Abstract
Background: Acute respiratory infection (ARI) accounts for over two-thirds of total antibiotic prescriptions although most are caused by viruses that do not benefit from antibiotics. Most antibiotics are prescribed in the outpatients setting. Antibiotic overuse leads to antibiotic-related adverse events (AEs), inclusive of secondary infections, resistance, and increased costs. Point-of-care tests (POCT) may reduce unnecessary antibiotics. A cost analysis was performed to assess diagnostic POCT options to identify patients with an ARI that may benefit from antibiotics in a United Kingdom (UK) outpatient setting.
Methods: Healthcare savings were estimated using a budget impact analysis based on UK National Institute for Health and Care Excellence (NICE) data and direct costs (antibiotics, AEs, POCTs) derived from published literature. Otitis media, sinusitis, pharyngitis and bronchitis were considered the most common ARIs. Antibiotic-related AE costs were calculated using re-consultation costs for anaphylaxis, Stevens-Johnson syndrome, allergies/diarrhea/nausea, C. difficile infection (CDI). Potential cost-savings from POCTs was assessed by evaluating NICE guideline-referenced POCTs (CRP, FebriDx, Sarasota, FL) as well as a target product profile (TPP).
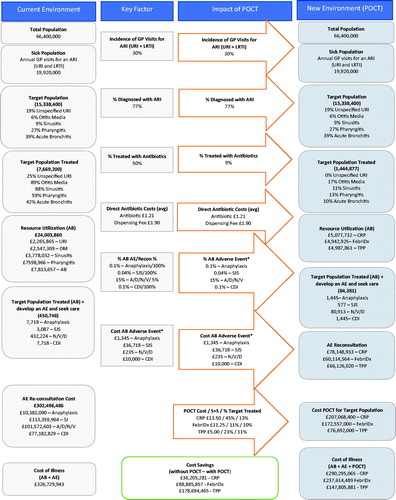
Results: Fifty-percent (7,718,283) of ARI consultations resulted in antibiotics while guideline-based prescribing suggest appropriate antibiotic prescriptions are warranted 9% (1,444,877) of ARI consultations. Direct antibiotic costs for actual ARI consultations associated with antibiotics was £24,003,866 vs. £4,493,568 for guideline-based, “appropriate” antibiotic prescriptions. Antibiotic-related AEs and re-consultations for actual vs. appropriate prescribing totaled £302,496,486 vs. £63,854,269. ARI prescribing plus AE costs totaled £326,729,943 annually without the use of delayed prescribing practices or POCT while the addition of delayed prescribing plus POCT totaled £60,114,564–£78,148,933 depending on the POCT.
Conclusions: Adding POCT to outpatient triage of ARI can reduce unnecessary antibiotics and antibiotic-related AEs, resulting in substantial cost savings. Further, near patient diagnostic testing can benefit health systems and patients by avoiding exposure to unnecessary drugs, side effects and antibiotic resistant pathogens.
Many patients are unnecessarily treated with antibiotics for respiratory infections.
Antibiotic misuse leads to unnecessary adverse events, secondary infections, re-consultations, antimicrobial resistance and increased costs.
Point-of-care diagnostic tests used to guide antibiotic prescriptions will avoid unnecessary adverse health effects and expenses.
Key points for decision makers
1. Introduction
Acute respiratory infection (ARI) comprised of otitis media, sinusitis, pharyngitis, acute cough and bronchitis account for more than two-thirds of the total antibiotic prescriptions for all medical conditions, even though most are caused by viruses for which antibiotics have no role in treatment. Most of these prescriptions occur in outpatient general practitioner (GP) or out of hours clinics. Antibiotic overuse leads to antibiotic-related adverse events (AEs), inclusive of antibiotic-associated secondary infections, antibiotic resistance, and increased healthcare costsCitation1.
Antibiotics are a common cause of medication-related severe AEs, ranging from mild (e.g. nausea, diarrhea or rash) to life-threatening (e.g. anaphylaxis, C. difficile infection (CDI))Citation2–5. Globally, it is estimated that nearly 700,000 people die annually, and 12,000 in the United Kingdom (UK) alone, as a direct result of resistant infectionsCitation6 and the economic burden of antimicrobial resistance (AMR) is estimated to range from $20,000 to $2,15,000 United States Dollar (USD) per case, with a predicted $3 trillion USD in global gross domestic product (GDP) loss by 2050Citation7. Due to the threat of rising antibiotic resistance, the UK government intends to reduce inappropriate antibiotic prescribing by 50% by 2020Citation6. Efforts aimed at reducing unnecessary antibiotic prescriptions for uncomplicated ARIs could result in the most sizable contribution to achieving the goalCitation8,Citation9.
Diagnostic uncertainty coupled with the fear of missing a serious infection and patient expectations frequently drive unnecessary antibiotic prescriptionsCitation10–14. Diagnostic test offerings in the outpatient setting are limited and thus clinicians rely on history, physical exam and sometimes scoring criteria to evaluate severity of illness and need for antibiotics. Physical examination alone has a sensitivity ranging from 50 to 70% and specificity of 60–75% for diagnosing pneumoniaCitation15. The Centor criteria for identifying bacterial pharyngitis requiring antibiotics demonstrate 40–55% accuracy at identifying group A streptococcus compared to bacterial throat cultureCitation16. A FeverPAIN criteria score of 4 or 5 has a 62–65% probability of having a bacterial infection, which is slightly higher than people with a Centor score of 4 who have a 55% probability of a bacterial infectionCitation17. Further, antibiotic prescriptions are also overprescribed because they have no direct cost to the GP surgery itself and GPs often do not consider their own prescribing practices as contributing to increasing antibiotic resistanceCitation18. Of more than 1,000 UK GPs surveyed, 55% felt patient/parent pressure to prescribe antibiotics, even when unsure antibiotics were necessary, while 44% had prescribed antibiotics to get a patient to leave the clinic or surgeryCitation19. This misuse of antibiotics has been associated with antibiotic-related AEs, the emergence of resistant pathogens and rising healthcare costs.
Interventions which reduce diagnostic uncertainty would likely be effective in promoting prudent antibiotic use about appropriate ARI management in primary care. Several laboratory based tests are available to detect pathogen etiology of a suspected ARI but are not frequently used in the UK due to concern over suboptimal diagnostic performance, high costs, and turnaround times that exceed the decision window for initiating treatment often result in prescription of antibiotic therapy in the absence of a bacterial infectionCitation20,Citation21. Unfortunately, this has led to continued overprescribing of antibiotics that carries direct prescribing costs, increased re-consultations and the major threat of antibiotic resistanceCitation20,Citation21.
Novel point-of-care tests (POCT) have the potential to improve outcomes in primary care by optimizing prescribing decisions, reducing referrals, improving efficiency of care and decreasing costsCitation22. The effectiveness of POCTs to limit antibiotic prescribing in lower respiratory tract infection (LRTI) by 21% using standalone CRP has been demonstrated in a recent studyCitation23,Citation24. GP surveys showed that at least 50–60% of respondents expressed interest in using POCTs (e.g. C-reactive protein (CRP), influenza, and Group A streptococcus) which suggests a need for a more widely accessible range of POCTs to aid clinicians with immediate decisions (urgent referrals, or immediate treatment decisions such as the decision to treat with antibiotics)Citation25.
Use of diagnostic tests has been recognized as a pathway to reduce the overuse of antibiotics, particularly simple, low-cost tests that can guide antibiotic prescription at the first point-of-contactCitation6,Citation26. Host biomarkers, such as CRP and Myxovirus resistance protein A (MxA), are indicative of the body’s systemic immune response to a clinically significant infection, whereas the lack of a systemic immune response suggests a carrier state or colonizationCitation27. CRP is nonspecific for inflammation while MxA is specific for viral infection and increases the specificity of standalone CRP tests. When paired together, host-response biomarkers, such as CRP and MxA, represent a systemic immune response to a clinically significant infection, whereas the lack of a systemic immune response in the presence of a detected pathogen suggests a carrier state or colonizationCitation27. Although there are no diagnostic tests that demonstrate 100% accuracy, standalone CRP has a median sensitivity of 86%, specificity of 69%, and overall accuracy of 80%Citation19,Citation28 for identifying a patient requiring antibiotic therapy, while the dual biomarker (CRP, MxA) FebriDx test has 95% sensitivity, 94% specificity, and overall accuracy of 92%Citation29.
A 1-year budget impact cost analysis model was developed using data from publicly available datasets and published literature to assess the costs incurred and cost savings associated with and without the use of POCTs in the diagnosis of ARI in the outpatient setting. Specifically, two POCTs included in National Institute for Health and Care Excellence (NICE) guidance for respiratory infection (standalone CRP; dual biomarker (CRP, MxA) FebriDx) as well as a hypothetical test which met a target product profile (TPP) defined by the global health community in 2015Citation26 were compared to no POCTs and to one another.
2. Materials and methods
2.1. Data collection
A structured approach for costing, using the NICE clinical guidelines, NHS websites, and peer reviewed literature, was used as the basis of this cost analysis. Published data related to ARI, including all upper respiratory infections including cough and bronchitis diagnosed in GP and urgent care settings and was applied to a derivation of the NICE costing modelCitation30.
2.2. Patient population
According to the 2018 UK office of National Statistics, the UK population was assumed to be 66,400,000Citation31. More patients consult GPs for ARI (30%) than for any other single diseaseCitation32. In the UK, ARI accounts for 300–400 registered patients for GP consultations annually per 1,000 registered patientsCitation33.
Based on data from the UK GP Research Database, it was conservatively assumed that 300 patients/1,000 registered patients, or approximately 19,920,000 patients, visit a GP for an ARI annuallyCitation33. Consultations for lower respiratory infection inclusive of bronchitis was found to be 70 consultations per 1,000 registered patients each year, or approximately 4,648,000 patientsCitation34. This suggests 77% or approximately 15,338,400 patients, would be defined as an ARI, inclusive of non-specific ARI (e.g. common cold, laryngitis, tracheitis, and flu like illness), acute otitis media, acute rhinosinusitis, and acute pharyngitis without acute bronchitisCitation32.
The number and proportion of actual vs. appropriate antibiotic prescriptions per year was calculated for the following conditions: non-specified ARI (common cold, laryngitis and tracheitis), acute otitis media, acute rhinosinusitis, acute pharyngitis/sore throat and acute bronchitis/coughCitation35 (). Actual and appropriate antibiotic prescriptions were calculated based on antibiotic prescription rates established by a recent analysis of 3.7 million active patient cases, representing approximately 7% of the general UK population, that were extracted from The Health Improvement Network (THIN) databaseCitation35. The THIN database uses data from 550 GPs throughout the UK and is considered the reference standard for capturing GPs patient management patterns. Due to the broad database coverage and experience in modeling and healthcare economics, the 2018 analysis of ARI antibiotic prescribing practices in primary care was used as the basis for calculating actual antibiotic prescriptions. Analysis of GP prescribing demonstrates that approximately 50% of all ARI visits (7,718,283) patient visits lead to an antibiotic prescription. The proportion of consultations that resulted in an actual antibiotic prescription consisted of 25% for non-specified ARI (common cold, laryngitis, tracheitis and flu-like illness), 89% for acute otitis media, 88% for acute rhinosinusitis, 59% for acute pharyngitis/sore throat and 42% for acute bronchitis/coughCitation35. The number and proportion of consultations that warrant “appropriate” antibiotic prescriptions average of 9% (1,444,287) of ARI consultations per year derived from publications that evaluated appropriate antibiotic prescription rates based on current guidelinesCitation1, Citation35. The consultations necessitating an appropriate antibiotic prescription consisted of 17% for acute otitis media, 11% for acute rhinosinusitis, 13% for acute sore throat and 10% for acute bronchitis/coughCitation35. Considering antibiotics are not indicated for non-specified ARI (common cold, laryngitis and tracheitis), it is assumed that the appropriate number of antibiotic prescriptions should be zeroCitation1,Citation35.
Table 1. Consultations resulting in antibiotic prescription: actual vs. appropriate prescribing assumptions calculated from published data (2014, 2018), combined for all age groups.
A direct and indirect cost for antibiotics used in ARI (defined below) as well as the rate and costs associated with antibiotic associated AEs were determined. Within the ARI population, the number of actual patients treated with antibiotics and the presumed appropriate number of patients that should be treated with antibiotics were multiplied against the total number of patients receiving antibiotics to drive the base cost analysis. It was assumed that POCT would lead to an absolute reduction in antibiotics and proportionately reduce both their direct and indirect costs associated with re-consultations.
2.3. Antibiotic cost assumptions
Cost assumptions were categorized as direct costs (i) antibiotics plus dispensing fee at the primary encounter and (ii) indirect costs (a) re-consultations and subsequent treatments due to mild or non-severe antibiotic-related AEs (allergies, nausea, diarrhea) as well as (b) severe antibiotic-related AEs (anaphylaxis, Stevens-Johnson syndrome (SJS), CDI, and (c) diagnostic tests (throat culture and POCTs).
To determine the direct costs of antibiotics, average antibiotic costs per episode for otitis media, sore throat, and cough were extracted from the NICE guidelines which were also used to determine the appropriate antibiotic treatment duration by condition, pharyngitis a 10-day course, otitis media a seven day course, and cough a five day courseCitation36–39. The 2019 Framlington Place Newcastle upon Tyne Regional Drug and Therapeutics Centre therapeutic costs were used to determine the specific antibiotic cost of treatment for each ARI condition ()Citation40. Antibiotic prescriptions for sinusitis and pharyngitis were assumed to be similar and were treated the same in the cost assumption (). Since published data indicate that patients older than 16 years of age represent the majority of patients receiving antibiotics, the most frequently prescribed antibiotics for patients 16 years and older was used to generate an average antibiotic cost of £1.21 per ARI episodeCitation40. A dispensing fee of £1.90 was added to the average antibiotic cost and was assumed to be included for every prescription, based on the mean dispensing rate per item for pharmacistsCitation41. Out-of-pocket payments for prescription charges applicable to these medicines were not included.
Table 2. Antibiotic cost of treatment for each ARI condition.
2.4. Adverse event and secondary infection assumptions
Four categories of antibiotic-related AEs were assumed to lead to re-consultations: anaphylaxis, SJS, allergies consisting of skin rashes, and gastrointestinal upset inclusive of nausea and diarrhea. Based on a literature analysis, an antibiotic-related AE rates were assumed to be 0.01% for anaphylaxisCitation42, 0.04% for SJSCitation43, 10% for allergiesCitation44–46, 25% for diarrhea and nauseaCitation47,Citation48. These rates were multiplied by the total amount of antibiotics prescribed to determine estimates for each subsequent AE. Secondary infections, such as CDI also contribute to significant costs. To estimate the frequency of CDI cases, only the community onset CDI rate of 12.1/100,000, or 7,865 annual cases was used and multiplied by the annual percentage of antibiotics prescribed for ARI (7,865 cases × 63.6%) to determine the incidence of 5,002 cases or (0.1%) serve as the 2019 base caseCitation49.
2.5. Diagnostic POCT assumptions
The cost of each POCT was derived from literature, guidelines, or directly from the test manufacturer (). The TPP is reported to cost between £1 and 5Citation26. The cost calculations assumed a cost of £5 based on the availability of current, similar diagnostics that cost nearly 2–10 times this price range. The cost of each CRP test was determined to be £13.50, inclusive of the testing device, reagents, disposables, calibrators and controlsCitation17. The cost of each FebriDx test was determined to be £11.25 (direct correspondence with manufacturer).
Annual costs of diagnostic tests were estimated based on the direct costs of the tests multiplied by the prevalence of ARI, and the indirect costs from post-diagnostic antibiotic prescriptions, re-consultations due to AEs. For the purposes of illustrating a complete picture of the potential costs incurred and saved, POCT utilization was presumed to be 100%.
Diagnostic performance of each POCT was determined by review of published literature describing minimal and optimal test characteristics of TPP as well as currently available, regulatory approved POCTs mentioned in NICE guidanceCitation26. Diagnostic performance characteristics of standalone CRP and FebriDx consisting of the sensitivity (true positive rate) and specificity (true negative rate) of each test was determined based on published evidenceCitation29,Citation50. The hypothetical tests based on the TPP used a sensitivity of 90% and specificity of 85%Citation26 ().
Table 3. Assumptions for the cost and accuracy of diagnostic tests.
The number of post-diagnostic antibiotic prescriptions was estimated based on diagnostic performance of each POCT. It was assumed that 100%-true sensitivity for bacterial infection represents patients that should have received an antibiotic and were more likely to return to clinic for disease progression and required antibiotic therapy. Similarly, it was also assumed that 100%-true specificity represents patients that were falsely prescribed unnecessary antibiotics when an antibiotic was not necessitated.
2.6. Sensitivity analysis
A one-way sensitivity analysis was performed on major cost drivers such as the number/cost of antibiotics, number of antibiotic related AEs the cost of the POCTs and total ARI population. Total annual cost to the health system with and without POCT were calculated based on the aforementioned assumptions included a 25% increase and decrease in major cost drivers (antibiotics, AEs, total ARI population). POCT costs were varied by 20% as it would not be feasible to cover the cost of goods for most POCTs if costs were reduced by 25%. Results are presented in .
To further test the validity of the model, the total costs of antibiotics, AEs, and re-consultations related to antibiotic use was determined. It was assumed that all patients with anaphylaxis, SJS and CDI would reconsult to the health facility for care. Thus, the number and cost of anaphylaxis, SJS and CDI were considered to be constant when considering different prescribing strategies (i.e. immediate, delayed, and delayed plus POCT). Additionally, it was assumed that although the incidence of non-severe AEs (allergies, diarrhea/nausea) ranges upwards of 25%, only a small proportion (111.98/100,000 = 5.6%) of patients would actually return to a healthcare practitioner for careCitation51. This was chosen as it is in line with published dataCitation51. Moreover, the GRACE study showed that the baseline re-consultation rate without antibiotics was 19.7% for worsening symptoms and increased to a re-consultation rate of 25.3% with immediate antibiotic useCitation51. Thus, a 5.6% re-consultation rate was considered to be the base case for mild AEs (allergies, nausea, diarrhea) and was the only variable adjusted when calculating the impact of different prescribing strategies on the cost of AE re-consultation.
3. Results
Over 15 million patients visit a GP for management of ARI conditions annually. Despite NICE recommendations advocating for delayed antibiotic prescribing, 7,718,283 (50.3%) consultations were associated with an actual antibiotic prescription. When considering guideline-based prescribing practices, 1,444,877 (9%) consultations would have resulted in an appropriate antibiotic prescription (). A difference of 6,273,405 (81%) consultations, which resulted in antibiotic prescriptions where not needed.
The corresponding antibiotic costs for actual ARI consultations resulting in antibiotic prescriptions amounted to £2,40,03,860 vs. £44,93,568 for guideline-based, appropriate antibiotic prescriptions.
Based on actual antibiotic prescriptions and the assumed rate of antibiotic-related AEs, it was calculated that 450,748 antibiotic-related AEs would occur annually under “status quo” prescribing practices. The annual cost of AEs resulting from actual antibiotic use was calculated to be £302,496,486. This was combined with the costs for antibiotics as well as the costs for throat cultures which amounted to a cumulative cost of £32,67,29,943 per year without the use of delayed prescribing practices or POCT ().
Based on guideline-based, appropriate prescribing practices the number of antibiotic-related AEs was estimated to be 115,131, a 75% reduction when compared to status quo prescribing practices. The annual cost of AEs resulting from appropriate antibiotic prescriptions was calculated to be £6,38,54,269 which amounted to an 79% reduction in AEs when compared to status quo prescribing practices. The annual cost of AEs resulting from appropriate antibiotic prescribing was combined with costs for antibiotics amounted to a cumulative cost of £68,347,837. Total annual costs for AEs were also calculated for delayed prescribing plus the addition of each POCT (CRP, TPP and FebriDx). The total cost for AEs re-consultation inclusive of antibiotics was £7,81,48,933 for delayed prescription plus CRP, £6,01,14,564 for delayed prescription plus FebriDx, and £6,61,26,020 for delayed prescription plus TPP ().
Total annual costs for each POCT prescription strategy was calculated by factoring in the cost of performing the POCT at each ARI consultation, influence of diagnostic performance on antibiotic prescription as well as antibiotic-related AEs resulting from delayed prescribing plus the POCT. FebriDx may avoid antibiotic prescription in 38–56% of ARI cases associated with a CRP ≥ 20 mg/LCitation29,Citation52.
The cumulative annual costs for CRP, FebriDx, and TPP were £29,02,95,065, £23,76,14,489, and £14,78,05,881, respectively, while status quo prescribing practices amounted to £32,65,00,346 in annual costs (). Use of the theoretical TPP resulted in the largest potential cost reduction when compared to status quo prescribing with an annual cost saving of £17,86,94,465 (54% reduction) followed by FebriDx with an annual cost saving of £8,88,85,857 (27% reduction). CRP POCT also resulted in annual cost savings of £3,62,05,281 (11% reduction) (). The sensitivity analysis also confirmed antibiotic prescribing without a POCT to be the highest cost prescription strategy followed by prescription guided by CRP testing. FebriDx and TPP were consistently found to be the lowest cost prescription strategy.
Table 4. Estimated total costs for ARI Consultation (2018) with and without POCT.
Table 5. Estimated annual cost for ARI consultation (2018) savings with POCT.
The sensitivity analysis showed that variation in the total ARI population assumptions, POCT cost and antibiotic-related AEs had the most impact on total cost to the healthcare system whereas antibiotic costs influenced total cost to a lesser extent ().
Table 6. Sensitivity analysis.
Antibiotic-related AE costs per episode were determined to be £1,345 for anaphylaxisCitation53, £36,718 for SJSCitation54, £235 for allergies and diarrhea/nausea (inclusive of ED, out of hours, and GP costs)Citation53, and £10,000 for CDICitation17,Citation55.
4. Discussion
AMR is a global threat that requires a multifaceted approach to combat spread of resistant pathogensCitation6. Patient concern for their symptoms and diagnostic uncertainty are the most common reasons that lead to an antibiotic prescription for ARI despite lack of evidence of clear benefitCitation24. POCTs capable of triaging patients that may not benefit from antibiotics have been proposed as an intervention to reduce over prescription in uncomplicated ARIs that are caused by a non-bacterial or self-limiting bacterial etiologyCitation6. This cost analysis confirmed that POCT can have a significant impact on healthcare savings. The primary drivers of the cost savings, and hence the sensitivity of the analysis, include direct antibiotic costs, GP re-consultations, and AEs inclusive of rashes, gastrointestinal complaints, anaphylaxis, SJS, and secondary infections such as CDI.
POCTs with high accuracy (sensitivity/specificity) and negative predictive value (NPV) can help drive antibiotic stewardship in the outpatient GP setting by helping to guide antibiotic therapeutic decisions, such as watchful waiting strategies, where the vast majority of unnecessary antibiotics are prescribed. Without POCT, the UK health system spends over £326 million annually and with POCT this can lead to over £36 million to over £178 million in annual savings depending on the type of POCT deployed.
Two review studies, including a Cochrane Review, have noted that delayed prescribing, in place of immediate antibiotic prescription may be a safe approach for uncomplicated ARI presentation to significantly reduce unnecessary antibiotics and antibiotic resistance while preserving patient safety and satisfactionCitation27,Citation28. This cost analysis showed that delayed prescription alone resulted in over £68 million cumulative cost savings and an 75% reduction in antibiotic-related AEs when compared to immediate prescription while delayed prescription plus POCT resulted in approximately £36–£178 million in cumulative cost savings.
When using the UK as an example, the findings suggest that the use of diagnostics to inform antibiotic prescription is associated with significant overall cost savings, but also the avoidance of antibiotic-related AEs which offer individual patient benefit. Thus, not only can point-of-care diagnostic testing help to prevent the misuse of antibiotics that leads to AMR, which in turn benefits public and global health, but is also beneficial to reduce healthcare spending and AEs-associated morbidity carried by the individual. Qualitative research exploring GPs attitudes toward POCTs found enthusiasm for a POCT finger-prick blood test that could distinguish viral from bacterial infectionCitation56. GPs emphasized that such a test would be most valuable in supporting the decision not to prescribe antibiotics in situations where patients are not likely to benefitCitation56.
Although the vast majority of antibiotics are prescribed in the outpatient setting while antibiotic stewardship is largely limited to the inpatient setting. A major benefit of POCT is the ability to improve antibiotic stewardship in the primary and urgent care setting by providing tangible results that both increase diagnostic certainty and confidence to delay or withhold antibiotics when bacterial infection is unlikely. Despite obvious benefits of POCTs, only a small number of POCTs, such as glucose testing and D-dimer testing, have broad utilization in the UK The National Health Service (NHS) model for primary care, which currently does not include POCT items of service payments, results in additional work and costs for initial purchase and subsequent maintenance and consumablesCitation24. This lack of reimbursement is the major limiting factor to accessing POCTs in the outpatient setting; however, other considerations include space to accommodate a range of technologies, time to train staff, regulatory requirements and uncertainty about test accuracyCitation22, Citation57–59. However, these costs may be partially or completely offset by enhanced workflow and payment strategies. An accurate, inexpensive test that facilitates high throughput to support patient flow in the clinic could be used as a triage tool to guide treatment of uncomplicated patients with respiratory symptoms. This scenario would avoid cumulative costs and morbidity associated with AEs that are significantly higher in the scenario without POCT. Additionally, Johnson et al. suggest that increased utilization of POCT could be operationalized by the introduction of an opt-in program that provides a separate fee for the delivery of additional “locally enhanced” services (LES), such as POCT, in management of ARI. An LES reimbursement plan may provide the needed financial incentive to uptake POCT for the management of ARI in an NHS primary care settingCitation24.
Re-consultations may indicate persistent symptoms or that the main concerns were not adequately addressed during the first consultation and often increase the pressure to prescribe. POCTs offer a strategy to address patient concerns during the initial visit and therefore may reduce unnecessary re-consultations. Moreover, antibiotic misuse puts individual patients at risk for preventable side effects, and exposes both patients and society to pathogens that develop resistance to antibioticsCitation18.
Significant changes in facilities and infrastructure are not needed to incorporate POCTs in the outpatient setting, particularly for the simple lateral flow technologies. POCTs in GP clinics could reduce unnecessary antibiotic use and provide an objective measure to reassure patients and providers that antibiotics are not needed. CRP is produced in response to inflammation and high (>20 mg/L) levels can be indicative of a bacterial infection or potentially other causes of inflammationCitation60, but CRP has low specificity to differentiate a viral from bacterial infection at lower concentrationsCitation27. Despite these limitations, standalone CRP testing has been accepted by patients and has also been shown to reduce antibiotic prescribing in primary careCitation27. The effectiveness of CRP testing to reduce antibiotic prescribing has been demonstrated in multiple settings, including primary care, ranging from 36 to 43% reduction in the UKCitation61, 25% decrease in a meta-analysis from European countries and the United StatesCitation62, and a 10–20% decrease in South East AsiaCitation63.
The UK data presented here serve as an example for other industrialized health systems, particularly those that are moving toward universal healthcare coverage where health spending is covered by the government rather than the individual. In addition to cost savings and implications for public health, the avoided ∼5.7–6 million antibiotics prescriptions improved the outcome and immediate health of ∼1 million patients a year, assuming each AEs episode is related to an individual patient. Outside of the UK, similar antibiotic pressures are felt in lower- and middle-income countries and access to inexpensive testing may have an even greater impact on resource limited regionsCitation64. While host immune biomarker responses have high NPVs for bacterial infection and may directly guide therapeutic management in settings without malaria, these tests would likely need to be performed subsequent to a rapid diagnostic malaria negative test confirmation prior recommending antibiotic withholding. Even the cost of performing two rapid tests pales in comparison to the threat of increasing rates of antibiotic resistant infectionsCitation65.
This study was designed as a simple benefit analysis focusing on the direct costs associated with antibiotic overuse in order to make it more easily comparable between higher income countries.
This study has a number of limitations as it significantly simplified both the antibiotic prescribing environment and the associated costs. Primary limitations that may lead to an under-estimation of the testing benefits include lack of long-term cost implications of antibiotic overuse on global health, higher costs associated with novel therapies required to treat resistant infections, time associated with phone consultations, inpatient or hospital emergency diagnostic testing, emergency department costs, radiographic or laboratory tests, and inclusion of only direct costs. The costs for AMR are estimated to range from £3 to £45,000 ($5 to $55,000) per patient treatedCitation66 and annual costs ranging from $2.2 billionCitation7,Citation67 to upwards of $55 billion inclusive of direct healthcare service costs ($35 billion) and indirect loss of productivity ($20 billion)Citation66,Citation68. Considering that 81% of antibiotics are prescribed for ARI inappropriately, POCT-guided antibiotic prescribing has the potential to significantly reduce both the incidence and associated cost of AMR in in the outpatient setting.
Additionally, prescribing practices and hospitalization costs change depending on age groupsCitation36 with higher prescriptions in children and elderly populations. Age stratification was not performed due to a lack of age stratified data for all the different symptom groups and follow up studies to address this limitation is needed. Further, the cost analysis did not account for potentially reduced test usage in higher priced diagnostics. Bearing the limitations in mind, the potential savings highlighted by this work makes a fiscal as well as patient-centered case for the value of diagnostics to inform antibiotic prescribing.
Conclusions
The majority of antibiotics are prescribed to ARI patients in the outpatient setting. Use of POCTs may reduce antibiotic misuse and reduce the spread of AMR while preserving healthcare funding and ensuring the best individual patient outcome.
Transparency
Declaration of funding
Support for FIND’s work on improved fever triage diagnostic is provided by the Government of the Netherlands, UK aid from the UK government, and the Australian Government.
Declaration of financial/other relationships
The authors declare no competing interest. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
The authors thank Dr. Rob Sambursky (Lumos Diagnostics) for support with data collection and critical discussions of the manuscript and data. Support for FIND’s work on improved fever triage diagnostic is provided by the Government of the Netherlands, UK aid from the UK government, and the Australian Government.
References
- Harris AM, Hicks LA, Qaseem A.; for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425–434.
- Shehab N, Lovegrove MC, Geller AI, et al. US Emergency Department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316(20):2115–2125.
- Olson SC, Smith S, Weissman SJ, et al. Adverse events in pediatric patients receiving long-term outpatient antimicrobials. J Pediatric Infect Dis Soc. 2015;4(2):119–125.
- Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–1315.
- Geller AI, Lovegrove MC, Shehab N, et al. National estimates of emergency department visits for antibiotic adverse events among adults-United States, 2011–2015. J Gen Intern Med. 2018;33(7):1060–1068.
- Tackling drug-resistant infections globally: Final report and recommendations (Review on Antimicrobial Resistance, 2016); 2016. http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
- Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7(1):58.
- Smieszek T, Pouwels KB, Dolk FCK, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(Suppl. 2):ii36–ii43.
- Pouwels KB, Hopkins S, Llewelyn MJ, et al. Duration of antibiotic treatment for common infections in English primary care: cross sectional analysis and comparison with guidelines. BMJ. 2019;364:l440.
- Ackerman SL, Gonzales R, Stahl MS, et al. One size does not fit all: evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Serv Res. 2013;13(1):462.
- Fletcher-Lartey S, Yee M, Gaarslev C, et al. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 2016;6(10):e012244.
- Dempsey C, Wojciechowski S, McConville E, Drain M. Reducing patient suffering through compassionate connected care. J Nurs Admin. 2014;44(10):517–524.
- Harbarth S, Samore MH. Antimicrobial resistance determinants and future control. Emerg Infect Dis. 2005;11(6):794–801.
- Davidson M. FebriDx point-of-care testing to guide antibiotic therapy for acute respiratory tract infection in UK primary care: a retrospective outcome analysis. J Infect Dis Prev Med. 2017;05(03):165.
- Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med. 1999;159(10):1082–1087.
- Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–246.
- National Institute for Health and Care Excellence. FebriDx for C-reactive protein and Myxovirus resistance protein A testing in primary care Medtech innovation briefing [MIB114]; 2017. p. 1.
- Stanton N, Francis NA, Butler CC. Reducing uncertainty in managing respiratory tract infections in primary care. Br J Gen Pract. 2010;60(581):e466–e475.
- Cole A. GPs feel pressurised to prescribe unnecessary antibiotics, survey finds. BMJ (Clin Res ed.). 2014;349:g5238.
- ten Oever J, Tromp M, Bleeker-Rovers CP, et al. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J Infect. 2012;65(6):490–495.
- World Health Organization. WHO global strategy for containment of antimicrobial resistance. 2nd ed. Geneva, Switzerland: World Health Organization (WHO); 2001. p. 105.
- Brown DK, Jennings I, Munroe-Peart S, et al. Point of care D-dimer testing in practice: survey results for D-dimer testing performed in patient care within the United Kingdom; 2015.
- Gwyn L, Harris S, Clarke C. Evaluating a point-of-care C-reactive protein test to support antibiotic prescribing decisions in a general practice. Prevention. 2019;18. https://www.pharmaceutical-journal.com/research/research-article/evaluating-a-point-of-care-c-reactive-protein-test-to-support-antibiotic-prescribing-decisions-in-a-general-practice/20201688.article.
- Johnson M, Cross L, Sandison N, et al. Funding and policy incentives to encourage implementation of point-of-care C-reactive protein testing for lower respiratory tract infection in NHS primary care: a mixed-methods evaluation. BMJ Open. 2018;8(10):e024558.
- Howick J, Cals JWL, Jones C, et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open. 2014;4(8):e005611.
- Dittrich S, Tadesse BT, Moussy F, et al. Target product profile for a diagnostic assay to differentiate between bacterial and non-bacterial infections and reduce antimicrobial overuse in resource-limited settings: an expert consensus. PLoS One. 2016;11(8):e0161721.
- Joseph P, Godofsky E. Outpatient antibiotic stewardship: a growing frontier-combining myxovirus resistance protein a with other biomarkers to improve antibiotic use. Open Forum Infect Dis. 2018;5(2):ofy024.
- Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev. 2017;9:CD004417.
- Shapiro NI, Self WH, Rosen J, et al. A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever. Ann Med. 2018;50(5):420–429.
- National Institute for Health and Care Excellence (NICE). NICE clinical guideline 69 – antibiotic prescribing – respiratory tract infections. London: NICE; 2008.
- Office for National Statistics. Overview of the UK population: August; 2019. p. 1–14.
- Morice A, McGarvey L, Pavord I. Recommendations for the management of cough in adults. Thorax. 2006;61:i1–i24.
- Ashworth M, Cox K, Latinovic R, et al. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health (Oxf). 2004;26(3):268–274.
- Macfarlane J. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax. 2001;56(2):109–114.
- Pouwels KB, Dolk FCK, Smith DRM, et al. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018;73(Suppl. 2):19–26.
- Dolk FCK, Pouwels KB, Smith DRM, et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73(Suppl. 2):ii2–ii10.
- Excellence NIFHaCo. Guideline sore throat (acute): antimicrobial; 2017.
- Excellence NIFHaC. Guideline cough (acute): antimicrobial prescribing NICE guideline draft for consultation; 2018.
- Excellence NIoHaC. Guideline otitis media (acute): antimicrobial prescribing; 2017.
- Costs FPNuTRDaTCT. Regional drug and therapeutics centre (Newcastle) – cost comparison charts; 2019.
- Holmes E, Harris S, Hughes A, et al. Cost-effectiveness analysis of the use of point-of-care C-reactive protein testing to reduce antibiotic prescribing in primary care. Antibiotics. 2018;7(4):106.
- National CGCU. Drug allergy: diagnosis and management of drug allergy in adults, children and young people. London: National Institute for Health and Care Excellence; 2014.
- Blumenthal KG, Wickner PG, Lau JJ, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a cross-sectional analysis of patients in an integrated allergy repository of a large health care system. J Allergy Clin Immunol Pract. 2015;3(2):277–280e1.
- Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35(6):489–494.
- Macy E, Ho NJ. Multiple drug intolerance syndrome: prevalence, clinical characteristics, and management. Ann Allergy Asthma Immunol. 2012;108(2):88–93.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778e1–778e7.
- Kuehn J, Ismael Z, Long PF, et al. Reported rates of diarrhea following oral penicillin therapy in pediatric clinical trials. J Pediatr Pharmacol Ther. 2015;20(2):90–104.
- Tahtinen PA, Laine MK, Huovinen P, et al. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364(2):116–126.
- England PH. Quarterly epidemiological commentary: mandatory MRSA, MSSA and E. coli bacteraemia, and C. difficile infection data (up to January–March 2019). London: Public Health England; 2019. p. 1–34.
- Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217.
- Little P, Stuart B, Smith S, et al. Antibiotic prescription strategies and adverse outcome for uncomplicated lower respiratory tract infections: prospective cough complication cohort (3C) study. BMJ. 2017;357:j2148.
- Self W, Rosen J, Sharp S, et al. Diagnostic accuracy of FebriDx: a rapid test to detect immune responses to viral and bacterial upper respiratory infections. J Clin Med. 2017;6(10):94.
- National Institute for Health and Care Excellence (NICE). Costing statement: drug allergy: diagnosis and management of drug allergy in adults, children and young people. Implementing the NICE guideline on Drug allergy in adults, children and young people (CG183); 2014.
- Oen IMMH, van der Vlies CH, Roeleveld YWF, et al. Epidemiology and costs of patients with toxic epidermal necrolysis: a 27-year retrospective study. J Eur Acad Dermatol Venereol. 2015;29(12):2444–2450.
- Wiegand PN, Nathwani D, Wilcox MH, et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81(1):1–14.
- Butler CC, Simpson S, Wood F. General practitioners’ perceptions of introducing near-patient testing for common infections into routine primary care: a qualitative study. Scand J Primary Health Care. 2008;26(1):17–21.
- Jones CH, Howick J, Roberts NW, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Fam Pract. 2013;14(1):117.
- Turner PJ, Van den Bruel A, Jones CHD, et al. Point-of-care testing in UK primary care: a survey to establish clinical needs. Fam Pract. 2016;33(4):388–394.
- Hickner J, Thompson PJ, Wilkinson T, et al. Primary care physicians’ challenges in ordering clinical laboratory tests and interpreting results. J Am Board Fam Med. 2014;27(2):268–274.
- Kapasi AJ, Dittrich S, González IJ, et al. Host biomarkers for distinguishing bacterial from non-bacterial causes of acute febrile illness: a comprehensive review. PLoS One. 2016;11(8):e0160278.
- Andreeva E, Melbye H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam Pract. 2014;15(1):80.
- Huang Y, Chen R, Wu T, et al. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: a systematic review and meta-analysis of primary care studies. Br J Gen Pract. 2013;63(616):e787–e794.
- Do NTT, Ta NTD, Tran NTH, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health. 2016;4(9):e633–e641.
- Escadafal C, Nsanzabana C, Archer J, et al. New biomarkers and diagnostic tools for the management of fever in low- and middle-income countries: an overview of the challenges. Diagnostics. 2017;7(3):44.
- Lubell Y, Blacksell SD, Dunachie S, et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect Dis. 2015;15(1):511.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ (Clin Res ed.). 2013;346:f1493.
- Thorpe KE, Joski P, Johnston KJ. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Affairs. 2018;37(4):662–669.
- Oppong R, Smith RD, Little P, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract. 2016;66(650):e633–e639.