Abstract
Objective
Identification of the phenotypic transition from relapsing-remitting multiple sclerosis (RRMS) to secondary progressive multiple sclerosis (SPMS) is often delayed due to disease complexity and an unwillingness to withdraw RRMS disease-modifying therapies (DMTs), driven by limited SPMS treatment options. Despite the paucity of clinical evidence for efficacy in patients with SPMS, DMTs licensed for RRMS are frequently continued into the early stages of SPMS. The cost-effectiveness of oral siponimod, an active SPMS DMT, versus continued oral or infused RRMS DMTs for patients with active SPMS, was evaluated.
Methods
A cohort Markov model based on disease progression through Expanded Disability Status Scale health states, with annual cycles and lifetime horizon, was employed to determine the cost-effectiveness of siponimod from a UK National Health Service (NHS) perspective for patients with active SPMS. Baseline characteristics, health state utility values, hazard ratios for time to 6-month confirmed disability progression, annualized relapse rate ratios and adverse events for siponimod were obtained from the phase 3 EXPAND clinical trial, supplemented by published literature. Published costs, resource use data and comparator efficacy data were obtained from the literature and, in the absence of data, reasonable assumptions were made.
Results
Quality-adjusted life years (QALYs) were greater for siponimod versus all comparators (3.45 versus 2.69–2.83). Incremental cost-effectiveness ratios (ICERs), calculated as cost per QALY, for siponimod versus natalizumab (dominant), ocrelizumab (£4,760), fingolimod (£10,033) and dimethyl fumarate (£15,837) indicated that siponimod was cost-effective at the commonly accepted willingness-to-pay threshold of £30,000/QALY.
Conclusions
Recognition of active SPMS and treatment of this phenotype with siponimod offers a cost-effective and clinically beneficial treatment approach compared with the continuation of oral or infused RRMS DMTs.
Introduction
Multiple sclerosis (MS) is a chronic, neurodegenerative, autoimmune disorder of the central nervous systemCitation1,Citation2. In the United Kingdom (UK), approximately 130,000 people have a diagnosis of MS, with around 7,000 new diagnoses each yearCitation3.
Approximately 85% of patients with MS begin with relapsing-remitting multiple sclerosis (RRMS), characterized by relapses, followed by complete or partial recovery and separated by stable periods of remissionCitation1,Citation4. For the majority with RRMS, progressive disability eventually becomes apparent, independent of relapse activity. This marks the transition to secondary progressive multiple sclerosis (SPMS), characterized by cumulatively increasing disability with or without superimposed relapsesCitation5.
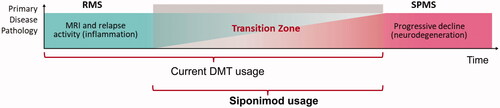
In untreated MS, the transition from RRMS to SPMS occurs in approximately half of patients with RRMS within 15 years; however, it is insidious and difficult to recognize, with a typical delay in recognition of three years retrospectivelyCitation1,Citation5,Citation6. Limited disease-modifying therapies (DMTs) are approved for use in SPMS, having been unable to demonstrate efficacy in clinical trials. This, and an unwillingness to withdraw DMTs initiated in the RRMS stage, has led to a reluctance to change the phenotypic labelCitation7,Citation8. Therefore, treatment with RRMS DMTs, which have not demonstrated efficacy in patients with SPMS, is often continued into the early stages of SPMS ()Citation7,Citation8.
Figure 1. A schematic representation of the transition between RRMS and SPMS over time along with the current scope of DMT use and that anticipated for siponimod. Abbreviations. DMT, disease modifying therapy; RMS, relapsing multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Siponimod, a sphingosine-1-phosphate receptor modulator with a central and peripheral mechanism of actionCitation9, is an oral DMT that has been shown to be clinically effective at slowing disability progression in SPMS, measured on the Expanded Disability Status Scale (EDSS) – a scale dominated by physical function, particularly ambulationCitation10. Data from the EXPAND clinical trial demonstrated that siponimod was also effective at reducing the annualized relapse rate (ARR) and maintaining cognitive function in patients with SPMS, compared with placeboCitation11,Citation12.
Post hoc subgroup analyses of patients with active SPMS (defined as having had relapses in the two years prior to screening and/or ≥1 gadolinium-enhancing lesion at baseline) highlighted more pronounced clinical efficacy, with respect to disability progression and ARR reduction than in the general SPMS populationCitation13–15. Furthermore, siponimod treatment was associated with maintenance of cognitive functions at Months 12 and 24, as measured by the symbol digit modalities test (SDMT; a measure of attention, concentration, and processing speed), versus placebo in the active subgroupCitation14,Citation15.
Siponimod has been approved by the National Institute for Health and Care Excellence (NICE) and the Scottish Medicines Consortium (SMC) for the subgroup of adult patients with active SPMS evidenced by relapses or imaging features of inflammatory activityCitation16,Citation17.
It is likely that the availability of a DMT licensed and reimbursed for SPMS will prompt earlier recognition and diagnosis of the phenotype, leading to earlier initiation of, or switch to, an effective specific therapyCitation6. Interferon beta-1b is the only alternative DMT currently reimbursed for SPMS with continuing relapses, and during the NICE appraisal of siponimod, it was confirmed by the National Health Service (NHS) commissioning expert that the use of this treatment is negligibleCitation16.
During the NICE appraisal, comparisons to no treatment and treatment with injectable interferon beta-1b found siponimod to be cost-effective; however, the true alternative scenario for many patients will be continuation of their current RRMS DMT (oral or infused) into the secondary progressive phaseCitation7,Citation8,Citation16,Citation18.
Here, we evaluate the cost-effectiveness of siponimod in this scenario, using DMTs established for the treatment of RRMS in the UK as comparators (natalizumab, ocrelizumab [both infused], and fingolimod, dimethyl fumarate and teriflunomide [all oral])Citation19–23. These were considered relevant comparators for this assessment based on prescribing patterns at the time of study conception. Interferon beta and glatiramer acetate were not considered relevant to this analysis given the paucity of use at this disease stage within the NHSCitation16.
Methods
Patient population
Patients considered in the model were those in the active SPMS subgroup of the EXPAND phase 3 clinical trialCitation14. EDSS scores for these patients were in the range of 3.0–6.5 and active SPMS was evidenced by relapses in the two years prior to the study and/or the use of magnetic resonance imaging (MRI) to identify gadolinium-enhancing lesions at trial entry. The data cut associated with this definition of active SPMS was assessed by the European Medicines Agency (EMA) and informed the marketing authorization of siponimodCitation24. Baseline characteristics of patients with active SPMS provided a cohort comprised of 64% female patients with a mean age of 46.6 years (standard deviation: 8.26) and a median EDSS score of 6.0Citation14.
Structure of the economic model
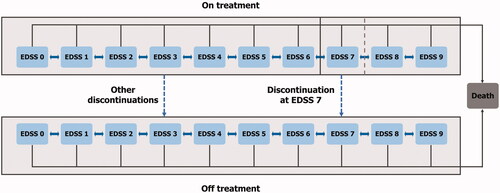
The cost-effectiveness of siponimod was assessed using a cohort Markov model based on disease progression through EDSS health states, as has previously been described ()Citation25. The model took the perspective of the UK NHS and Personal Social Services with 1-year cycles and a lifetime time horizon.
Figure 2. The structure of the cohort Markov model shows the EDSS health states (0–9) through which patients could transition, or remain, during each cycle. An EDSS score of 10 is considered a death state. At any time, patients could discontinue treatment and move to the off-treatment arm, thus losing any treatment benefit. Upon reaching an EDSS health state of 7, treatment is discontinued. This figure has been redrawn from Schur et al.Citation25. Abbreviation. EDSS, Expanded Disability Status Scale.
The EDSS is a composite score (0–10) combining clinical signs of neurological impairment and assessment of function, predominantly driven by the loss of ambulation for the range 3.0–6.5. A lower score corresponds to reduced disability (or no disability for EDSS 0) and an EDSS score of 10 is indicative of death occurring due to MS; this was treated separately from the other health states.
In alignment with recent NICE appraisals, a half-point EDSS score was rounded down to the nearest whole number to give 10 EDSS states (0–9). During each cycle of the model, patients could remain at the same EDSS state or transition to a higher/lower EDSS state, as well as experience a relapse. As per the NHS England treatment algorithm for MS, it was assumed that patients reaching an EDSS health state of 7 or higher discontinued their DMT, receiving best supportive care (BSC) until deathCitation26. These guidelines provide a conservative assumption of treatment regimes in SPMS and have not yet been updated to reflect the introduction of an SPMS DMT. Therefore, this stopping rule was applied as a modeling assumption to provide a direct comparison between siponimod and RRMS DMTs in current clinical practice.
Patients could be moved to the off-treatment cohort due to discontinuation for any reason, as per the EXPAND clinical trialCitation11. At the point of treatment discontinuation, any treatment benefit associated with the intervention was no longer applied and it was assumed that patients would not commence or revert to any other DMT; patients who discontinued were modeled to receive BSC until death.
Death was the absorbing state for the model and patients could transition to this state from any EDSS state. Mortality was incorporated based on gender-averaged mortality rates derived from the Office for National Statistics (2017–2019) and modified based on the EDSS state using a modifier derived from Pokorski et al. in the base caseCitation27,Citation28. Treatment with DMTs was not assumed to have a direct survival benefit.
Clinical inputs
Natural history probabilities for transitioning between EDSS health states 3–6 were obtained from the placebo arm of the EXPAND clinical trial; the values of which are reported in previous cost-effectiveness analysesCitation11,Citation25. Transition probabilities between EDSS health states 0–2 and 7–10 were extracted from the London Ontario database, to give the complete transition matrix (Supplementary Table 1) which was normalized and calculated, as previously describedCitation25. In general, concordance was observed in the overlapping regions of the two data sets.
The hazard ratio (HR) for time to 6-month confirmed disability progression (CDP) for siponimod was applied as a treatment effect on the transition probabilities of disability worsening, but not applied to disability improvement. 6-Month CDP was chosen as the base case for the model since it is less likely to be impacted by incomplete recovery from relapses than 3-month CDP and is the preferred outcome for NICE appraisals in this disease area.
Natalizumab was the only comparator with clinical trial data for patients with SPMS, reporting an odds ratio of 1.060 (standard error: 0.74–1.53) versus placebo for the proportion of patients with 6-month CDP at Week 96 of ASCENDCitation29. The ASCEND trial did not report time to 6-month CDP (as measured by EDSS scores), thus requiring data for the proportion of patients with 6-month CDP at 96 weeks to be used. The results suggested that, while a highly effective DMT for RRMS, natalizumab was not observed to reduce 6-month CDP (measured by EDSS scores), versus placebo, in patients with SPMSCitation29.
In the absence of clinical efficacy data for other comparators in patients with SPMS, it was considered reasonable to assume that no effect on disability progression in SPMS would apply, in line with the ASCEND trial results. Further support for the validity of this assumption can be found in an observational study published by Roos et al.Citation30, reporting similar efficacy of both high- and low-efficacy RRMS DMTs on CDP in patients with active SPMS. Taken with the lack of efficacy reported for natalizumab with respect to 6-month CDP (as measured by EDSS scores), a treatment effect was not applied to the natural history of EDSS disability progression for comparators.
The effect of each intervention on the frequency of relapses was also considered, and for siponimod the ARR ratio (versus placebo) was sourced from the active SPMS subgroup of the EXPAND clinical trialCitation14. The ARR ratio used for natalizumab was reported in the ASCEND clinical trial for patients with SPMSCitation29. For the remaining interventions without available SPMS-specific data, the ARR ratios used in the model were those reported by Samjoo et al.Citation31 in a recent network meta-analysis (NMA) conducted for DMTs in patients with relapsing forms of MS. As a result, it was assumed that RRMS DMTs continue to have a positive effect on relapses in active SPMS, as observed by Roos et al.Citation30, but disability progression is unaffected, in line with results from the ASCEND trialCitation29.
Treatment benefits were applied to the natural history of ARRs by EDSS health state, obtained from the placebo arm of the EXPAND trial (for EDSS 3–7) and a study by Patzold et al. from a UK population of patients with MS (for EDSS 0–2)Citation29,Citation32. These values are summarized in Supplementary Table 2. Values for EDSS 8 and 9 were assumed to be the same as for EDSS 7, aligning with Patzold et al. and recent NICE appraisalsCitation32. The values for 6-month CDP HRs and ARR ratios for each intervention are summarized in .
Table 1. Clinical efficacy inputs for the DMTs, versus placebo.
For siponimod, the all-cause discontinuation rate was obtained from the EXPAND clinical trialCitation11. An exponential function was chosen as the most appropriate fit for the data for the long-term extrapolation of discontinuation rate. Discontinuations for natalizumab were obtained by applying the relative risk from a discontinuation indirect treatment comparison (ITC), between the EXPAND and ASCEND trials, to the discontinuation rate of siponimod obtained from the parametric curve for the respective year, as in a previous cost-effectiveness analysis of siponimodCitation25. In the absence of treatment discontinuation data in SPMS patients for comparators other than natalizumab, assumptions were required. Given the lack of established efficacy on disability progression, combined with guidelines to discontinue such DMTs once SPMS is formally recognized, it was assumed that the discontinuation rate would be 30% higher for such comparators relative to siponimod; this assumption of higher discontinuation was found to be conservative in the model. The exponential plot for siponimod and relative risk of discontinuation for comparators, versus siponimod, are given in Supplementary Figure 1 and Supplementary Table 3, respectively.
Adverse events (AEs) for siponimod included those reported in ≥5% of siponimod-treated patients, by preferred term, during the placebo-controlled treatment phase of the EXPAND clinical trial, as with the NICE technology appraisal of siponimodCitation16. The nature and probabilities of AEs associated with comparator DMTs, accepted by NICECitation16, are shown in Supplementary Table 4.
Cost inputs
Treatment costs considered in the model included acquisition (list prices obtained from the British National Formulary [BNF]) and administration, using the most recently published NHS references costs (for the year 2019–2020) and Personal Social Services Research Unit (PSSRU) costs (for the year 2020) (Supplementary Table 5)Citation33,Citation34. Monitoring resources and costs were sourced from the relevant NICE appraisals of each DMTCitation16,Citation19–23.
All relapses were modelled identically with average costs and utility decrements applied; the proportion of these requiring hospitalization was taken from the EXPAND clinical trial and the costs were obtained from Tyas et al., inflated to the year 2020 (Supplementary Table 6a)Citation35. AE costs were estimated based on the resources required to manage each AE and were dependent on whether the AE was classified as serious or non-serious (the proportions of which were taken from the EXPAND clinical trial and were 0.858 and 0.142, respectively). Resource use was obtained from recent RRMS NICE appraisals and used NHS reference and PSSRU costs (Supplementary Table 6b). EDSS health state costs were obtained by inflating those reported in the 2005 UK MS Survey to 2019–2020 costs, consistent with the NICE committee preference (Supplementary Table 7)Citation36.
Utility inputs
Utility inputs for EDSS health states 3–7 were calculated from the active SPMS subgroup of the EXPAND clinical trial while those for EDSS health states 0–2 and 8–9 were extracted from data published by Orme et al. (Supplementary Table 8)Citation37. The values derived from the study by Orme et al. were obtained by the application of utility decrements, dependent on EDSS state and due to SPMS, to the coefficient calculated for patients with RRMS and EDSS 0. Data from EXPAND and those derived from Orme et al. showed concordance in the overlapping EDSS states.
Disutility values for relapse events were those calculated from the active SPMS subgroup of the EXPAND clinical trial (Supplementary Table 9). AE utility decrements were considered for each treatment and sourced from the relevant NICE appraisals (Supplementary Table 10). Caregiver utility decrements by EDSS health state were included in the model; data for which were obtained from the NICE appraisal for natalizumab (Supplementary Table 11)Citation19.
Model outcomes
Results generated from the model are provided in terms of incremental cost-effectiveness ratios (ICERs), calculated as cost per quality-adjusted life year (QALY); ICERs are estimated for siponimod versus each comparator. Costs and benefits are discounted by 3.5% annually, aligned with the NICE reference caseCitation38.
Sensitivity analyses
Probabilistic and deterministic sensitivity analyses (PSA and DSA) were undertaken. PSAs were generated by assigning distributions to all model inputs and randomly sampling from these over 1,000 simulations. These analyses allowed calculation of the probabilities of siponimod being cost effective at the willingness-to-pay (WTP) threshold of £30,000 versus each comparator.
One-way DSAs were also carried out for each comparator to determine the impact of individual model inputs on the outcomes. Where available, data for the upper and lower confidence intervals (or credible intervals for ARR ratios taken from the NMA) were used from the relevant source. In the absence of data, an assumed change of ±20% was applied.
Scenario analyses
Several scenarios were explored to investigate the effects of changes to input choices and assumptions in the model. Such scenarios included variations on the sources of inputs used for the natural history of disease progression and relapse, utility values, and relapse disutilities. Additional scenarios investigated the exclusion of treatment discontinuation, relapses and adverse events, as well as varying the model time horizon. Based on the assumptions made for the relative risk of discontinuation for comparators, the change to ICERs when using the upper and lower bounds of these values (based on an assumed change of ±20%) were explored.
A further scenario, whereby patients with SPMS were treated up to an EDSS score of 8 (as opposed to 7 in the NHS England treatment algorithmCitation26), was also evaluated for siponimod versus all comparators, based on the possibility of a change in clinical practice due to the introduction of a DMT licensed and reimbursed for active SPMS.
Results
Base case
The results of the base case scenarios for siponimod versus each comparator are presented in . The ICERs estimated by the model predicted that siponimod was estimated to be cost-effective at the commonly accepted WTP threshold of £30,000/QALY when compared with natalizumab, ocrelizumab, fingolimod and dimethyl fumarate.
Table 2. Base case outcomes of the model for each DMT.
When comparing siponimod with teriflunomide, the model predicted an ICER of £33,689, marginally above the commonly accepted WTP threshold of £30,000/QALY. Despite this, the QALYs predicted for siponimod treatment (3.45) were greater than those for the comparators in all cases, including teriflunomide (2.69–2.83).
Sensitivity analysis
The results from the PSAs for each comparator are shown in and were aligned to the deterministic results reported in . The results show that siponimod has a high probability of being cost-effective at a WTP of £30,000 versus each comparator, with the exception of teriflunomide. Scatter plots containing further results from the PSAs for each comparator are given in Supplementary Figure 2 and cost-effectiveness acceptability curves are provided in Supplementary Figure 3.
Table 3. Average probabilistic results obtained from the PSAs.
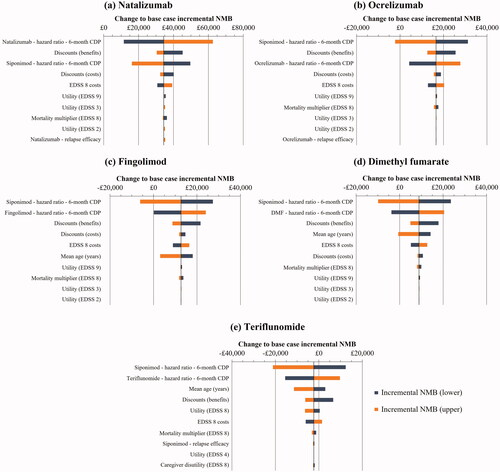
Tornado plots of the DSAs for each comparator are given in showing the parameters with the largest impact on the net monetary benefit (NMB). In all cases, the greatest uncertainty was found in HRs of disability progression for both siponimod and/or the comparator.
Scenario analysis
The scenario analyses and resulting ICERs are summarized in . Exploration of the use of literature data in place of those from the EXPAND trial allowed evaluation of the effect on the resulting ICERs for each comparator. The natural history of disability progression (from the London Ontario and British Columbia databases), relapse (from Patzold et al.Citation32) and utility values (from Orme et al.Citation37) were all explored as scenarios.
Table 4. Scenario analyses performed for siponimod versus each comparator.
The use of the London Ontario database, data from which were not specific to patients with active SPMS, for natural history of disease progression through all EDSS health states resulted in increased ICERs versus all comparators. A more pronounced increase was observed when implementing the British Columbia database; however, these data are predominantly from patients with RRMS. The effect of changing the source of relapse data was minimal, and minor differences resulted from the use of literature utility values. These results reflect the concordance between the EXPAND data and those reported in the literature for these parameters.
Interestingly, the exclusion of treatment discontinuation resulted in a significant increase in the ICER (to £1,986 from dominant in the base case) versus natalizumab. This increase was due to siponimod incurring higher total costs than natalizumab in this scenario (siponimod: £454,055; natalizumab: £449,342 [incremental costs: £4,714]) and an incremental QALY of 2.37, resulting from total QALYs of 4.82 and 2.45 for siponimod and natalizumab, respectively. This change in outcome was primarily driven by the change from negative to positive incremental costs, resulting in a positive ICER.
Continuation of siponimod and comparators to EDSS 8 was also explored, with the results from this analysis provided in . This scenario found siponimod to be slightly more cost-effective versus natalizumab, ocrelizumab, fingolimod and dimethyl fumarate when compared with stopping treatment at EDSS 7 as in the base case analysis.
Table 5. Model outcomes with treatment discontinuation at EDSS health state 8.
Discussion
The analysis reported here examines the cost-effectiveness of siponimod in an important real-world decision problem, taking into account current clinical practice in the NHS, as reported by healthcare professionals during the NICE technology appraisalCitation16. Within the NHS it is common for DMTs, reimbursed for use in patients with RRMS but not recommended for those with SPMS, to be continued during the transition to, and initial stages of SPMSCitation7,Citation16. This continuation may be a conscious decision so as not to disadvantage the patient; however, may also be a result of difficulties identifying the phenotypic transition to SPMSCitation7,Citation8.
RRMS DMTs were modeled as comparators with the respective ARR ratios obtained from the ASCEND trial for natalizumab and the NMA published by Samjoo et al. for all other comparatorsCitation29,Citation31. Induction therapies cladribine and alemtuzumab, although approved by NICE for use in some forms of RRMS, were not considered as comparators (as with the NICE appraisal of siponimod) as these are given as induction doses only and are not an ongoing treatment. Thus, if a patient begins the transition to SPMS having been treated with these drugs, retreatment with additional courses of the original drug would not be considered.
Ofatumumab, recently approved by NICE and the SMC, and ozanimod, approved by the SMC (but not recommended by NICE) for use in RRMS were not considered relevant comparators for this analysis as they are yet to be established in current clinical practice and were not approved at the time of study conception. Interferon beta and glatiramer acetate were also excluded from the model due to limited use within the NHSCitation16.
The reported lack of clinical efficacy of natalizumabCitation29, a highly efficacious treatment for RRMSCitation31,Citation39, in reducing 6-month CDP for patients with SPMS provided the basis of the key assumption that continuation of RRMS DMTs does not slow disability progression (an increase in EDSS score) in patients with SPMS. No clinical trial data are currently available for the other comparators, requiring an assumption to be made about the treatment effect in the modeled population. Since Roos et al.Citation30 found no difference between high-efficacy (including natalizumab, ocrelizumab and fingolimod) and low-efficacy (including interferons, glatiramer acetate and teriflunomide) RRMS DMTs on CDP, it was assumed that the lack of clinical efficacy observed for natalizumab in the ASCEND study was applicable to all comparators, thus no effect on disability progression was applied.
The results of the model estimated that siponimod is likely to be cost-effective for the treatment of patients with active SPMS at the usual NICE WTP thresholds when compared to the continuation of RRMS DMTs, driven by its clinical efficacy in slowing disability progression. The one exception was teriflunomide; however, considering the lack of evidence and usual positioning of the drug, expert clinical opinion deemed it to be an inappropriate and unlikely clinical choice in patients transitioning to or identified as having SPMS, despite the low list price which is likely to have influenced the ICER value.
While the results demonstrate the cost-effectiveness in this context, the true values are uncertain given that DMTs are known to be provided to the NHS with confidential discounts. Despite this uncertainty, the therapeutic benefit of siponimod is evident from the incremental QALY results which drive the predicted cost-effectiveness, despite higher total costs versus all comparators (excluding natalizumab). Treatment with natalizumab (which does not have a confidential discount and is commissioned at list price) was predicted to incur higher costs (total and incremental) with a reduced therapeutic benefit (reduced QALYs) when compared with siponimod, leading to the dominant cost-effectiveness result and large NMB at WTP of £30,000 ().
Additional clinical benefits reported with siponimod use, such as the improved cognitive measures versus placeboCitation14,Citation15, were not included in the model. Such inclusion is not trivial and may not be consistently or accurately reported in cost-effectiveness modelsCitation40; however, it is likely that the conversion of these scores to utility values would improve the overall clinical benefit predicted for siponimod (and be reflected in the QALY values). This is, however, uncertain and further assumptions for comparator treatments would be required due to the limited comparative data and inconsistent use of the SDMT measure in clinical trials.
In common with many economic models, one limitation of this evaluation is the use of a clinical trial patient population, based on pre-specified and stringent inclusion criteria. The generalizability of the data and model outcomes to the general population of patients with active SPMS could be queried on these grounds; however, in practice, these data have been considered generalizable by the EMACitation24.
The paucity of clinical trial data pertaining to the efficacy of RRMS DMTs in SPMS populations was also a limitation, leading to a lack of direct comparative evidence. Only the ASCEND trial of natalizumab evaluated the use of a comparator in patients with SPMS, with no reduction in 6-month CDP observed versus placeboCitation29; results that were further supported by Roos et al. Citation30. Extrapolation of this lack of efficacy across all comparators was therefore required and assumed to be reasonable given that natalizumab is known to be a highly efficacious DMT in RRMSCitation31,Citation39 and that there are differences in the mechanism of action compared with siponimodCitation9. This assumption was further explored in the DSA, with intervention and comparator HRs for 6-month CDP found to be the most influential parameters on the model outcomes. Despite this, in cases where siponimod was predicted to be cost-effective, large deviations from the HRs used in the model would be required for negative NMBs to be observed, which would be inconsistent with the available evidence.
As a further limitation, no clinical trial data are available for comparator use in the active SPMS subgroup; in particular, the ASCEND trial of natalizumab does not explore efficacy with respect to 6-month CDP, as measured by EDSS scores, in this subgroup of patients. Therefore, a comparison in this sub-population cannot be made, requiring the assumption that the treatment effect (with respect to 6-month CDP) and ARR ratio observed in the ASCEND trial of the overall SPMS population are relevant to patients with active SPMS.
The ARR ratios reported in the RRMS population are used in the model under the assumption that the treatment benefit, with respect to relapse reduction, in the RRMS population is identical in patients transitioning to, or identified as having, active SPMSCitation31. Roos et al.Citation30 support this assumption, highlighting that a reduction in relapses is observed in patients with active SPMS when treated with high-efficacy RRMS DMTs compared with low-efficacy RRMS DMTs. Equal effectiveness on relapses in SPMS and RRMS was considered to be a conservative assumption. The requirement for the abovementioned assumptions relating to the efficacy of DMTs in this patient cohort highlights a key data gap pertaining to a prospective comparison of RRMS DMTs in the transition to, and early stages of, SPMS. This may reflect the difficulty in identifying the transition point, which remains a challenge in clinical practice and cannot be addressed by this study.
The relative risk of discontinuation for RRMS DMTs, other than natalizumab, versus siponimod required assumptions to be made as no clinical trial data in an SPMS population was available. The assumption of a relative rate 30% above the discontinuation rate for siponimod was conservative in the model, as was reflected in the ICER values obtained in the scenario analyses using the upper and lower bounds of this value (±20% of the relative risk value of 1.3). Despite an increase in the values of the predicted ICERs when using the upper limit of the relative risk of discontinuation (a relative rate 56% above that of siponimod), siponimod remained cost-effective versus all comparators except for teriflunomide, as in the base case. The use of the lower limit, assuming a similar rate of discontinuation, gave predicted ICERs well below the WTP threshold of £30,000/QALY, however, appeared to be an unfair representation of the real-world scenario. The use of this assumption represents an additional limitation of this model, as well as highlighting the sparsity of clinical data for RRMS DMTs in a secondary progressive patient cohort.
Finally, results from the scenario analysis exploring the continuation of treatment to EDSS 8 showed very little deviation from the base case results (within the limitations outlined, extrapolating clinical efficacy data from patients in EDSS health states 3–7). This suggests that cost-effectiveness considerations may not present a significant barrier to a potential future policy change in which NHS patients are allowed to continue on treatment for longer, if the assumptions underpinning the analysis are valid.
The consideration of continued RRMS DMT use versus the earlier identification of the transition to SPMS, and treatment with siponimod is an important real-world decision problem in which to assess the cost-effectiveness of siponimod. This approach, focusing on continued RRMS treatment, has predicted that siponimod provides a cost-effective and therapeutically beneficial treatment option for patients with active SPMS (demonstrated by incremental QALY benefits of 0.62–0.76), treated in the NHS. Earlier identification of the transition to SPMS and delaying disability progression as a result of siponimod treatment are key influences in the outcome of this model and offer a distinction between this treatment strategy and the continuation of ineffective RRMS DMTs.
Conclusions
The introduction of siponimod, a clinically effective DMT licensed for active SPMS, may result in earlier recognition and acknowledgement of the transition from RRMS to SPMS, thus displacing continued RRMS DMT use. The analysis presented here shows that siponimod provides a cost-effective alternative to current treatments (continued RRMS DMTs) used within the NHS in the UK. Siponimod is estimated to be cost-effective in most cases and was predicted to show improved QALYs versus all comparators.
Transparency
Declaration of funding
This research was funded by Novartis Pharmaceuticals UK Ltd.
Declaration of financial/other interests
SM and FW are employees of Costello Medical, who were contracted by Novartis Pharmaceuticals UK Ltd to undertake the work. UV is an employee of Novartis Ireland Limited. KG is an employee of Novartis Healthcare Private Limited. MD has received honoraria for advisory boards, speaker’s fees, research funding and expenses to attend educational events from Novartis, Biogen, Sanofi Genzyme, Teva, Mylan, Roche and TG Therapeutics. MK was an employee of Novartis Pharmaceuticals UK Ltd when this analysis was conducted and is now an employee of Novartis Pharma AG and a Novartis shareholder.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Substantial contributions to study conception and design: SM, FW, UV, KG, MK; substantial contributions to analysis and interpretation of the data: SM, FW, UV, KG, MD, MK; drafting the article or revising it critically for important intellectual content: SM, FW, UV, KG, MD, MK; final approval of the version of the article to be published: SM, FW, UV, KG, MD, MK.
Previous presentations
An interim analysis and the corresponding results were presented at virtual ISPOR Europe 2021.
Supplemental Material
Download MS Word (447.3 KB)Acknowledgements
The authors would like to acknowledge Nicholas Adlard, Novartis Pharma AG, Basel, Switzerland, for support with model development. Medical writing assistance was provided by Luke Green, Costello Medical, Cambridge, UK.
References
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):669–1517.
- Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;184(1–2):37–44.
- Multiple Sclerosis Trust – Prevalence and Incidence of Multiple Sclerosis [cited 2021 June 08]. Available from: https://mstrust.org.uk/a-z/prevalence-and-incidence-multiple-sclerosis.
- MS Society. Types of MS. [cited 2021 June 03]. Available from: https://www.mssociety.org.uk/about-ms/types-of-ms/relapsing-remitting-ms.
- Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler. 2008;14(3):314–324.
- Inojosa H, Proschmann U, Akgun K, et al. A focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition. J Neurol. 2021;268(4):1210–1221.
- Duddy M, Wilkinson C, Medhurst S, et al. Results from SPECTRUM: a survey of healthcare professionals to understand current diagnosis and management practices for secondary progressive multiple sclerosis in the United Kingdom. Mult Scler Relat Disord. 2021;55:103174.
- Caseby SCL, Woodhouse FA, Montgomery SM, et al. Transition to secondary progressive multiple sclerosis: The consequences for patients and healthcare systems, a healthcare professional survey. Health Sci Rep. 2022;5(1):e474.
- Yong HYF, Yong VW. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol. 2022;18(1):40–55.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452.
- Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273.
- Kappos L, Giovannoni G, Gold R, et al. Long-term efficacy and safety of siponimod in patients with SPMS: EXPAND extension analysis up to 5 years (4128). Neurology. 2020;94(15 Supplement):4128.
- Gold R, Kappos L, Benedict R, et al. Siponimod slows physical disability progression and decline in cognitive processing speed in SPMS patients with active disease: a post hoc analysis of the EXPAND study. Eur J Neurol. 2020;27:328–329.
- Gold R, Kappos L, Bar-Or A, et al. Efficacy of siponimod in secondary progressive multiple sclerosis patients with active disease: the EXPAND study subgroup analysis. ECTRIMS Online Library. 2019;279110:P750.
- Benedict RHB, Tomic D, Cree BA, et al. Siponimod and cognition in secondary progressive multiple sclerosis: EXPAND secondary analyses. Neurology. 2021;96(3):e376–e386.
- National Institute for Health and Care Excellence. Siponimod for treating secondary progressive multiple sclerosis (TA656). 2020. [cited 2021 June 03]. Available from: https://www.nice.org.uk/guidance/ta656.
- Scottish Medicines Consortium. Siponimod (Mayzent®) 2020. [cited 2021 July 05]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/siponimod-mayzent-full-smc2265/.
- Transition to secondary progressive multiple sclerosis: When is SPMS identified in the UK and what are the consequences for patients and the National Health Service? Presented at the MS Trust Conference 2019 [cited 2021 July 05]. Available from: https://mstrust.org.uk/sites/default/files/ms_conference_posters_2019_Caseby_FINAL.pdf.
- National Institute for Health and Care Excellence. Natalizumab for the treatment of adults with highly active relapsing–remitting multiple sclerosis (TA127) 2007. [cited 2021 June 08]. Available from: https://www.nice.org.uk/guidance/ta127.
- National Institute for Health and Care Excellence. Fingolimod for the treatment of highly active relapsing–remitting multiple sclerosis (TA254) 2012. [cited 2021 June 08]. Available from: https://www.nice.org.uk/guidance/ta254.
- National Institute for Health and Care Excellence. Dimethyl fumarate for treating relapsing-remitting multiple sclerosis (TA320) 2014. [cited 2021 June 08]. Available from: https://www.nice.org.uk/guidance/ta320.
- National Institute for Health and Care Excellence. Teriflunomide for treating relapsing–remitting multiple sclerosis (TA303) 2014. [cited 2021 June 08]. Available from: https://www.nice.org.uk/guidance/ta303.
- National Institute for Health and Care Excellence. Ocrelizumab for treating relapsing–remitting multiple sclerosis (TA533) 2018. [cited 2021 June 08]. Available from: https://www.nice.org.uk/guidance/ta533.
- European Medicines Agency. Mayzent: EPAR – Public assessment report. 2020. [cited 2021 November 02]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/mayzent-epar-public-assessment-report_en.pdf.
- Schur N, Gudala K, Vudumula U, et al. Cost effectiveness and budget impact of siponimod compared to interferon beta-1a in the treatment of adult patients with secondary progressive multiple sclerosis with active disease in Switzerland. Pharmacoeconomics. 2021;39(5):563–577.
- Treatment Algorithm for Multiple Sclerosis Disease-Modifying Therapies (NHS England Reference: 170079ALG) 2019 [cited 2021 July 15]. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/03/Treatment-Algorithm-for-Multiple-Sclerosis-Disease-Modifying-Therapies-08-03-2019-1.pdf.
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101–106.
- Office for National Statistics. National Life Tables, England. 2017–2019. 2021 [cited 2021 May 21]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables.
- Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405–415.
- Roos I, Leray E, Casey R, et al. Effects of high- and low-efficacy therapy in secondary progressive multiple sclerosis. Neurology. 2021;97(9):e869–e880.
- Samjoo IA, Worthington E, Drudge C, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–1274.
- Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976-1980. Acta Neurol Scand. 1982;65(4):248–266.
- Curtis L, Burns A. Personal social services research unit. Canterbury: University of Kent; 2020.
- NHS National Tariff 2019–2020 London: NHS; 2021 [cited 2021 July 08]. Available from: https://www.england.nhs.uk/national-cost-collection/.
- Tyas D, Kerrigan J, Russell N, et al. The distribution of the cost of multiple sclerosis in the UK: how do costs vary by illness severity? Value Health. 2007;10(5):386–389.
- National Institute for Health and Care Excellence. Beta interferons and glatiramer acetate for treating multiple sclerosis (TA527) 2018. [cited 2021 June 11]. Available from: https://www.nice.org.uk/guidance/ta527.
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60.
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal. 2013. [cited 2021 June 10]. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword.
- Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660–668.
- Lloyd A, Schofield H, Adlard N. Cognitive decline may not be adequately captured in economic evaluations of multiple sclerosis: are new treatments being undervalued? Curr Med Res Opin. 2020;36(4):609–611.