. In any given patient, the numerical ABR tends to be higher than the AsBR; therefore, the overall treatment effect is usually detected utilizing the ABR. However, AsBR is not affected by lifestyle to the same extent as is the ABR, and, therefore, AsBR is sometimes viewed as a truer marker of disease severity. Annualized joint bleeding rate (AjBR) is another important measure, as recurrent joint bleeds can result in progressive joint damage and functional disabilityCitation3.
Maintaining FIX trough levels above 1% is often the target of prophylactic therapy, as this has been associated with a lower bleeding rateCitation1. Frequent administration of standard half-life FIX concentrate (2 times per week) is often required to maintain this trough level, and poor adherence to a regimen involving frequent infusions can compromise treatment effectivenessCitation1,Citation4,Citation5. Extended half-life (EHL) recombinant FIX (rFIX) products may reduce the burden of frequent injections and potentially improve treatment adherenceCitation6,Citation7.
rIX-FP (IDELVION) is a fusion protein genetically linking recombinant human coagulation FIX with recombinant human albumin indicated for on-demand and prophylactic treatment of bleeding in hemophilia BCitation8. In clinical trials, use of rIX-FP has resulted in a median AsBR and AjBR of 0, with up to 14-day dosing in adults and adolescents, and a trough level of 12% for 14-day dosingCitation8. Fc-fusion and PEGylation have also been used to develop EHL FIX concentrates, with rFIXFc and N9-GP both developed for use in hemophilia BCitation9,Citation10. Currently, there is no direct head-to-head study comparing the efficacy of available rFIX products in hemophilia B. Therefore, we performed a systematic literature review and statistical analysis that indirectly compared the efficacy of rFIX products for prophylaxis in adult hemophilia B patients in comparison with rIX-FP. This analysis focused on published Phase III clinical trials reporting on ABR, AsBR, and AjBR.
Methods
In this analysis, we compared ABR, AsBR, and AjBR reported for rIX-FP in adults and adolescentsCitation8, with data reported in published Phase III clinical trials of other rFIX products identified by a systematic literature review.
Search strategy and study selection
A systematic literature search was conducted in both PubMed and EMBASE on October 17, 2018. Search terms were designed to select publications according to the patient population, treatment administered, and outcomes reported (). The search was limited to articles published in English, with a date range of 1966 (PubMed) or 1968 (EMBASE) to present. All publications retrieved by this search strategy were individually assessed against pre-defined inclusion and exclusion criteria (). The goal was to identify full original articles detailing the results of Phase III clinical trials of prophylactic rFIX treatment in previously treated adult and adolescent hemophilia B patients who were diagnosed with moderate-to-severe hemophilia B (endogenous FIX levels ≤2%). Eligible studies were also required to report at least one of the outcomes of interest (ABR, AsBR, or AjBR). Phase III trials were selected as these studies require regulatory approval with specified criteria and are more likely to be similar in sample size and study rigor, and the data obtained from these trials is included within the product label. The patient number and the quality of other clinical trial phases may also have greater variability between products.
Table 1. Literature search terms.
Table 2. Inclusion and exclusion criteria for the analysis.
Publications underwent an initial screen based on the title and abstract using these inclusion and exclusion criteria. Potentially relevant publications then underwent a second screen based on the full text of the article.
The relevant data from all eligible publications was collected and aggregated to allow further analysis. Data was extracted using a standardized data extraction form according to the following outcome measures: ABR, AsBR, and AjBR.
Statistical analysis
The analyses took the approach of comparing each individual study to the rIX-FP studyCitation8. Studies which used the same products were not pooled together. The mean and standard deviation (SD) were extracted or calculated for each study and, subsequently, a z-test was performed for comparison. The mean difference in outcome between studies was calculated, along with a corresponding confidence interval.
Where multiple prophylaxis regimens were reported, a decision was taken regarding which treatment groups should be included in the analysis. For Santagostino et al.Citation8, patients could transfer between prophylaxis regimens during the study; therefore, only data from the 7-day prophylaxis regimen was analyzed in order to prevent including individual patients in the analysis more than once. For the Powell et al.Citation9 study, the mean (SD) data was only available for the 7-day prophylaxis regimenCitation11. This also enabled a comparison of the same regimen between products, as 7-day dosing was the only common regimen reported across all studies for the EHL products.
All outcomes were continuous in nature, and, therefore, mean and standard deviation (SD) values were required for the data analysis, as there are no suitable methods for comparing median valuesCitation12. Some studies presented some outcomes as only median and interquartile range (IQR)/data range. Thus, outcomes not reported as mean/SD were excluded from the analysis. Powell et al.Citation9 reported only median data for AsBR and AjBR, and so these values were excluded from the analysis; ABR data was included in the analysis, as mean/SD data was reported for this study in Iorio et al.Citation11. Collins et al.Citation10 reported only median data for ABR, AsBR, and AjBR; this study was, therefore, excluded from the statistical comparison as mean/SD data was not available for any of the three outcomes. The standard deviation for ABR was not reported by Lambert et al.Citation13; however, this was estimated by Iorio et al.Citation11, and the equivalent value was used in this analysis.
Results
Literature review
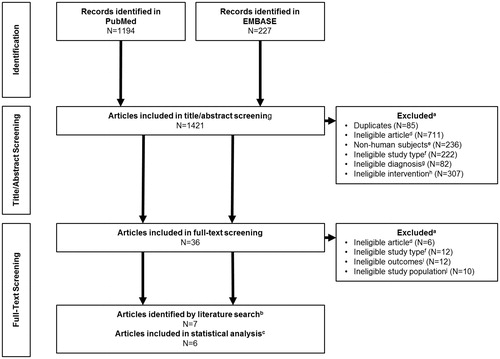
The search identified a total of 1,421 articles, with 36 passing the initial title and abstract screen. Following full-text review, nine articles met the inclusion criteria (). These studies included EHL rFIX products, with one study of rIX-FP (IDELVIONCitation8), one study of rFIXFc (AlprolixCitation9), and two studies of N9-GP (RefixiaCitation10,Citation14). Standard-acting rFIX products were also included, with two studies of rFIX (BeneFIXCitation13,Citation15), two studies of BAX 326 (RixubisCitation16,Citation17), and one study of IB1001 (IxinityCitation18). Following further evaluation, one of the articles reporting on BAX 326 was excluded, as this publication included all patients who had completed a pre-treatment study, followed by pivotal trials in adults and pediatricsCitation17. As the results of the adult pivotal trial were separately reported by Windyga et al.Citation16, the Solano Trujilo et al.Citation17 study was excluded from further review and analysis to prevent including duplicate patients. Similarly, Oldenburg et al.Citation14 reported pooled results from the paradigm trials, and so was excluded from further review to prevent patient duplication, as the results of the adult trial were reported separately by Collins et al.Citation10.
Figure 1. PRISMA diagram for the systematic literature review of articles that evaluated the treatment of previously treated adult human subjects diagnosed with moderate-to-severe hemophilia (FIX levels ≤2 %) receiving rFIX either prophylactically or on-demand. aSome articles met more than one criterion. bNine articles met the inclusion criteria initially, but Solano Trujillo et al.Citation17 and Oldenburg et al.Citation14 were excluded from further analysis as they included duplicate patients from Windyga et al.Citation16 and Collins et al.Citation10, respectively. cOf the remaining seven articles, Collins et al.Citation10 was excluded from the statistical analysis as it did not include mean/SD data for analysis. dIneligible articles consisted of manuscripts which did not describe results of a Phase III study, including post-hoc analyses, meta-analyses, and post-marketing surveillance. eExclusions for non-human subjects included any study using animals or animal tissues or in vivo, in vitro, or ex vivo studies. fIneligible study types were those that were not Phase III clinical trials, including surgical sub-studies and extension studies. gAny study where the patients were not diagnosed with hemophilia B were excluded. hStudies not treating participants with a recombinant FIX product were excluded. iEligible studies must include outcomes from at least one of the following; ABR, AsBR, AjBR. jPediatric studies were excluded, including those who combined adult and pediatric data.
The key characteristics for the included publications are summarized in . Subject ages ranged from 12–71 years, and the overall study duration ranged from 50 exposure days to up to 18 months ( and ). All of the studies included patients with endogenous FIX levels of ≤2% (moderate-to-severe hemophilia B; ). Additionally, three publications reported the proportion of patients with severe hemophilia B (endogenous FIX levels of ≤1%) in their study: 87.5% for rIX-FP in Santagostino et al.Citation8, 82.4% of patients for rFIXFc in Powell et al.Citation9, and 53.4% for BAX 326 in Windyga et al.Citation16. One publication only included patients who had received on-demand therapy prior to the studyCitation15, while five studies included patients who had received either prophylaxis or on-demand therapyCitation8–10,Citation16,Citation18; one publication did not specify the pre-study treatment regimenCitation13. The prophylaxis regimens used ranged from >3-times weekly to a 14-day regimen; the most common was a 7-day prophylaxis regimen, reported in five of the publications ().
Table 3. Study characteristics for included articles.
Table 4. Details of bleeding rates of prophylaxis patients in rFIX studies identified by the systematic review.
Median and mean ABRs for prophylaxis treatment with rIX-FP and the comparison products reported in the identified publications are presented in . Reported median values in the included studies ranged from 0–3.0 for ABR, 0–1.0 for AsBR, and 0–1.1 for AjBR. When comparing among the 7-day regimens for the EHL products and the regimens for the standard-acting products, median bleeding rates were lower with rIX-FP than with other products (all 0 for ABR, AsBR, and AjBR with rIX-FP), which was only equaled by BAX 326 (0 for AsBR and AjBR), rFIX (0 for AjBR) and N9-GP (40 IU/kg dose only, 0 for AsBR). Mean values ranged from 0.82–4.26 for ABR, 0.13–2.6 for AsBR, and 0.34–2.85 for AjBR. When comparing among the 7-day regimens for the EHL products and the regimens for the standard-acting products, rIX-FP achieved lower values for ABR, AsBR, and AjBR than other products.
Statistical comparisons
The data available for statistical comparisons of each individual study vs the Phase III rIX-FP trial was collated; a summary of the analysis results is given in and . As noted in the methods, the AsBR and AjBR data for rFIXFcCitation9 and the ABR and AsBR data for N9-GPCitation10 were excluded from the statistical comparison, as mean/SD data was not available for these outcomes. For each outcome, and for each study reporting data with the specific dosing regimen, the mean and standard deviation are reported. The final two columns summarize the difference in outcome between the study and rIX-FP. The mean difference between studies is reported, along with corresponding confidence intervals. P-values indicating the significance of the differences between studies are also given.
Table 5. Summary of statistical analysis results.
The results suggested a significant difference in ABR between rIX-FP and the majority of the other studies (using rFIXFc, rFIX, IB1001, and BAX 326). Except for borderline significance observed between rIX-FP and rFIX in the Lambert et al.13 study (p = 0.05), all other rFIX studies showed a significant difference vs rIX-FP (p = 0.02 or less). ABR was at least 1.8 episodes lower for the rIX-FP study compared to all other studies.
AsBR was found to be significantly lower for rIX-FP compared to rFIX (p = 0.04), with patients experiencing an average of 2.1 fewer spontaneous bleeding episodes per year with rIX-FP than with FIX. rIX-FP also appeared to have a lower AsBR than BAX 326 (by an average of 1.2 episodes); however, these results did not reach statistical significance.
AjBR was significantly lower with rIX-FP compared with BAX 326 (p = 0.001), by an average of 2.0 episodes per year.
Discussion
This review identified suitable data for an indirect comparison of rFIX products. The results of the systematic review and analysis demonstrated that rIX-FP has comparable or better efficacy in comparison to other EHL and standard rFIX products. Indeed, mean ABR was lower (with or approaching statistical significance) for rIX-FP 7-day prophylaxis compared to prophylaxis with both standard and EHL rFIX products demonstrating the excellent efficacy of rIX-FP.
A study was conducted by Iorio et al.Citation11 in which a systematic review and meta-analysis were used to compare the efficacy of rFIXFc with standard rFIX products. They concluded that rFIXFc showed comparable efficacy to standard rFIX products, as demonstrated by a slightly lower, but comparable, ABR when treating weekly with rFIXFc. In our study, when comparing the reported bleed rates for rIX-FP with rFIXFc, we saw a further significant improvement in efficacy, indicating improved efficacy for rIX-FP over both rFIXFc and standard rFIX products. In line with Iorio et al.Citation11, the conclusions in our study are based on results reported from clinical trials. In a real-world setting, adherence levels are lower and may differ depending on the individual product’s dosing regimen, possibly affecting the reported efficacyCitation11,Citation19,Citation20. Iorio et al.Citation11 carried out a mathematical modeling analysis to evaluate the impact of the improved treatment adherence that may be expected to accompany the reduced dosing frequency of EHL products. They concluded that improved real-world adherence with EHL products may be associated with significantly reduced ABRs compared to standard rFIX products.
rIX-FP has a longer half-life than standard rFIXCitation8, offering the potential for longer dosing intervals, reducing the patient burden and potentially increasing adherence. A long half-life and high sustained FIX trough levels means rIX-FP is efficacious, even during an extended 14-day prophylaxis regimenCitation8. As discussed above, the use of EHL rFIX products may improve treatment adherence, and therefore efficacy. In our review, good efficacy for rIX-FP in comparison with both standard and EHL rFIX was reported, even at extended dosing intervals.
There were some limitations relating to our analysis. The low number of suitable studies identified, with only one study found for each of the majority of rFIX products, limits the quantity of data available for comparison. The limited number of appropriate trials available for comparison also restricted the use of methods of adjustment for variance in study design or patient characteristics, and so potential confounders may affect the results. Data was further restricted as, although seven studies were included in the statistical analysis, not all studies provided data for analysis for each outcome. Another limitation is that suitable data was not always directly reported in the studies. Estimations were sometimes used to obtain the figures required for analysis, and lack of access to patient-level data prevented us from confirming the accuracy of these estimations. Whilst mean and standard deviations were estimated for some studies, these statistics can be influenced by the data distributions. The differences in outcomes seen between studies could, therefore, be due to differential patient characteristics and treatment methods rather than due to the study products under analysis. A number of these limitations are shared with Iorio et al.Citation11 and similar analyses; in particular, when specific data are missing or inappropriate, values are frequently estimated or converted based on reasonable assumptionsCitation11,Citation21. Another limitation common to literature searches is publication bias, although the systematic search methods selected were designed to minimize the impact of such bias.
Conclusions
In conclusion, this systematic review and statistical analysis has shown that treatment with rIX-FP in general compares favorably vs other rFIX products for prophylactic treatment of hemophilia B in adult patients. rIX-FP 7-day prophylaxis displayed significantly lower ABR than most other rFIX products, with ABR at least 1.8 episodes per year lower compared to all other products. AsBR and AjBR also showed statistical significance or trending in favor of rIX-FP, where data were available for analysis. Given the lack of direct, head-to-head trials of rFIX products, this analysis provided a summary and indirect statistical comparison of the efficacy between different rFIX products. Further analysis with real-world data regarding bleed rates, treatment regimens, and adherence in hemophilia B patients could provide additional information regarding the benefits of treatment with rIX-FP.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Transparency
Declaration of funding
This study was funded by CSL Behring LLC, King of Prussia, PA, USA.
Declaration of financial/other interests
JD received clinical research funding from CSL Behring, and has participated in scientific advisory boards for CSL Behring and Genentech; SY is employed by CSL Behring; TM has acted as a paid consultant to CSL Behring, and has received funding for research not related to this work; LA has received grants/research support from Bayer, CSL Behring, Novartis, NovoNordisk, Roche, Shire, and Sobi, support for the hemophilia nurses program from CSL Behring, Bayer, NovoNordisk, Octapharma, Roche, Shire, and Sobi, and honoraria for participating in scientific advisory boards for Bayer, Boehringer Ingelheim, Daiichi Sankyo, NovoNordisk, OrPha Swiss, Pfizer, Roche, Shire/Baxalta, and Sobi; PB has received financial support for the analysis included in this study; ES has acted as a speaker or member of advisory boards for Bayer, Shire, CSL Behring, NovoNordisk, Grifols, Kedrion, Octapharma, Roche, Sobi, Bioverativ, and Pfizer. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
The results of this literature search were presented at the International Society for Thrombosis and Haemostasis (ISTH) meeting in 2018 as a poster.
Acknowledgements
The authors thank Claire Crouchley of Meridian HealthComms, Plumley, UK for providing medical writing support, which was funded by CSL Behring LLC, King of Prussia, PA, USA in accordance with Good Publication Practice (GPP3). The authors would also like to thank Moshe Fridman of AMF Consulting, Inc. for his input regarding the statistical analysis.
References
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47.
- Franchini M. Current management of hemophilia B: recommendations, complications and emerging issues. Expert Rev Hematol. 2014;7:573–581.
- Forsyth AL, Rivard GE, Valentino LA, et al. Consequences of intra-articular bleeding in haemophilia: science to clinical practice and beyond. Haemophilia. 2012;18:112–119.
- Djambas Khayat C. Once-weekly prophylactic dosing of recombinant factor IX improves adherence in hemophilia B. J Blood Med. 2016;7:275–282.
- Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169:777–786.
- Mahdi AJ, Obaji SG, Collins PW. Role of enhanced half-life factor VIII and IX in the treatment of haemophilia. Br J Haematol. 2015;169:768–776.
- Mannucci PM, Franchini M. Emerging drugs for hemophilia B. Expert Opin Emerg Drugs. 2014;19:407–414.
- Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127:1761–1769.
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313–2323.
- Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124:3880–3886.
- Iorio A, Krishnan S, Myren KJ, et al. Continuous prophylaxis with recombinant factor IX Fc fusion protein and conventional recombinant factor IX products: comparisons of efficacy and weekly factor consumption. J Med Econ. 2017;20:337–344.
- Higgins J, Deeks J. Chapter 7: Selecting studies and collecting data. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011): The Cochrane Collaboration. Chichester, England: John Wiley & Sons, Ltd.; 2011.
- Lambert T, Recht M, Valentino LA, et al. Reformulated BeneFix: efficacy and safety in previously treated patients with moderately severe to severe haemophilia B. Haemophilia. 2007;13:233–243.
- Oldenburg J, Carcao M, Lentz SR, et al. Once-weekly prophylaxis with 40 IU/kg nonacog beta pegol (N9-GP) achieves trough levels of >15% in patients with haemophilia B: pooled data from the paradigm trials. Haemophilia. 2018;24:911–920.
- Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia. 2016;22:381–388.
- Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicentre phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level </=2%) haemophilia B. Haemophilia. 2014;20:15–24.
- Solano Trujillo MH, Stasyshyn O, Rusen L, et al. Safe switching from a pdFIX (Immunine®) to a rFIX (Bax326). Haemophilia. 2014;20:674–681.
- Collins PW, Quon DVK, Makris M, et al. Pharmacokinetics, safety and efficacy of a recombinant factor IX product, trenonacog alfa in previously treated haemophilia B patients. Haemophilia. 2018;24:104–112.
- Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7:392–396.
- Armstrong EP, Malone DC, Krishnan S, et al. Adherence to clotting factors among persons with hemophilia A or B. Hematology. 2015;20:148–153.
- Yeung CH, Santesso N, Pai M, et al. Care models in the management of haemophilia: a systematic review. Haemophilia. 2016;22:31–40.