Abstract
Aim: This study aimed to assess patients’ preferences for HIV treatment in an urban Colombian population.
Methods: A Discrete Choice Experiment (DCE) was conducted. Urban Colombian HIV patients were asked to repetitively choose between two hypothetical treatments that differ in regard to five attributes ‘effect on life expectancy’, ‘effect on physical activity’, ‘risk of moderate side effects, ‘accessibility to clinic’ and ‘economic cost to access controls’. Twelve choice sets were made using an efficient design. A Mixed Logit Panel Model was used for the analysis and subgroup analyses were performed according to age, gender, education level and sexual preference.
Results: A total of 224 HIV patients were included. All attributes were significant, indicating that there were differences between at least two levels of each attribute. Patients preferred to be able to perform all physical activity without difficulty, to have large positive effects on life expectancy, to travel less than 2 h, to have lower risk of side-effects and to have subsidized travel costs. The attributes ‘effect on physical activity’ and ‘effects on life expectancy’ were deemed the most important. Sub-analyses showed that higher educated patients placed more importance on the large positive effects of HIV treatment, and a more negative preference for subsidized travel cost (5% level).
Limitations: A potential limitation is selection bias as it is difficult to make a systematic urban/rural division of respondents. Additional, questionnaires were partly administered in the waiting rooms, which potentially led to some noise in the data.
Conclusions: Findings suggests that short-term efficacy (i.e. effect on physical activity) and long-term efficacy (i.e. effect on life expectancy) are the most important treatment characteristics for HIV urban patients in Colombia. Preference data could provide relevant information for clinical and policy decision-making to optimize HIV care.
Introduction
The Human Immunodeficiency Virus (HIV) is a virus that damages the immune system which weakens the ability to fight everyday infections and diseases. HIV continues to be a major global health problem. The World Health Organisation (WHO) estimated 36.7 million people to be infected with HIV worldwide at the end of 2016Citation1. Of this number, 1.8 million people are estimated to live in the Latin America regionCitation2 of which around 150,000 people in ColombiaCitation1. In 2016, the estimated HIV incidence rate was at 0.12 per 1,000 inhabitants, the prevalence rate at 0.25 per 1,000 inhabitants and AIDS-related deaths at approximately 11,000 in ColombiaCitation2.
Colombia has a free and universal health care system, meaning that there is free health care under the subsidized system. Antiviral therapy is used to combat the consequences of HIV. This therapy causes a decline in fatality and morbidity relating to HIV infection, and can prevent further progression from HIV to AIDSCitation3. However, treatment requires lifelong intake and is characterized by a risk of development of virus resistance when adherence is sub-optimalCitation4. Typical adherence rates for medications prescribed over long periods of time are approximately 50–75%, which is considered suboptimal for HIV treatmentCitation5. Therefore, improving adherence is a key determinant in successful antiretroviral therapy, showing a strong interrelation between adherence and diminished rates of resistance, an increase in survival and a higher quality of lifeCitation5. Adherence to antiretroviral treatment is, however, challenging, not only because the treatment for HIV is lifelong but also since patients are in relative good health and mostly without symptoms when starting therapyCitation4. It is, therefore, important to optimize treatment intake, and improve adherence with HIV treatment.
Patient preferences studies are nowadays increasingly used to inform policy decision makingCitation6. Including patient preferences when designing and evaluating healthcare programs, can prove beneficial, and help broaden the perspective on new or existing technologiesCitation7. Incorporating needs of the target population is paramount to enhance adoption and sustained use of an interventionCitation8, such as antiviral therapy adherence. To elicit patient preferences in health care, discrete choice experiments (DCE) have been increasingly conducted in recent years. A DCE is a stated preference method to elicit how respondents’ trade-off between attributesCitation9. An advantage of DCE is the ability to assess the relative importance of different treatment characteristics that influence the patient’s choiceCitation10. This model is based on the assumption that choice differences between levels show the preferences of patientsCitation7.
Previous studies have already explored patient preferences for HIV treatment. For instance, Muhlbacher et al.Citation11 conducted a DCE in a German population, and suggested that patients valued emotional quality of life the most. Terris et al.Citation12 also showed that patients are more likely to use new HIV prevention technology if the effectiveness was higher. Since the majority of DCE studies are performed in western countriesCitation9, it would be worthwhile to investigate to what extent results of these studies can be transferred to low and middle income countries. We previously conducted a best-worst scaling to elicit the most important HIV/AIDS treatment characteristics in Colombia. This study revealed that Colombian patients mostly valued drug efficacy, maximum prolongation of life and long duration of efficacyCitation13.
To further investigate how people make trade-offs between treatment attributes, a DCE was conducted as a second step. The aim of this study is, therefore, to assess patients’ preferences for HIV treatment in an urban Colombian population. Results of this study could form a basis to better cater clinical and policy decision making regarding HIV treatment to the preferences of urban Colombian people living with HIV.
Methods
A DCE was used to elicit patients’ preferences for HIV treatment of the urban population in Colombia. HIV patients were asked to repetitively choose between two treatment profiles, represented by several treatment attributes. By varying these levels within each attribute and for every question, scenarios for each choice were developed; referred to as choice sets. This study followed guidelines described by Lancsar and LouviereCitation9 and the ISPOR Good Research Practices for Conjoint Analysis Task ForceCitation7.
Attributes and levels
A two-step approach was used to identify and select attributes and levels that were estimated to be the most important treatment characteristics of HIV patients in Colombia.
First, to examine which attributes were used in previous DCEs on patients’ preferences in HIV treatment, a literature review was conducted in PubMedCitation11–17. This was aided by a previous best-worst scaling studyCitation18, which identified the most important treatment characteristics of HIV patients in Colombia. Based on the review and previous study, two independent researchers (A. G. and E. S.) blindly consulted which five attributes were preferred for inclusion. Additionally, a senior expert (M. H.) and a Colombian HIV clinician (R. C.) were consulted until consensus was reached. Following, each attribute was assigned with three associated levels, based on existing literature and the expertise of a Colombian HIV clinician (R. C.). Then four researchers (A. G., E. S., M. H. and R. C.) discussed the attribute levels until consensus was reached, which was externally validated by a Colombian professor of Rosario (J. G.).
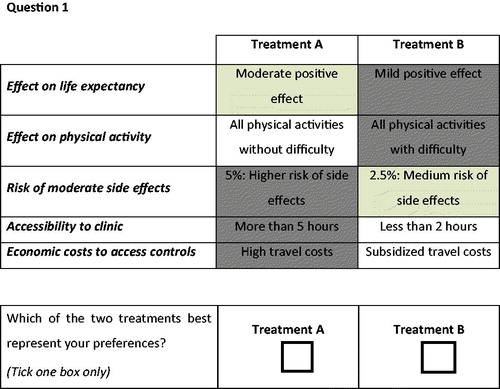
Second, to further gather information about important treatment barriers and side effects as deemed by the HIV patients, and to validate the five attributes and their levels, a focus group with six HIV patients of Assistencia Cientifica de Alta Complejidad (ACAC), a HIV clinic in Bogota Colombia was conducted on April 2018. The focus group confirmed that patients were concerned by treatment outcomes, side-effects and costs. As outcomes, the effect of treatment on their life expectancy and on their daily physical activity were deemed relevant to the patients, confirming the first two attributes (effect on life expectancy, effect on physical activity). In addition, the risk of side-effects and the accessibility to the clinic were relevant consideration for the patients. The last attribute was, however, changed after the focus group, i.e. the initially chosen attribute on frequency of visits was replaced by a new attribute on economic costs. Patients reported to be concerned by the economic costs of their treatment, while the frequency of visits was not deemed important attribute for them. Based on the two-step approach, a final list of five attributes were included in our DCE: effect on life expectancy, effect on physical activity, risk of moderate side effects, accessibility to clinic and economic costs to access controls (e.g. travel costs). details the complete list of the five attributes and their associated levels, which were presented as they are in the survey.
Table 1. List of attributes and levels.
Questionnaire
The DCE questionnaire was first drafted in English by E. S. and A. G. After review/approval by two additional experts (M. H. and R. C.), the questionnaire was translated to Spanish. A pilot with 8 HIV patients was then conducted to check understandability, and assess the applicability and practicality of the questionnaire, in semi-structured interviews. Based on their comments and recommendations, minor clarifications were added to wording.
The choice sets were developed using the software NgeneCitation19. An efficient design (fractional factorial) was used to identify the choice sets, assuming that respondents have preferences certain accompanying levels over others (e.g. those patients prefer a large positive effect on life expectancy, and low levels of side effects). These expectations were based on intuitive assumptions and existing studiesCitation11–17.
The final questionnaire started with an informed consent, followed by an explanation of the assignment, as well as an example of the choice sets (). Block design was applied through constructing two versions of the questionnaire, both with 12 different choice sets. Choice sets had no option to opt-out, since opting for no treatment is not a rational optionCitation19. The final part of the questionnaire assessed the respondent regards the perceived difficulty of the survey difficulty on a 7-point Likert scale, as well as eleven questions regarding age, socioeconomic status and patients views on treatment. The questionnaires were composed in a paper version as well as an online version. The online version was made using QualtricsCitation20.
Data collection and patient recruitment
The questionnaires were administered at a HIV clinic “Asistencia Cientifica de Alta Complejidad”, located in the city Bogota. Data were collected in May 2018 through self-administered questionnaires. Researchers AG and ES approached people in the waiting room of the clinic, and included all patients with HIV (without restrictions) if they (1) filled in the informed consent, (2) passed the dominance question, (3) filled in eight or more choice set questions, and (4) were considered urban (see description of urban patients below).
The filtering of the urban population was based two items: “Where do you live; city and barrio?” and “How much time does it take you to get to your clinic?” The city of Bogota consists of twenty municipalities (“Localidades”), which are in turn made up of more than a thousand neighborhoods (“Barrios”). Respondents were classified urban if they lived in one the “Localidades” deemed urban. For respondents from the “Localidades” San Cristobal, Bosa and Suba, the Barrio determined the urban/rural classification. If the Barrio was unknown for these specific “Localidades”, any travel time less than 2 h was classified as urban. In case the “Localidad” was unknown altogether, the classification was made on basis of time spent to get to the clinic, with 2 h being the cut-off point. Any travel time under 2 h in combination with a missing value for question five was categorized as urban (see Supplementary material for the map of “Localidades” Bogota and the urban classification).
Data analysis
Data analysis was performed with Nlogit software (Econometric Software, Inc, Plainview, NY) to estimate the strength of the preferences for attributes and levels, the trade-offs between attributes and how preferences varied by individual respondent characteristics. The analysis of the data was based on random utility theoryCitation7. The data were assessed with a mixed multinomial logit model (also called random parameter model)Citation7. This analysis allows for preferences for treatment to vary across our population. A standard deviation (SD) parameter is estimated, revealing the heterogeneity between patients.
All attributes except “risk of moderate side effects” were categorical variables. Effect coding describes the different levels by only using ones, zeros and minus ones. Effects coding was chosen over dummy coding of categorical variables as with dummy coding the parameter estimate for the baseline (omitted) category cannot be recovered. Effects coding has desirable properties in modeling conjoint-analysis data and is widely used in many conjoint-analysis applicationsCitation7. When interpreting the analysis, the sign of the coefficients indicates whether the level has a positive or negative effect compared to the mean of the attribute. The magnitude of the coefficients indicates the size of this effect. The parameter for the omitted category is the negative sum of the included-category parameters and the 95% CI of the omitted category was also based on the standard deviation and covariance of the other levelsCitation7. One of the choice sets was the dominance question, where one treatment is assumed to have better utility levels for all attributes (i.e. higher positive effect on life expectancy and physical activity, lower risk on side effects, better accessibility and lower economic costs to access the clinic) to test whether the respondents correctly understood the questionnaire. If respondents failed the test they were excluded for the analysisCitation21.
All parameters were drawn from a normal distribution and the estimation was conducted using 1,000 Halton draws. The relative importance of the attributes was then estimated with the magnitude of the coefficients using the range of the magnitude coefficients per attribute. This conditional relative importance of the attributes was estimated using the coefficients’ magnitude via the range of the magnitude coefficients per attribute. Preferences estimate from the model was then used to estimate the conditional relative importance of attributes; overall and per country. The range of attribute-specific levels is calculated via the range approach by measuring the difference between the highest and lowest coefficient for the levels of the respective attribute. By dividing the attribute-specific level range by the sum of all attribute level ranges, the conditional relative importance is calculated, via this method, the relative attribute importance always depends on the range of levels chosen per attribute and on the other attributes included in the experiment.
The MIXL model identifies for which attributes there is a significant preference variation. However, it does not provide insight into why this variation exists. Therefore, additional subgroup analyses were conducted to examine if the preferences differ among subgroups in specific covariates (i.e. age, gender, education and sexual preference). Age was classified according to patients with an age of 38 or younger and patients older than 38, with 38 being the average age of the study population. Gender was reflected by male and female, excluding the category “other”. Education was classified according to low educated (i.e. primary and secondary school) and high educated (i.e. engineer and university degree). Sexual preference was classified according to heterosexual and homosexual, with the category bisexual and other being excluded due to the limited number of participants reflecting these categories. Analyses were based on interaction terms within the final model to investigate differences per attribute between each subgroup pair. A joint model using dummy variables was, therefore, used to estimate significant differences in treatment preferences between subgroups. The data analyzed during the current study are available from the corresponding author on request.
Results
Patients’ characteristics
details the respondent characteristics. The questionnaire was completed by 257 respondents of which 33 were excluded after failing the dominance test. Eight respondents were additionally excluded based on missing values for “urban/rural”. The final dataset included 216 respondents. Forty-one (19%) respondents completed the questionnaire online, while 175 (81%) completed paper versions. Eight-nine (41%) participants rated the perceived difficulty less than four on a seven-point scale. An analysis including patients who failed the dominance test provided highly similar results.
Table 2. Patient characteristics.
Patients’ preferences
shows the patient preferences for all attributes. All attributes were significant and thus important for patients. All attributes were significant, indicating the importance for patients, as there were significant differences between at least two levels for each attribute (i.e. the 95% CI did not overlap). Patients preferred to be able to perform all physical activity without difficulty, to have large positive effects on life expectancy, to travel less than 2 h, to have lower risk of side-effects and subsidized travel costs. The standard deviation parameters, suggesting preference variation among patients per level, were significant for all parameters.
Table 3. Results from Mixed Multinomial Logit model.
Subgroup analysis
No significant differences were found between subgroups according to age, gender and sexual preference. Significant differences were however observed between education subgroups. details the coefficients and standard deviation for both low educated and high educated respondents. The results showed significant differences for the effect on life expectancy and the economic cost to access controls. First, the higher the educated group significantly value, the more the large positive effects of HIV treatment (p < .10). Second, higher educated patients significantly valued less subsidized travel cost than low educated patients (p < .05).
Table 4. Subgroup analysis on education: low education versus high education.
Discussion
This study is the first to elicit patient preferences for HIV treatment of the urban population of Colombia, and provide insights into the weightings of the attribute levels. It also provides insights into the differences in trade-off between subgroups. It is important to understand the preferences of specific populations as needs may vary across groups. Even the disproportionately affected by HIV, may be less likely to take antiretroviral therapiesCitation22. Understanding the patients’ preferences for HIV treatment could contribute to better health communication to enhance uptake of treatment. HIV patients valued a treatment with effect on physical activities without difficulty, with large positive effects on life expectancy, with less than 2 h of travel time and being subsidized travel costs. The effect on physical activity was deemed the most important attribute followed by the effect on life expectancy and the risk of moderate side effects. The performed subgroup analyses showed no significant differences for age, gender and sexual preference. However, patients with a higher education had a relatively higher preference for the large positive effects of HIV treatment, and a stronger dispreference for subsidized travel cost.
These results are mostly in line with prior studiesCitation11–17. Muhlbacher et al.Citation11 also suggested that patients valued the attribute “emotional quality of life” the most. In the previously conducted best-worst scaling in ColombiaCitation13, patients mostly valued drug efficacy, maximum prolongation of life and long duration of efficacy.
Moreover, a similar DCE study conducted in the rural population of Bogota, showed similar results. Both, urban and rural, populations deemed the attributes “effect on physical activity” and “effects on life expectancy” as most important. For rural as well as urban patients, compared to patients categorized as low educated, patients categorized as high educated preferred large effects regards life expectancy and deemed travel costs as less important. The results of our study are therefore in line with other literature, which hints at transferability to a Colombian population. However, more research is needed to understand why physical activity was found to be relatively important to the higher educated subgroup in the rural population, while this difference was not found in the urban population.
Understanding patients’ preferences for HIV treatment may inform future consensus or design meetings regards HIV treatment policy, designing medication adherence education or creating patient centered HIV treatment. Results may therefore contribute to the development of future policies taking into account the preferences of HIV patients (e.g. “all physical activity without difficulty”, “large positive effects on life expectancy” and less than 2 h travel time”). Incorporating patient needs may enhance adherence to therapy (i.e. antiretroviral treatment). Policy interventions are needed to address serious problems in public transport (e.g. with large congestions) due to the lack of underground mass transport, which is reflected by the value patients attach to a reduced travel time to access specialized points of care. In turn, enhancing adherence by incorporating patient needs may lead to societal impact. Literature shows adherence to antiretroviral therapy, prolongs and improves the lives of HIV patientsCitation23,Citation24, which positively affects health outcomes, care efficiency, and productivity, which may reduce societal costs. Patients’ preferences are nowadays increasingly investigated and used as they can provide relevant insights for policy and clinical decision making. Our study showed that HIV treatments benefits (i.e. effect on physical activity and the effect on life expectancy), risks but also costs and the access to the clinics are important for patients. Incorporating the needs regards these attributes may enhance treatment adherence, which in turn improves the lives of HIV patientsCitation23,Citation24.
This study has several limitations. One potential limitation is the risk of selection bias. The questionnaire only reached urban patients that visited one HIV clinic. Bogota officially has more than nine million inhabitantsCitation25, and a large part of these are displaced citizens from rural parts. It is challenging to make a systematic urban/rural division of respondents at the HIV clinic. For example, many displaced people live in Bogota but still have a traditional rural way of living. This increases the likelihood of rural respondents being included in the urban study population. Another limitation is that the attribute levels may be considered ambiguous, such as “large” and “moderate” which may reflect different meanings for different individuals. Attributes were also presented as they are without providing a detailed description per attribute, which means that attributes may be interpreted differently across participants. Moreover, significant differences were identified in the subgroup analyses, reflecting real differences between groups. Yet, due to a small sample size, the lack of differences between other subgroup pairs could be due to a lack of power. Furthermore, some bias in the data collection may have occurred as the questionnaire was partly administered in the waiting room, which may include distractions such as noise and time of appointment.
In conclusion, the present study revealed that all included attributes were important for HIV treatment. The effect on physical activity and the effect on life expectancy were the most valued attributes. This information could help decision makers to set up appropriate programs to fit with patients’ preferences.
Transparency
Declaration of funding
No funding was received for this study.
Declaration of financial/other relationships
There are no financial or other relationships to be declared. The authors declare that they have no competing interests. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
ES planned and managed the work, analysed and interpreted results and produced the first draft of the manuscript with support from MH, KLC, AG, RC, and JG. Different versions of the manuscript have been reviewed and conceptualized by all co-authors. KLC and ES produced the final manuscript and MH is the corresponding author. All authors have read and approved the final manuscript.
Supplemental Material
Download MS Word (164.1 KB)Acknowledgements
The authors are indebted to all participating patients of this study. The views expressed and any errors in this article are those of the authors.
References
- World Health Organisation. Number of people (all ages) living with HIV Estimates by WHO region 2017. Available from: http://apps.who.int/gho/data/view.main.22100WHO?lang=en
- UNAIDS. UNAIDS data 2017. 2017.
- Case KK, Ghys PD, Gouws E, et al. Understanding the modes of transmission model of new HIV infection and its use in prevention planning. Bull World Health Org. 2012;90:831–838A.
- Dybul M, Fauci AS, Bartlett JG, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents: the panel on clinical practices for treatment of HIV. Ann Intern Med. 2002;137:381–433.
- Grierson J, Koelmeyer R, Smith A, et al. Adherence to antiretroviral therapy: factors independently associated with reported difficulty taking antiretroviral therapy in a national sample of HIV‐positive Australians. HIV Med. 2011;12:562–569.
- Lancsar E. Deriving welfare measures from stated preference discrete choice modelling experiments, CHERE Discussion Paper No. 48. 2002.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413.
- Eldredge LKB, Markham CM, Ruiter RA, et al. Planning health promotion programs: an intervention mapping approach. Hoboken (NJ): John Wiley & Sons; 2016.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;26:661–677.
- Ryan M, Gerard K, Amaya-Amaya M. Discrete choice experiments in a nutshell. Using discrete choice experiments to value health and health care. Dordrecht: Springer; 2008. p. 13–46.
- Mühlbacher AC, Stoll M, Mahlich J, et al. Evaluating the concordance of physician judgments and patient preferences on AIDS/HIV therapy-a Discrete Choice Experiment. Health Econ Rev. 2013;3:30.
- Terris-Prestholt F, Hanson K, MacPhail C, et al. How much demand for new HIV prevention technologies can we really expect? Results from a discrete choice experiment in South Africa. PLoS One. 2013;8:e83193.
- Hendriks A, Wijnen B, van Engelen R, et al. A best-worst scaling in Colombian patients to rank the characteristics of HIV/AIDS treatment. The Netherlands:Maastricht University; 2016.
- Brégigeon-Ronot S, Cheret A, Cabié A, et al. Evaluating patient preference and satisfaction for human immunodeficiency virus therapy in France. Patient Prefer Adherence. 2017;11:1159–1169.
- Quaife M, Eakle R, Cabrera M, et al. Preferences for ARV-based HIV prevention methods among men and women, adolescent girls and female sex workers in Gauteng Province, South Africa: a protocol for a discrete choice experiment. BMJ Open. 2016;6:e010682.
- Gazzard B, Ali S, Muhlbacher A, et al. Patient preferences for characteristics of antiretroviral therapies: results from 5 European countries. J Int AIDS Soc. 2014;17:19540.
- Safarnejad A, Pavlova M, Son VH, et al. Criteria for prioritization of HIV programs in Viet Nam: a discrete choice experiment. BMC Health Serv Res. 2017;17:719.
- Hendriks A, Wijnen B, van Engelen R, et al. A best–worst scaling in Colombian patients to rank the characteristics of HIV/AIDS treatment. J Med Econ. 2018;21:468–473.
- Veldwijk J, Lambooij MS, de Bekker-Grob EW, et al. The effect of including an opt-out option in discrete choice experiments. PLoS One. 2014;9:e111805.
- Qualtrics I. Qualtrics. com. Provo (UT); 2013.
- Tervonen T, Schmidt-Ott T, Marsh K, et al. Assessing rationality in discrete-choice experiments in health: an investigation into the use of dominance tests. Value Health. 2018;21:1192–1197.
- Hauber AB, Mohamed AF, Watson ME, et al. Benefits, risk, and uncertainty: preferences of antiretroviral-naïve African Americans for HIV treatments. AIDS Patient Care STDs. 2009;23:29–34.
- Cohen MS, Gay C, Kashuba AD, et al. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1 antiretroviral therapy for preventing the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804.
- The World Factbook [Internet]. United States. 2018. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/co.html