Abstract
Objectives
Antiviral treatments for early intervention in patients with mild-to-moderate COVID-19 are needed as a complement to vaccination. We sought to estimate the impact on COVID-19 cases, deaths, and direct healthcare costs over 12 months following introduction of a novel, antiviral treatment, RD-X19, a light-based, at-home intervention designed for the treatment of mild-to-moderate COVID-19 infection.
Methods
A time-dependent, state transition (semi-Markov) cohort model was developed to simulate infection progression in individuals with COVID-19 in 3 US states with varying levels of vaccine uptake (Alabama, North Carolina, and Massachusetts) and at the national level between 1 June 2020 and 31 May 2021. The hypothetical cohort of patients entering the model progressed through subsequent health states after infection. Costs were assigned to each health state. Number of infections/vaccinations per day were incorporated into the model. Simulations were run to estimate outcomes (cases by severity, deaths, and direct healthcare costs) at various levels of adoption of RD-X19 (5%, 10%, 25%) in eligible infected individuals at the state and national levels and across three levels of clinical benefit based on the results from an early feasibility study of RD-X19. The clinical benefit reflects a decline in the duration of symptomatic disease by 1.2, 2.4 (base case), and 3.6 days.
Results
In the base case analysis with 10% adoption, simulated infections/deaths/direct healthcare costs were reduced by 10,059/275/$69 million in Alabama, 21,092/545/$135 million in North Carolina, and 16,670/415/$102 million in Massachusetts over 12 months. At the national level, 10% adoption reduced total infections/deaths/direct healthcare costs by 686,722/17,748/$4.41 billion.
Conclusion
At-home, antiviral treatment with RD-X19 or other interventions with similar efficacy that decrease both symptomatic days and transmission probabilities can be used in concert with vaccines to reduce COVID-19 cases, deaths, and direct healthcare costs.
Introduction
The effect of the coronavirus disease 2019 (COVID-19) pandemic on daily life is unprecedented. In the United States (US), more than 77 million confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and over 900,000 COVID-19 deaths have been recorded through February 2022Citation1. As the world vaccinates against SARS-CoV-2, the transformation from pandemic phase to endemic phase has begun; however, that transformation will not end the threat or need for complementary treatments. The Institute for Health Metrics and Evaluation projected that the 2021–2022 winter surge in the Northern hemisphere would be similar to that of 2019–2020, with transmission continuing throughout 2022Citation2. This surge was observed with the rapid spread of the omicron variant, and future variants could result in similar case escalationCitation3. In the absence of broadly available therapeutic interventions, even with widespread vaccination and without new virulent strains, vaccine hesitancy, waning vaccine efficacy, immunosuppression from other diseases and their treatment, and clinical risk factors (e.g. obesity and older age) pose a risk for serious COVID-19 illness in as much as 40% of the adult populationCitation4–6.
Beyond the human toll, the economic impact of COVID-19 is similarly staggering. Globally, the pandemic has caused supply chain disruptions, labor shortages, and the deepest worldwide recession since World War IICitation7. In the second quarter of 2020, the US experienced its largest decline in gross domestic product (GDP) in 70 years as a result of the pandemicCitation8. Economic losses directly attributable to the COVID-19 pandemic include lost jobs, reduced industrial output, increased health care costs, and massive economic stimulus distributions. Although a definitive cost has not been calculated, the COVID-19 economic toll in the US is estimated to be in the tens of trillions of United States dollars (USD)Citation9.
COVID-19 vaccines are cost-effective, but not uniformly: the cost per quality-adjusted life year (QALY) gained is estimated at $8,200 for individuals over 65 years of age but $94,000 for those with low risk of hospitalization and deathCitation10. The first approved pharmacologic treatment, remdesivir, is now available in both the outpatient and inpatient settings, and though clinically proven to reduce time to discharge/recovery and to reduce hospitalizations in high risk patients, this drug requires multiple days of intravenous infusion and the price of remdesivir is at least several thousand dollars for a typical six vial treatment course for those with private insuranceCitation11. Monoclonal antibody (mAb) therapies were also only authorized for patients at high risk for developing severe disease, leaving a gap in treatment options for the broader population of patients with mild-to-moderate disease. Further, the uptake of mAbs (e.g. casirivimab/imdevimab and bamlanivimab/etesevimab) has been challenged by difficulty in getting both patients and mAbs to suitable outpatient treatment facilities and by the decreased efficacy against omicron and other variants leading to the selective withdrawal of certain emergency use authorizationsCitation12. Therapies that can be used at-home for mild-to-moderate COVID-19 and made readily available through large scale, equitable deployment are also neededCitation13. Recently, two orally administered antivirals (nirmatrelvir/ritonavir tablets and molnupiravir capsules) have been authorized for the treatment of individuals with mild-to-moderate COVID-19 who are at high risk for progressing to severe diseaseCitation14,Citation15. A course of treatment for both of these therapies, each comprising a 5-day course of pills taken at home, resulted in reductions in viral load, hospitalizations, and deaths.
Empirical evidence supports the use of limited wavelength light to both prevent and treat a number of diseases including psoriasisCitation16, Barrett’s esophagusCitation17, microbial infections such as tuberculosisCitation18,Citation19, and Propionibacterium acneCitation20. Similarly, blue light treatment represents a novel approach to mitigate the threat of SARS-CoV-2, and light delivery devices for use at-home may serve as a new treatment option for COVID-19Citation21. Accessing supply chains orthogonal to those used by pharmacological interventions, these devices could be manufactured in high volume, made available through standard distribution channels, and be rapidly deployed and readily stockpiled for future need. One such light-based treatment, the EmitBio RD-X19, is an investigational device designed to enable patients with mild-to-moderate COVID-19 to self-administer precisely engineered doses of blue light through the mouth to the oropharynx and surrounding tissuesCitation22.
In a randomized, double-blind, sham-controlled early feasibility study of RD-X19, subjects were enrolled based on a positive SARS-CoV-2 rapid antigen test within 3 days of symptom onset and having at least 2 moderate COVID-19 symptoms at baseline (NCT04662671). Subjects self-administered 5-minute, twice-daily treatments with RD-X19 for 4 days and were medically monitored for the duration of the study (8 days). For safety and tolerability, assessments included local site reactions (pain, induration, erythema) and the presence, type, severity, and attribution of any device-related treatment-emergent adverse events. Efficacy outcome measures included assessments of SARS-CoV-2 saliva viral load and clinical assessments of COVID-19.
At the end of study (Day 8), the mean change in log10 saliva viral load was −3.29 for RD-X19 and −1.81 for sham, demonstrating a treatment benefit of −1.48 logs or approximately a 95% reduction compared to placebo. Among the clinical outcome measures, differences between RD-X19 and sham were also observed, with a shortened duration of symptomatic disease of 2.4 days in RD-X19 treated subjects compared to sham treated subjectsCitation23. There were no local application site reactions and no device-related adverse events.
In this manuscript, we present a simulation model to estimate the population-level benefits resulting from the introduction of the RD-X19 treatment based on the results of the RD-X19 early feasibility study. More broadly, this simulation also is useful to assess how any intervention used as a complement to vaccines that reduces the duration of COVID-19 symptomatic disease and transmission probabilities impacts healthcare costs, incidence, and mortality due to COVID-19. Simulation models provide an approach to estimate the clinical and economic benefits from the introduction of interventions like RD-X19 to mitigate infectious disease outbreak. As such we simulated estimates of the reductions in COVID-19 disease (mild, moderate, and severe), mortality, and direct health care costs for introduction of the treatment prior to the 2020 winter surge in 3 US states with varying degrees of COVID-19 vaccinations and at the US national level.
Methods
Cost-consequence analysis
A cost-consequence analysis was performed using a time-dependent, state transition (semi-Markov) cohort model to retrospectively estimate benefits (compared with standard practice) associated with the use of RD-X19 for the treatment of mild-to-moderate COVID-19 in each state and nationally (US) in accordance with best practice standards for economic evaluationsCitation24. The impact of the device was modeled over 12 months of the pandemic from 1 June 2020 to 31 May 2021. A US healthcare payer perspective was adopted. Three states were selected to represent variability in infection, control and vaccination rates (as of 30 May 2021): Alabama (29.2% fully-vaccinated), North Carolina (36.1% fully-vaccinated), and Massachusetts (53.4% fully-vaccinated)Citation25. Similarly, 3 levels of reduced symptomatic days relative to the standard were evaluated (1.2, 2.4, and 3.6 fewer symptomatic days); the base case of 2.4 days reflected the time to symptom resolution in the RD-X19 early feasibility studyCitation23. The lower boundary of clinical benefit represented a 50% reduction in efficacy from the results (50% of 2.4 days = 1.2 days) of the study and is found to be similar to that of the approved influenza antiviral baloxavir marboxilCitation26. The upper boundary of 3.6 days represents a benefit 150% greater than expected (150% of 2.4 days = 3.6 days) from the device efficacy results from the early feasibility study.
Model structure
The patients in the hypothetical cohort entering the model progressed through a series of 15 health states after SARS-CoV-2 infection based on calculated transition probabilities (). The proportion of the model cohort that became infected with SARS-CoV-2 in each cycle was based on the number of newly reported COVID-19 cases, the number of individuals fully-vaccinated in each cycle, and the current number of infected individuals. The length of time that an individual remains contagious impacts the number of new cases observed each model cycle. A detailed description of the model is provided in the Online Supplement. When reporting results, the model ran from the first timepoint (March 2020), but no results were recorded before 1 June 2020. The reason for running the model from the first timepoint was to populate all model 15 health states with realistic values before beginning collection of results and to verify that the model accurately represented disease progression. A summary of the calculated transition probabilities for standard practice and RD-X19 arms (which are the same for all modelled US states) of the model are provided in Supplemental Figures S1 and S2.
Figure 1. Cost-consequence model schematic. Abbreviations. COVID-19: coronavirus disease 2019; MV, mechanical ventilation.
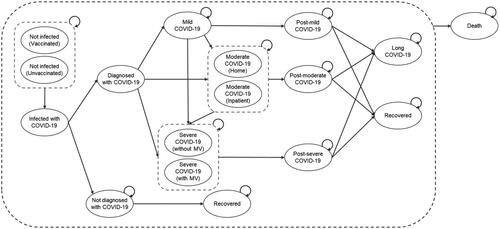
Infectious health states covered mild, moderate, and severe COVID-19 infections, are aligned to definitions outlined by the National Institutes of Health (NIH), and incorporate guidance provided by the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration (FDA) ()Citation27. Within moderate infection, the model further differentiates between management at home or in the inpatient setting, whereas severe infection was split between those requiring mechanical ventilation (MV) or not. Following recovery from acute infection, any infection severity could potentially lead to long COVID-19. Mortality from any model health state is possible. Further details of the model structure and modeling assumptions can be found in the Online Supplement. All model inputs were identified through targeted searches of either peer-reviewed published literature or publicly available data provided by state and federal government. Model inputs and selected references are provided in .
Table 1. COVID-19 testing, disease severity, and symptomsCitation27.
Table 2. Model inputs.
The primary cost outcomes were simulated estimates of the total direct incremental cost (total cost when the intervention was adopted minus total standard practice cost) and total direct incremental cost per individual that received the intervention. Clinical outcomes included number of COVID-19 infections and deaths, both in total and stratified by infection severity. Deterministic sensitivity analysis (DSA), probabilistic sensitivity analysis (PSA) randomly sampling model inputs over 1,000 iterations, and scenario analyses (varying uptake, efficacy, and date of introduction of intervention) were performed. No formal statistical testing was conducted.
COVID-19 characteristics
The proportions of diagnosed COVID-19 infections that were mild (74.2%), moderate (14.19%), and severe (11.57%) were fixed across the model time horizonCitation31, as were the proportions of moderate cases managed at home, severe cases requiring MV, and the probabilities of experiencing infection progression and long COVID-19Citation31,Citation33,Citation36,Citation37. Mean duration of infectious and recovery days and time to infection progression differ by infection severity. The time course of long COVID-19 is fixed. Vaccine efficacy was assumed to be 95% for the prevention of laboratory confirmed infection and 86% for vaccine efficacy against COVID-19-related hospitalization (partially vaccinated individuals were not considered to be vaccinated within the context of the model, nor was any drop in efficacy modeled with reduced protections against disease caused by emerging variants of concern, making estimates more conservative)Citation40,Citation41.
RD-X19 treatment (anti-viral intervention)
The base case was modeled on results from an early feasibility trial of the RD-X19 device, designed as an anti-viral treatment to be used as a complement to vaccines to reduce COVID-19 symptomatic days of disease and duration of SARS-CoV-2 infectivity. RD-X19 was made available to patients with mild-to-moderate COVID-19, ages 18 years and up, who were also eligible for at-home disease management as identified via model assumptions. The course of treatment was twice-daily dosing for 4 days. The model base case assumed that the intervention was available to 10% of all eligible infected individuals starting in the fourth month (1 June 2020 after 3 months model run without intervention) of the model time horizon and that the intervention reduced symptomatic days by 2.4 relative to the standard.
Healthcare costs
A cost was assigned to each infected health state (excluding long COVID-19) and post-COVID-19 health states (excluding post-mild COVID-19). Each health state cost was assumed to account for all aspects of care. In order to identify the costs impacted by the intervention over the cost of the intervention itself, intervention cost was assumed to be $0. All costs, reported in 2020 USD ($), are shown in .
Results
Cost analysis
In the base case analysis, use of the intervention led to simulated estimates of total direct health care cost savings ranging from $68,788,691 to $135,299,936 across the 3 US states modeled and $4,408,120,862 at the national level (). The cost saving per person receiving intervention ranged from $2,015 to $2,515 (). presents results of scenario analyses that varied the proportion of eligible infected individuals receiving intervention (5%, 10% [base case], and 25%) alongside alternative intervention clinical benefit scenarios where the time to no or mild symptoms was assumed to be 50% above ([3.6 days [150%]) and 50% below (1.2 days, [50%]) the base case of 2.4 days (100%). Adoption of the intervention results in cost savings per person treated for all combinations with results ranging from a low of $997 (5%, 1.2 days, Alabama) to a high of $3,969 (25%, 3.6 days, Massachusetts). Maximum cost savings per state evaluated with 25% uptake and 150% efficacy (3.6-day reduction) were $250,164,905 in Alabama, $490,498,555 in North Carolina, and $365,729,833 in Massachusetts.
Table 3. Total cost saving and cost saving per person receiving RD-X19.
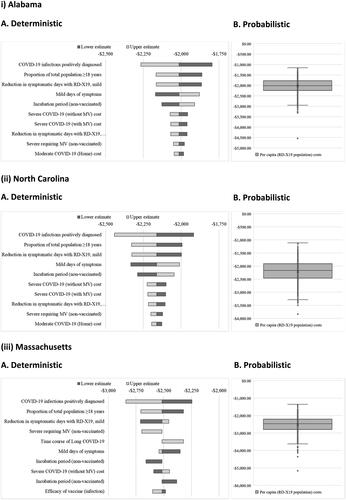
Results of deterministic and probabilistic sensitivity analyses are shown in . In a deterministic sensitivity analysis, model inputs were varied to identify those with the greatest changes in cost savings per person receiving the intervention. The interventional efficacy (reduction in symptomatic days) and the proportions of the total population over the age of 18 years and of confirmed COVID-19 infections were shown to drive the greatest changes in the cost savings per person receiving the intervention. For Alabama, North Carolina, and Massachusetts, the average probabilistic savings per person receiving intervention (tornado plots) were $2,028 (interquartile range [IQR]: $1,772–$2,242), $2,207 (IQR: $1,909–$2,458), and $2,521 (IQR: $2,204–$2,788), respectively.
COVID-19 incidence and mortality
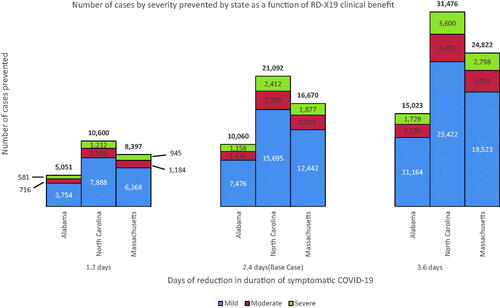
The base case simulation analysis revealed that intervention with RD-X19 led to an estimated reduction in the cumulative number of mild, moderate, and severe COVID-19 infections across all states as well as a reduction in mortality; the reduction in COVID-19 infections ranged from 10,060 to 21,092 (), whereas between 275 and 545 deaths were prevented across all modeled US states (). At the national level, introduction of the device reduced cases by 686,722 and deaths by 17,748 ().
Table 4. Lives saved and cases reduced as a function of efficacy and date of introduction of RD-X19 (base case with 10% penetration).
Additionally, the same alternative RD-X19 clinical benefit levels (1.2 days, 2.4 days, and 3.6 days) are presented alongside scenarios for intervention availability in (June 2020 before the second wave [base case], October 2020 before the third wave, and February 2021 at the tail end of the third wave once vaccines had become available). For each scenario, including the shortest intervention availability time combined with the lowest level of clinical benefit, 10% adoption of RD-X19 reduced both confirmed COVID-19 infections and deaths at each of the individual state and national levels.
Discussion
The COVID-19 pandemic has catalyzed scientific innovation to prevent infection as well as reduce disease burden and transmission in those who develop COVID-19. Although no formal global estimates have been published, the effort has likely prevented millions of symptomatic cases and hundreds of thousands of deaths worldwide. In the US alone, it is estimated that vaccines prevented approximately 139,000 deaths in the first 5 months they were availableCitation44. However successful, the approved prevention and control strategies continue to leave many individuals at risk of serious illness and deathCitation45,Citation46. Given the distrust of traditional medicine that has emerged in some populationsCitation47,Citation48 during the pandemic, novel antiviral innovations such as RD-X19 are needed in addition to conventional antiviral therapies (e.g. nirmatrelvir/ritonavir tablets and molnupiravir capsules) that may abrogate social, political, operational, and economic constraints of embracing early treatment interventions.
Our model describes how any antiviral treatment with efficacy similar (+/‒ 50%) to RD-X19, as demonstrated in an early feasibility study, can prevent infections, deaths, and associated direct health care costs in outpatients with mild-to-moderate COVID-19 by reducing viral load, symptomatic days, and secondary transmission.
We recreated the course of the pandemic in 3 states representing those with lower, middle, and high vaccine uptake and at the US national level through a mathematical model using data from the CDC. Our results showed that the introduction of RD-X19 to only 10% of eligible patients (base case) with a diagnosis of mild or moderate (outpatient) in North Carolina, a state near the midpoint of vaccine adoption during the modeled period, could have resulted in between 10,600 and 31,476 fewer COVID-19 cases and between 274 and 812 fewer deaths, depending upon real-world effectiveness. The model shows similar cases averted and deaths prevented for the low- and high- vaccine status states of Alabama (cases reduced: 5,051–15,023; deaths averted: 138–410) and Massachusetts (cases reduced: 8,937–24,822; deaths averted: 209–615). In addition, our model describes how early intervention can meaningfully reduce direct health care expenditures. With 25% of the mild-to-moderate (outpatient) infected population using RD-X19 as a complement to vaccines, direct health care expenditures were reduced by approximately $332 million in North Carolina over 12 months. Even at an uptake as low as 5%, direct health care costs were reduced by approximately $68 million in the state. For the other states considered, reductions ranged from a low of approximately $35 million (5%, Alabama) to a high of approximately $249 million (25%, Massachusetts). Using the model to estimate the impact at the national level, subject to the limitations of extrapolation, revealed that at 10% utilization (2.1 million RD-X19 treatments deployed over 12 months), not only were the aggregate number of symptomatic days of illness reduced by 5.04 million but health care expenditures were reduced by approximately $4.4 billion, total infections by 686,722, and total deaths by 17,748.
Finally, our model also shows that antiviral therapeutics like RD-X19, which target delivery to infected individuals early in the disease process (within 3 days of symptom onset and following a positive COVID test), are a necessary and important complement to vaccines broadly delivered to healthy individuals. For example, over the first 5 months that they were available, 124 million full vaccine courses were delivered, preventing an estimated 139,000 deaths (1 life saved for every 892 full courses)Citation44. In our model, over the same period and in the base case of 10% uptake, 2.1 million antiviral RD-X19 interventions were self-administered to sick individuals, preventing an additional 17,748 deaths (1 life saved for every 118 interventions, 0.85%). We acknowledge that beyond the model time horizon (May 2021), vaccination rates have increased across the US and globally. However, the emergence of the omicron variant and the likelihood that other variants will emerge, the lack of a robust booster program, booster hesitancy, and breakthrough infections in the US highlight the continued need for new treatment options.
Further, the lifesaving benefits of antiviral therapeutics have now been demonstrated in large, international randomized controlled trials and validate the disease progression model reported in the current work. For example, Merck reported a treatment benefit over placebo of 1 life saved for every 83 courses (1.2%) of molnupiravir administered in their Phase 3 trialCitation15. Similarly, Pfizer reported a treatment benefit over placebo of 1 life saved for every 90 courses (1.1%) of nirmatrelvir/ritonavir administeredCitation14.
Early interventions such as RD-X19 that not only reduce days of symptomatic disease but also interrupt infection transmission could have a profound impact on the course of the pandemic. Over the past 2 years, we have experienced how the introduction of control measures resulted in significant benefits but also had unintended consequences. While we cannot model or predict the effects on human behavior with the introduction of this type of strategy, we believe that deployed as a complement to vaccines, interventions be they a device like RD-X19 or other antiviral therapies, can accelerate the transition from pandemic to endemic, helping the world return to pre-pandemic conditions. Further, they can maintain utility during the endemic stage by continuing to reduce morbidity, mortality, and direct health care costs, thereby providing relief for infected individuals and their families and reducing societal burdens.
Limitations
This simulation of the impact of a novel, light-based, antiviral intervention administered at home for COVID-19 has several limitations inherent to any modeling exercise. Where possible in our model, we have accounted for these limitations with a combination of conservative assumptions and sensitivity analyses. First, we assumed that treatment benefit was uniform across all adult populations in the modeled cohort. Although this is an exemplary target, it may not be the case in practice for all interventions, especially during the early stages of implementation (e.g. RD-X19 has to-date been evaluated only in the population aged 18–65 years). Second, risk of reinfection is not considered in the model, although evidence suggests up to a 7% risk of symptomatic reinfectionCitation49. Third, though infectiousness varies over the COVID-19 disease trajectory, we assumed it was constant. We verified that this did not change the findings by providing sensitivity analyses showing that even with intervention efficacy reduced to 50% of the base case, a decline in the incidence of overall infections and deaths was still observed. Fourth, we assumed that uptake of the treatment was instantaneous to modeled levels (5%, 10% and 25%) early in the modeled period. Even with a conservative estimate of 5% uptake, the effects of the device on the trajectory of the pandemic and resultant outcomes were meaningful. Fifth, we assumed that all vaccines, regardless of manufacturer, were equally efficacious (95%), with efficacy instantaneous upon completion of the course of the vaccine (2 doses of the mRNA vaccines and 1 dose of the Johnson & Johnson), that efficacy was constant for the complete duration of the 12-month model period, and that all recipients were protected from infection, not just symptomatic disease. These assumptions estimate that a higher proportion of the population is protected at a higher level than in practice, and therefore drive fewer individuals available for infection in both arms of the model. Additionally, this simulation was based on the observed COVID-19 incidence and vaccination data available at the conclusion of the modeled period. As such, we were not able to project into the future and can only hypothesize the potential impact of introducing RD-X19 or comparable antiviral treatments on potential future waves of COVID-19. Finally, the early feasibility study providing the reference for the base case of shortened duration of symptomatic disease by 2.4 days compared with sham-treated subjects is small (NCT04662671). Although larger studies are needed to confirm the results, and one such study is on-going (NCT04966013), this limitation is considered in the current work through the inclusion of a lower boundary condition of 1.2 days. Further, beyond RD-X19, the model can be used to evaluate the impact of other antiviral therapies on the course of the current pandemic and following endemic, regardless of the method of administration.
Conclusions
Although COVID-19 is likely to evolve into an endemic disease requiring periodic vaccination, many will remain at risk of illness and death due to the emergence of SARS-CoV-2 variants as well as existing and evolving social, political, and economic constraints. Interventions capable of reducing the duration of symptomatic disease and decreasing secondary transmission are needed as a complement to vaccines. Using the novel light-based, antiviral treatment RD-X19 as an example, our simulations show that even with modest efficacy and uptake, the morbidity, mortality, and health care costs can be reduced via deployment of an at-home treatment for mild-to-moderate COVID-19.
Transparency
Declaration of funding
This study was funded by EmitBio, Inc.
Declaration of financial/other relationships
RS, RTT are employees of Coreva Scientific; SG was an employee of Coreva Scientific during model design, interpretation, and initial manuscript development. DE, NS and JO are employees of EmitBio, Inc. MO was an employee of EmitBio, Inc. during model design. CB, JKK, and BF are employees of Cardinal Health.
DE reports personal fees from EmitBio, Inc., its parent, KnowBio LLC, and its Affiliates, outside the submitted work. In addition, Dr. Emerson has a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 17/410,154 issued, a patent ORAL ILLUMINATION DEVICE, 29/757,911 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128935 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210138259 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128936 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128937 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128938 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 11,147,984 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 2021-518715 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, PCT/US21/19785 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210379400 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 2021239894 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290970 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290975 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290971 pending, and a patent ORAL ILLUMINATION DEVICE, 29/780,830 pending.
JO reports personal fees from EmitBio, Inc. its parent, KnowBio LLC, and its Affiliates, outside the submitted work.
NS reports personal fees from EmitBio, Inc., its parent, KnowBio LLC, and its Affiliates, outside the submitted work; In addition, NS has a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 17/410,154 issued, a patent ORAL ILLUMINATION DEVICE, 29/757,911 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128935 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210138259 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128936 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128937 pending, a patent PHOTOTHERAPEUTIC LIGHT FOR TREATMENT OF PATHOGENS, US20210128938 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 11,147,984 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 2021-518715 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, PCT/US21/19785 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210379400 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, 2021239894 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290970 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290975 pending, a patent ILLUMINATION DEVICES FOR INDUCING BIOLOGICAL EFFECTS, US20210290971 pending, a patent ORAL ILLUMINATION DEVICE, 29/780,830 pending, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 15/222,199 issued, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 15/222,243 issued, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, ZL 2016 8 00559361 issued, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, BR 11 2018 001874 0 pending, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, BR 12 2020 024964 1 pending, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 202010561507.X pending, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 16831333.6 pending, a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 16/709,550 pending, and a patent SYSTEMS AND METHODS FOR PHOTOTHERAPEUTIC MODULATION OF NITRIC OXIDE, 16/898,385 pending.
MO reports personal fees from EmitBio, Inc., its parent, KnowBio LLC, and its Affiliates, outside the submitted work
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
SG [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript]; RS [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript], DE [interpretation of data, translation of model results to real-world implementation, drafting manuscript, approval of final manuscript], NS [study concept and design, interpretation of data, approval of final manuscript], CS [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript], JO [interpretation of data, approval of final manuscript], MO [study concept and design], RTT [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript], JKK [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript], BF [study concept and design, interpretation of data, drafting manuscript, approval of final manuscript].
Supplemental Material
Download MS Word (68.5 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Centers for Disease Control and Prevention. COVID data tracker. [cited 2022 Feb 1]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- Institute for Health Metrics and Evaluation. New COVID-19 Projections. September 30, 2021. [cited 2021 Oct 1]. http://www.healthdata.org/covid/video/insights-ihmes-latest-covid-19-model-run.
- Callaway E. Beyond omicron: what’s next for COVID’s viral evolution. Nature. 2021;600(7888):204–207.
- Marist Poll. NPR/PBS newshour/marist national poll: COVID. [2021; cited 2021 Oct 1]. https://maristpoll.marist.edu/polls/npr-pbs-newshour-marist-national-poll-covid-september-3-2021/.
- Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208.
- Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773.
- ReYeyati EL, Filippini F. (Global Economy and Development Program at Brookings). Social and economic impact of COVID-19. Brookings Global Working Paper #158. 2021. Available from: https://www.brookings.edu/wp-content/uploads/2021/06/Social-and-economic-impact-COVID.pdf
- U.S. Congressional Research Service. Global Economic Effects of COVID-19 (R46270); 2021. By James K. Jackson, Martin A. Weiss, Andres B. Schwarzenberg, Rebecca M. Nelson, Karen M. Sutter, Michael D. Sutherland. Text in: LexisNexis® Congressional Research Digital Collection; [cited 2021 Nov 30].
- Bartsch MCF, JA, McKinnell KJ, O’Shea, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020;39(6):927–935.
- Padula WV, Malaviya S, Reid NM, et al. Economic value of vaccines to address the COVID-19 pandemic: a U.S. cost-effectiveness and budget impact analysis. J Med Econ. 2021;24(1):1060–1069.
- Inserro A. Gilead sciences sets US price for COVID-19 drug at $2340 to $3120 based on insurance. [2020; cited 2021 Oct 22]. https://www.ajmc.com/view/gilead-sciences-sets-us-price-for-covid19-drug-at-2340-to-3120-based-on-insurance..
- Coronavirus (COVID-19) Update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. 2022. [cited 2022 Feb 17]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron.
- National Academies of Sciences, Engineering, and Medicine. Rapid expert consultation on allocating COVID-19 monoclonal antibody therapies and other novel therapeutics (January 29, 2021). Washington (DC): The National Academies Press; 2021.
- Fact sheet for healthcare providers: emergency use authorization for PAXLOVID™. New York City (NY): Pfizer Inc.; 2021.
- Fact sheet for healthcare providers: emergency use authorization for MOLNUPIRAVIR™. Whitehouse Station (NJ): Merck & Co., Inc.; 2022.
- González E. PUVA for psoriasis. Dermatol Clin. 1995;13(4):851–866.
- Wang KK, Kim JY. Photodynamic therapy in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2003;13(3):483–489.
- Møller KI, Kongshoj B, Philipsen PA, et al. How Finsen’s light cured lupus vulgaris. Photoderm Photoimm Photomed. 2005;21(3):118–124.
- Wiehe A, O’Brien JM, Senge MO. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. 2019;18(11):2565–2612.
- Dai T, Gupta A, Murray CK, et al. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15(4):223–236.
- Enwemeka CS, Bumah VV, Masson-Meyers DS. Light as a potential treatment for pandemic coronavirus infections: a perspective. J Photochem Photobiol B. 2020;207:111891.
- Stasko N, Kocher JF, Annas A, et al. Visible blue light inhibits infection and replication of SARS-CoV-2 at doses that are well-tolerated by human respiratory tissue. Sci Rep. 2021;11(1):1–13.
- Stasko N, Cockrell AS, Kocher JF, et al. A randomized, controlled, feasibility study of RD-X19 in patients with mild-to-moderate COVID-19 in the outpatient setting. Clin Transl Sci. 2022. DOI:10.1111/cts.13249
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–122.
- Centers for Disease Control and Prevention. Trends in number of COVID-19 vaccinations in the US. [cited 2021 Sept 2]. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends.
- Baloxavir marboxil [package insert]. South San Francisco (CA): Genentech USA, Inc.; 2021.
- Clinical Spectrum of SARS-CoV-2 Infection. 2021. [cited 2021 Sept 1]. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
- Center for Drug Evaluation and Research. 2022. [cited 2022 Feb 18] https://toolkit.ncats.nih.gov/glossary/center-for-drug-evaluation-and-research/
- United States Census Bureau 2019. 2019 American Community survey 1-year estimates. http://www.census.gov/programs-surveys/acs.htm
- Centers for Disease Control and Prevention. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and juridiction. https://covid.cdc.gov/covid-data-
- Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstetr Gynecol. 2021;137(4):571–580.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207.
- Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). 2020;39(7):1253–1262.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 – final report. N Engl J Med. 2020;383(19):1813–1826.
- López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435.
- Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644.
- Wongvibulsin S, Garibaldi BT, Antar AAR, et al. Development of severe COVID-19 adaptive risk predictor (SCARP), a calculator to predict severe disease or death in hospitalized patients with COVID-19. Ann Intern Med. 2021;174(6):777–785.
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615.
- Grannis SJ, Rowley EA, Ong TC, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - Nine States, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291–1293.
- Chen J, Vullikanti A, Hoops S, et al. Medical costs of keeping the US economy open during COVID-19. Sci Rep. 2020;10(1):18422.
- Congly SE, Varughese RA, Brown CE, et al. Treatment of moderate to severe respiratory COVID-19: a cost-utility analysis. Sci Rep. 2021;11(1):17787.
- Gupta S, Cantor J, Simon KI, et al. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff (Millwood). 2021;40(9):1465–1472.
- Charumilind S, Craven M, Lamb J, et al. [McKinsey and Company]. When will the covid-19 pandemic end? 2021. Available from: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/when-will-the-covid-19-pandemic-end.
- del Rio C, Malani P. COVID-19 in 2021-continuing uncertainty. JAMA. 2021;325(14):1389–1390.
- Carson SL, Casillas A, Castellon-Lopez Y, et al. COVID-19 vaccine decision-making factors in racial and ethnic minority communities in Los Angeles, California. JAMA Netw Open. 2021;4(9):e2127582.
- Jaiswal J, LoSchiavo C, Perlman DC. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 2020;24(10):2776–2780.
- Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397(10283):1459–1469.