Abstract
The loss of sex steroids (e.g. estradiol, dehydroepiandrosterone [DHEA], progesterone) that causes menopause commonly affects a woman’s general health and produces bothersome physical changes that may interfere with normal sexual and genitourinary functioning. Although both over-the-counter and prescription treatments are available, there remains a large unmet need, as less than 10% of women are treated. Adrenal DHEA and its sulfate are the most abundant steroids in humans. Here we review the development of intravaginal prasterone, the synthetic equivalent to endogenous DHEA. Prasterone is approved by the US Food and Drug Administration for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. Prasterone has been shown to decrease the pain associated with dyspareunia, and to improve vaginal pH, as well as superficial and parabasal cell counts, while maintaining serum hormone levels within the range of those seen in normal postmenopausal women. Unlike other menopausal prescription therapies, intravaginal prasterone does not carry a boxed warning, thus allowing the clinician and patient to engage in meaningful and reassuring discussion around a new approach that treats this common, debilitating condition.
Chinese abstract
通常由绝经引起性激素(例如:雌激素、脱氢表雄酮[DHEA]、黄体酮)的缺乏会影响女性的健康, 并且会产生令人困恼的身体变化, 这些变化可能会干扰正常的性功能和生殖泌尿功能。尽管患者能够得到非处方药和处方药治疗, 但仍有大量的需求未能满足, 因为只有不到10%的女性得到治疗。脱氢表雄酮及其硫酸盐是人类体内含量最高的类固醇。在此, 我们回顾了阴道内使用的普拉斯酮的发展, 它是一种与内源性脱氢表雄酮(DHEA)相当的合成物。美国食品和药物管理局批准普拉斯酮用于治疗由于更年期出现的中重度的性交困难、外阴和阴道萎缩的症状。普拉斯酮已被证实可以降低性交困难相关的疼痛、改善阴道pH值, 以及浅表和基底旁细胞数量, 同时维持正常绝经后女性血清激素水平在正常范围内。不像其他治疗更年期的处方药, 阴道内使用的普拉斯酮并没有黑框警告, 因此允许临床医生和患者围绕这种常见的、使人衰弱的疾病进行有意义且安全的新的治疗方法进行讨论。
Introduction
Menopause is associated with an arrest of the ovarian synthesis of estrogen, progesterone, and dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S), which are produced primarily by the adrenal glands, but also by the ovaries (Supplementary Figure 1)Citation1. In the first year of menopause, women lose about 80% of their estrogens. Testosterone levels decline by about 50–75% from the early twenties to age 40–45 years but do not change significantly across the menopausal transitionCitation2. DHEA-S levels decline steadily with age and are not significantly related to menopause. By age 70 years, they are approximately 20–23% of their peak valueCitation3. Most importantly, serum testosterone simply results from the uncontrolled leakage of some testosterone made intracellularly from DHEA and is not a valid parameter of androgenic activityCitation4. Thus, menopause itself is not associated with dramatic decreases in androgens but postmenopausal women can have significantly less endogenous androgens compared with younger womenCitation5. The loss of these sex steroids causes physical changes that may interfere with normal sexual and genitourinary functioning in a significant number of postmenopausal womenCitation6,Citation7.
During perimenopause, menopause, and postmenopause, sex steroid-dependent tissues undergo atrophic changes including epithelium thinning, altered appearance and function of smooth muscle cells, increased density of connective tissue, and fewer blood vesselsCitation8,Citation9. Changes in vaginal flora associated with the loss of superficial cells, glycogen, and lactobacilli result in increased pH and the increased potential for vaginal and urinary tract infections and inflammationCitation10. Decreases in vaginal blood flow and lubrication often result in dryness and dyspareuniaCitation11,Citation12. In the vaginal epithelium, a lack of sex steroids causes a decrease in the proportion of superficial cells and an increase in the proportion of immature parabasal cellsCitation13. There is also less elasticity and vulvovaginal tissue is prone to petechiae, injury, and painCitation11.
Menopausal symptoms
A 2013 position statement by the North American Menopause Society (NAMS)Citation14 cited the REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs (REVIVE) survey in which the impact of vulvovaginal atrophy (VVA) symptoms is described in more than 3000 postmenopausal women: 85% of partnered women had ‘some loss of intimacy’; 59% indicated that VVA symptoms detracted from enjoyment of sex; 47% indicated that VVA interfered with their relationship; 29% reported that VVA had a negative effect on sleep; and 27% reported that VVA had a negative effect on their general enjoyment of life. Despite this, only 7% of the surveyed women indicated that their health-care professional (HCP) initiated a discussion about VVA.
In 2014, in response to a concern that the term VVA was inadequate to describe the range of menopausal urogenital symptoms, the International Society for the Study of Women’s Sexual Health and the NAMS proposed a new term – genitourinary syndrome of menopause (GSM)Citation15. GSM is defined as a collection of symptoms and signs associated with a decrease in estrogen and other sex steroids involving changes to the labia majora/minora, clitoris, vestibule/introitus, vagina, urethra, and bladder, including symptomatic VVACitation15. This consensus statement also recognizes that androgen receptors are widely distributed in the vestibule and within its glands, with urogenital tissues responsive to androgens as well as estrogens.
With the decline of circulating sex steroids and precursors, GSM can start in perimenopause, increase during the early menopausal period, and further increase in the 2–3 years after menopause. The symptoms range in severity from mildly bothersome to unbearableCitation16. Unlike vasomotor symptoms, the vaginal symptoms associated with GSM do not resolve spontaneously; rather, there is a progressive and cumulative negative effect over timeCitation9. Long-term therapy may be necessary to maintain urogenital healthCitation17.
Women spend more than one-third of their lives in the postmenopausal state. Over half of postmenopausal women report experiencing GSMCitation18–22, and more than 75% report an impact on their sexual livesCitation18. However, most postmenopausal women do not recognize these symptoms as part of a chronic condition associated with menopause that may require treatment. Many think the symptoms will subside over time or attribute them to a natural part of aging. Surveys have revealed that only 20–25% of women with GSM seek medical attentionCitation23,Citation24.
The burden of these symptoms is significant. Thinning of the vaginal wall and decreased vaginal secretions may cause vaginal itching, burning, dryness, and even bleeding. Decreased lubrication and associated dryness may lead to dyspareunia often accompanied by associated sexual dysfunctionCitation25.
Both HCPs and patients find vaginal symptoms to be a sensitive topic that can be difficult to discussCitation21, as evidenced by numerous surveysCitation21,Citation26,Citation27. Reasons given for not initiating the discussion included embarrassment (39%), belief that nothing could be done medically (26%), and belief that it is not an appropriate conversation to have with a HCP (23%). Only 36% of HCPs in the Revealing Vaginal Effects at Mid-Life (REVEAL) survey indicated that they ‘often’ discuss vaginal pain associated with sex with their patientsCitation28.
Available therapies
Currently available treatments for the symptoms of GSM include topical over-the-counter lubricants and moisturizers and prescription systemic and vaginal estrogen therapies. Non-hormonal lubricants and moisturizers can ameliorate dyspareunia, but do not address declining levels of sex steroids or anatomic changesCitation29. Although vaginal estrogen therapies can restore estrogen to the vaginal tissue and can reverse some atrophic changes, even low-dose estrogen therapies have been shown to increase serum estradiol above normal levelsCitation30–32. However, these products carry Food and Drug Administration (FDA) boxed warnings identical to the risks associated with systemic estrogen based on extrapolation of data from trials of either oral estrogen or combined oral estrogen–progestin therapy. Without large, long-term prospective studies that confirm a safer adverse event profile for vaginal estrogen compared to systemic therapies, the class label boxed warning for all estrogen-based products will remain. Thus, patient perceptions of safety based upon class labeling and the time needed by HCPs to convey information about these warnings are obstacles to acceptance and/or adherence to estrogen therapyCitation28. The oral selective estrogen receptor modulator ospemifene is another alternative for these symptoms; however, it carries a modified boxed warningCitation33.
Compounded hormones
FDA-approved prescription drugs are required to demonstrate safety and efficacy and to be manufactured according to current Good Manufacturing Practices to help ensure the identity, strength, quality, and purity of drugs by requiring adequate control of manufacturing operations. In contrast, compounded medications are not FDA approved, have not undergone rigorous testing, and do not contain labeling to support the discussion of risks and benefits for informed decision-making. One unintended consequence following the Women’s Health Initiative was the rise of compounded hormones, which may appeal to patients because of their perceived lack of risk, as they are not labeled in the same manner as FDA-approved estrogen therapiesCitation34. Both the American College of Obstetricians and Gynecologists and the NAMSCitation35 have publicly stated their concerns regarding the use of compounded hormone therapies. Further, the FDA has recently taken steps to regulate this area more aggressivelyCitation36.
FDA approval requirements for treatment of VVA symptoms
In 2003, the FDA published a draft guidance providing recommendations to industry concerning the conduct of clinical studies of estrogen and estrogen/progestin drug products for the treatment of moderate to severe vasomotor symptoms and moderate to severe symptoms of VVA associated with menopauseCitation37. These recommendations impact product approval and indications. For the treatment of moderate to severe symptoms of VVA, study subjects are required to have ≤5% superficial cells on a vaginal smear, to have a vaginal pH >5.0, and to have self-identified one moderate to severe symptom that is the ‘most bothersome symptom’ (MBS) to her. The MBS must be one of the five symptoms recognized by the FDA: vaginal dryness, vaginal and/or vulvar irritation/itching, dysuria, vaginal pain associated with sexual activity, or vaginal bleeding associated with sexual activity. As the enrollment criteria were restricted to a single MBS per clinical efficacy study, the indication for medications approved after 2003 have been based on a single symptom. Currently, only Osphena®Citation33 and Intrarosa®Citation38 have been approved and are available for prescribing, following release of this guidance for prescribing for moderate to severe dyspareunia. Medications approved prior to 2003 are labeled for ‘atrophic vaginitis’ or ‘vulvar and vaginal atrophy’.
The role of DHEA in the premenopausal and postmenopausal states
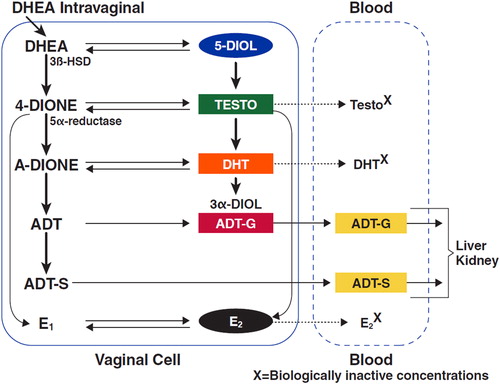
In menopause, approximately 80% of DHEA comes from the adrenals and 20% from the ovariesCitation4. DHEA is rapidly transformed into its more stable sulfate, DHEA-S; it is also a precursor for intracellular conversion by dehydrogenases, hydroxylases, aromatase, and other enzymes in specific target tissues into estrone, estradiol, testosterone, and dihydrotestosterone ()Citation39,Citation40. Final intracellular sex steroid inactivation occurs through glucuronidation by enzymes in the uridine diphosphate-glucuronosyltransferase familyCitation41. This proposed conversion and inactivation mechanism has been described as intracrinology to distinguish it from other endocrine mechanisms of hormone regulationCitation1.
Figure 1. Metabolism of dehydroepiandrosterone (DHEA) after its uptake in vaginal cellsCitation1. 4-DIONE, androstenedione; A-dione, 5a-androstane-3,17-dione; ADT, androsterone; ADT-G, androsterone glucuronide; ADT-S, androsterone sulfate; 3α-DIOL, 3α-androstanediol; 3β-HSD, 3β-hydroxysteroid dehydrogenase; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; Testo, testosterone.
Intravaginal prasterone
Prasterone (Intrarosa®) is a steroid approved by the US FDA in 2016 at a dose of 6.5 mg and a concentration of 0.50% for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopauseCitation38. Prasterone is administered as a vaginal insert once daily at bedtime, does not carry a boxed warning in its label, and has no restrictions on duration of use. Prasterone is contraindicated in the presence of undiagnosed abnormal genital bleeding. Postmenopausal women with undiagnosed, persistent, or recurring genital bleeding should be evaluated before considering treatment with prasterone. Prasterone has not been studied in breast cancer.
Preclinical studies
Androgens are involved in the regulation of vaginal and vestibular lubrication, smooth muscle activity, and blood flowCitation42,Citation43; they are also capable of inducing vaginal mucification in the absence of estrogensCitation44. In female rats 9 months post ovariectomy, DHEA (80 mg/kg) was associated with a highly mucified epithelium, and increased muscularis thickness by 50 ± 2 μm (mean ± standard deviation) and collagen fiber compactness in the lamina propriaCitation45. Histopathologic examination after application of DHEA (30 mg) to the dorsal skin of ovariectomized rats twice daily for 1, 3, or 6 months showed proliferation and mucification of the vaginal epithelium and complete reversal of the signs of vaginal atrophy. The endometrium remained atrophic at all time intervals during treatmentCitation46. In another study in ovariectomized rats, 2 weeks of daily intravaginal DHEA (0.33 mg, 0.66 mg, or 1 mg) increased serum DHEA, DHEA-S, and androstenediol; while serum testosterone, estradiol, estrone, and dihydrotestosterone remained below detectable levelsCitation47.
Clinical development of prasterone
The intravaginal prasterone clinical trials are detailed in Supplementary Table 1. The bioavailability of DHEA and its metabolites was assessed in a Phase 1/2 randomized, placebo-controlled, double-blind study in postmenopausal women (n = 40) receiving daily intravaginal applications of prasterone (0.5%, 1.0%, or 1.8%) or placebo. After 7 days of treatment, the maturation value of the vaginal epithelial cells was significantly increased, and vaginal pH was significantly decreased at all DHEA doses. Serum concentrations of estradiol and testosterone remained within the values found in normal postmenopausal womenCitation48–50 for the 0.5% and 1.0% dosesCitation49,Citation51.
Five randomized, multicenter, double-blind, Phase 3 studies of once-daily intravaginal prasterone were conducted to examine the dose and scheduling in more than 1500 postmenopausal women with VVA. One placebo-controlled trial assessed serum steroid levels in postmenopausal women (n = 218; age 42–74 years) during 12 weeks of daily intravaginal administration of prasterone (0.25%, 0.50%, or 1.0%) or placeboCitation52. The serum levels of DHEA and 11 of its metabolites remained at or below the normal postmenopausal limits at day 1 and weeks 2, 4, 8, and 12. At 12 weeks, 0.5% prasterone caused a 45.9 ± 5.31% (mean ± standard deviation) decrease in parabasal cells, a 6.8 ± 1.29% increase in superficial cells, a 1.3 ± 0.13 unit decrease in vaginal pH, and a 1.5 ± 0.14 score unit decrease in the severity of vaginal symptoms (all p < 0.0001 vs. placebo). Similar changes to vaginal secretions, color, epithelial surface thickness, and epithelial integrity were noted on gynecologic examination. One trial that evaluated a twice-weekly dosing regimen for vaginal dryness failed to meet statistical significanceCitation53. As a result, the pivotal registration trials focused on daily administration at bedtime.
Two pivotal, Phase 3, randomized, double-blind trials confirmed the efficacy of intravaginal prasterone for moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. In trial 1, postmenopausal women (n = 253; mean age 58.6 years) with the MBS of moderate to severe dyspareunia received prasterone (0.25% and 0.50%) or placebo as a vaginal insert once daily for 12 weeks. The coprimary endpoints were changes from baseline to week 12 in percent vaginal parabasal and superficial cells, vaginal pH, and dyspareunia score. Secondary endpoints included vaginal dryness and vulvovaginal irritation/itchingCitation54. As the 0.25% dose is not approved or available, we present here only the results for the FDA-approved 0.50% dose. After 12 weeks of daily treatment, 0.50% prasterone significantly decreased the percentage of parabasal cells, increased the percentage of superficial cells, and decreased vaginal pH compared with placebo (all p < 0.0001). By week 12, moderate to severe dyspareunia, self-identified as the MBS, significantly improved by 0.40 severity score units (46%; p = 0.013) versus placebo after treatment with prasterone (). The secondary endpoint of moderate to severe vaginal dryness improved significantly (p = 0.013); irritation/itching also improved, but the change was not significantly different from placebo. Pelvic examinations that included Pap smears noted improvements in secretions, epithelial integrity, and epithelial surface thickness and color.
Figure 2. Changes in (a) parabasal cells, (b) superficial cells, (c) vaginal pH, and (d) moderate to severe dyspareunia score in the intent-to-treat population (all women who received at least one dose of the study drug or placebo) using analysis of covariance, with treatment as the main factor and baseline value as the covariate. Score based on severity of dyspareunia: none = 0, mild = 1, moderate = 2, and severe = 3. *p < 0.0001 vs. placebo; †difference vs. placebo from baseline to week 12; ‡p < 0.05 vs. placebo; §p < 0.001 vs. placebo.
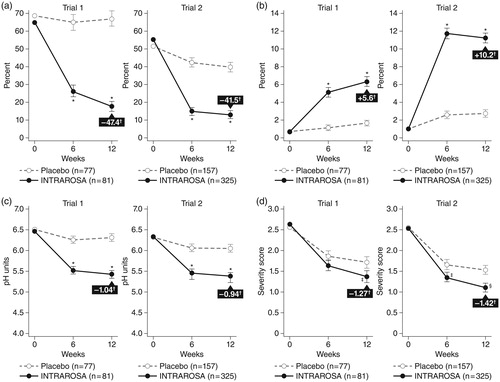
Pivotal trial 2, also in postmenopausal women (n = 554; mean age 59.5 years), employed a similar study design and endpoints as trial 1, except only the 0.5% dose of prasterone and placebo were usedCitation55. The results were similar to those of trial 1, with significant decreases in the percentage of parabasal cells (p < 0.0001 vs. placebo), increases in superficial cells (p < 0.0001), decreases in vaginal pH (p < 0.0001), and improvement of pain during sexual activity (p = 0.0002) at 12 weeks (). Although not a primary endpoint in the study, moderate to severe vaginal dryness (present in 84.0% of women) improved by 23% (p = 0.004 vs. placebo). On gynecological physical evaluation, vaginal secretions, epithelial integrity, and epithelial surface thickness and color all improved by 86–121% (p < 0.0001 vs. placebo). This separation of intravaginal prasterone from the placebo group is particularly noteworthy as the vehicle contains Witepsol, a fatty acid glycerol ester base that likely exerted a daily emollient effect in relieving some dyspareunia and dryness symptoms in the vehicle-only arm above and beyond that usually seen with a placebo effect alone. The vehicle alone had minimal effect on the objective parameters of pH and vaginal maturation index.
While there are no head-to-head trials of intravaginal prasterone versus vaginal estrogen, and cross-trial comparisons have limitations, one review of Phase 3 clinical trials of the effect of daily intravaginal prasterone 6.5 mg (0.50%), daily vaginal conjugated equine estrogens (CEE; 0.3 mg 21 days on/7 days off) or twice-weekly 0.3 mg CEE, and vaginal estradiol 10 μg daily for 2 weeks then twice-weekly for 10 weeks on moderate to severe dyspareunia showed a decrease in the total dyspareunia severity score of 1.27–1.63 units with prasterone, 1.4 units with CEE, and 1.23 units with estradiolCitation56.
Adverse events
In the four placebo-controlled, 12-week clinical trials of intravaginal prasterone, vaginal discharge was the most frequently reported treatment-emergent adverse event in the prasterone treatment group (incidence ≥2% and the only one greater than placebo)Citation38.
Fifty-two-week, open-label safety study
The fifth trial was a Phase 3, open-label, long-term safety study of intravaginal prasterone in postmenopausal women (n = 530; mean age 57.9 years, range 43–75 years) with mild, moderate, or severe dyspareunia, vaginal dryness, and/or irritation/itch due to VVA associated with menopause. Participants were treated daily with 0.50% intravaginal prasterone for up to 52 weeks. The symptoms and signs of vaginal atrophy were evaluated as a secondary objectiveCitation57,Citation58. A total of 435 women were exposed to prasterone for 52 weeks, 24 women for ≥26 weeks but <52 weeks, and 62 women for <26 weeks. Vaginal discharge (74 cases) and abnormal Pap smear at 52 weeks were the most frequently (incidence ≥2%) reported treatment emergent adverse events. An abnormal Pap smear was observed in 11 of 521 subjects (2.1%), with the majority (10 of 11 subjects) being atypical squamous cells of undetermined significance and five subjects being human papilloma virus negativeCitation38. Improvements in dyspareunia severity score (Supplementary Figure 2), percentage of parabasal cells and superficial cells, and vaginal pH were noted as early as 12 weeks. The improvements continued and were clinically and statistically significant at weeks 26 and 52Citation57(p<0.0001 vs. baseline).
Serum steroid concentrations
The upper limit for normal postmenopausal estradiol levelsCitation48, based on validated mass spectrometry, first determined as 9.3 pg estradiol/mlCitation49, and similar levels of 10 pg/ml have been recognized by the Mayo Clinical Medical LaboratoriesCitation48,Citation59. Using this parameter as background, there was no meaningful change in the serum levels of estradiol or in the metabolism of the DHEA-derived steroids at 12, 26, or 52 weeks of daily intravaginal administration of 0.50% DHEA compared to baseline. All measurements were done based on sensitive validated liquid chromatography–tandem mass spectrometryCitation58.
Endometrial safety
The endometrial safety of intravaginal prasterone has been examined in three Phase 3, multicenter, placebo-controlled trials and one long-term, open-label safety trial. In total, endometrial biopsies were obtained for 722 women aged 40–75 years who were treated with prasterone (0.25%, 0.50%, or 1.0%) for 12–52 weeks. Atrophic or inactive endometrium was observed in 668 women for whom there was sufficient material for histologic evaluation, with no proliferation or hyperplasia seen, confirming a lack of stimulatory effect due to intravaginal prasterone ()Citation54,Citation60. This is consistent with the absence of aromatase expression in the normal endometrium, which is necessary to convert testosterone into estradiol and androstenedione into estrone, and aligns with the lack of a boxed warning for endometrial hyperplasia or cancer in the prasterone label.
Table 1. Endometrial biopsy results in women treated with intravaginal prasteroneCitation60.
Summary
For many postmenopausal women, decreased sex steroids leads to a group of genital and urinary symptoms called GSM, which are usually chronic and progressive without treatment. Many women are unaware of GSM as a condition and assume symptoms are due to aging. Further, they are reluctant to discuss their vaginal health with their HCP and few HCPs initiate the discussionCitation61. There is a need for increased awareness and responsiveness on the part of HCPs to educate their patients and to assess, discuss, and adequately treat GSM symptoms in postmenopausal women.
Over-the-counter products can provide temporary relief for the symptoms of GSM, but they do not address the underlying pathophysiology of the condition. Although vaginal estrogen therapy is effective in relieving some of the symptoms of GSM, many women are uncomfortable with or not eligible for vaginal estrogen therapy based upon contraindications for use (e.g. history of thromboembolic disorder, such as stroke or myocardial infarction; history of deep vein thrombosis and/or estrogen-dependent neoplasia).
While not FDA approved, several studies have investigated intravaginal testosterone alone or in combination with CEEs as a potential treatment for VVA, recognizing the role that androgens may play in vaginal health. While some studies’ results have shown promise for GSM, studies have been limited. Adequately powered placebo-controlled trials are needed for intravaginal testosterone prior to clinical useCitation62.
Intravaginal prasterone provides a treatment for providers and patients that avoids the aforementioned estrogen contraindications, allowing the clinician to focus on counseling without distracting patients with fearful warnings. Moreover, prasterone is novel in its conversion into important androgens, as well as estrogens, and has undergone robust and formal drug development to confirm its efficacy and safety.
The efficacy and safety of prasterone has been evaluated in four placebo-controlled, 12-week trials and one 52-week, open-label safety study in postmenopausal women meeting the FDA criteria for moderate to severe dyspareunia. At a daily bedtime intravaginal dose of 0.50% (6.5 mg), prasterone, a synthetic version of endogenous DHEA, is converted intracellularly at the local tissue level to androgens and estrogens, while sex steroids remain within the normal range of postmenopausal women. Prasterone has shown clinically and statistically significant improvements. In both pivotal trials, treatment with intravaginal prasterone was associated with a significant reduction in moderate to severe dyspareunia due to menopause compared with placebo: −1.27 (0.40 severity score units over placebo, 46%; p = 0.013) and −1.42 (0.36 severity score units over placebo, 34%; p < 0.0002) in pivotal trial 1 and 2, respectivelyCitation54,Citation55.
Many postmenopausal women suffering with GSM symptoms identify painful intercourse as the MBS associated with VVA. Prasterone can provide a safe, effective, and innovative approach to this common and often debilitating conditionCitation62.
Conflict of interest
David J. Portman has received research support or consulting fees from Palatin Technologies, Sprout, Valeant, Endoceutics, AMAG Pharmaceuticals, and Agile Therapeutics; he is on the speakers’ bureau for AMAG and is currently CEO and shareholder of Sermonix Pharmaceuticals, LLC. Steven R. Goldstein has served as an advisory board member for Shionogi, Pfizer, JDS Therapeutics, Amgen, and Sermonix; served as a consultant for Cook OB/GYN and Philips Ultrasound; and has served on the speaker’s bureaus for Pfizer, Shionogi, and JDS Therapeutics. Risa Kagan has served as a consultant/advisory board member for Amgen, Allergan, AMAG Pharmaceuticals, Merck & Co, Pfizer, Shionogi, Palatin, TherapeuticsMD, Sprout, Valeant, Duschensay, and P&G; has received grants/research support (fees to Alta Bates Summit Medical Center, Jordan Research and Education Institute) from TherapeuticsMD and Endoceutics; has served on a speaker’s bureau for Shionogi, Pfizer, AMAG, and Valeant; and has received editorial writing support from Pfizer and Shionogi.
Source of funding
The preparation of this review and online open access of the publication were sponsored by AMAG Pharmaceuticals, Inc., Waltham, MA, USA.
Supplemental Material
Download MS Word (333.4 KB)Acknowledgements
Writing and editorial assistance was provided by Maria B. Vinall of The Curry Rockefeller Group, LLC, Tarrytown, NY, USA.
Additional information
Funding
References
- Labrie F, Belanger A, Pelletier G, et al. Science of intracrinology in postmenopausal women. Menopause 2017;24:702–12
- Labrie F, Martel C, Balser J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary?. Menopause 2011;18:30–43
- Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847–53
- Labrie F, Martel C, Belanger A, et al. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J Steroid Biochem Mol Biol 2017;168:9–18
- Traish AM, Vignozzi L, Simon JA, et al. Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev 2018;6:558-71
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA 2005;294:91–6
- Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28 Suppl 1: 129–42
- Johnston S. Urogenital concerns. J Obstet Gynaecol Can 2006;28:S33–S42
- Barton DL, Shuster LT, Dockter T, et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer 2018;26:1335–43
- Willhite LA, O'Connell MB. Urogenital atrophy: prevention and treatment. Pharmacotherapy 2001;21:464–80
- Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010;85:87–94
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric 2014;17:3–9
- Meisels A. The menopause: a cytohormonal study. Acta Cytol 1966;10:49–55
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013;20:888–902 quiz 903-4
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Climacteric 2014;17:557–63
- Freedman MA. Perceptions of dyspareunia in postmenopausal women with vulvar and vaginal atrophy: findings from the REVIVE survey. Womens Health (Lond Engl) 2014;10:445–54
- Chollet JA. Efficacy and safety of ultra-low-dose Vagifem (10 mcg). Patient Prefer Adherence 2011;5:571–4
- Erekson EA, Li FY, Martin DK, et al. Vulvovaginal symptoms prevalence in postmenopausal women and relationship to other menopausal symptoms and pelvic floor disorders. Menopause 2016;23:363–75
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) – results from an international survey. Climacteric 2012;15:36–44
- Simon JA, Kokot-Kierepa M, Goldstein J, et al. Vaginal health in the United States: results from the Vaginal Health: Insights, Views & Attitudes survey. Menopause 2013;20:1043–8
- Kingsberg SA, Wysocki S, Magnus L, et al. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med 2013;10:1790–9
- Nappi RE, Mattsson LA, Lachowsky M, et al. The CLOSER survey: impact of postmenopausal vaginal discomfort on relationships between women and their partners in Northern and Southern Europe. Maturitas 2013;75:373–9
- Wysocki S, Kingsberg S, Krychman M. Management of vaginal atrophy: implications from the REVIVE Survey. Clin Med Insights Reprod Health 2014;8:23–30
- Reiter S. Barriers to effective treatment of vaginal atrophy with local estrogen therapy. Int J Gen Med 2013;6:153–8
- Lara LA, Useche B, Ferriani RA, et al. The effects of hypoestrogenism on the vaginal wall: interference with the normal sexual response. J Sex Med 2009;6:30–9
- Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas 2010;67:233–8
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med 2017;14:413–24
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis. J Sex Med 2013;10:1567–74
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition?. Climacteric 2016;19:151–61
- Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric 2010;13:219–27
- Pickar JH, Amadio JM, Bernick BA, et al. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric 2016;19:181–7
- Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency-a pharmacokinetic and pharmacodynamic study. Maturitas 1992;15:121–7
- Shiongi Inc. Osphena (Ospemifene) Tablets. Prescribing Information. Florham Park, NJ; February 2015
- Simon JA. The Woman’s Health Initiative and one of many unintended consequences. Menopause 2016;23:1057–9
- The North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24:728–53
- United States Food and Drug Administration. 2018 Compounding policy priorities plan. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm592795.htm [last accessed 26 Mar 2018]
- United States Food and Drug Administration. Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms – recommendations for clinical evaluation. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071643.pdf) [last accessed 6 Dec 2017]; 2003
- AMAG Pharmaceuticals. Intrarosa (prasterone) vaginal insert. Prescribing Information. Waltham, MA; April 2017. Available from: http://www.intrarosahcp.com/wp-content/uploads/2017/07/INTRAROSA-PI-7-2017.pdf [last accessed 17 April 2018]; 2017
- Traish AM, Kang HP, Saad F, et al. Dehydroepiandrosterone (DHEA)-a precursor steroid or an active hormone in human physiology. J Sex Med 2011;8:2960–82; quiz 2983
- Crawford S, Santoro N, Laughlin GA, et al. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab 2009;94:2945–51
- Belanger A, Pelletier G, Labrie F, et al. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab 2003;14:473–9
- Berman JR, Almeida FG, Jolin J, et al. Correlation of androgen receptors, aromatase, and 5-alpha reductase in the human vagina with menopausal status. Fertil Steril 2003;79:925–31
- Bertin J, Dury AY, Ouellet J, et al. Localization of the androgen-synthesizing enzymes, androgen receptor, and sex steroids in the vagina: possible implications for the treatment of postmenopausal sexual dysfunction. J Sex Med 2014;11:1949–61
- Kennedy TG, Armstrong DT. Induction of vaginal mucification in rats with testosterone and 17beta-hydroxy-5alpha-androstan-3-one. Steroids 1976;27:423–30
- Berger L, El-Alfy M, Martel C, et al. Effects of dehydroepiandrosterone, Premarin and Acolbifene on histomorphology and sex steroid receptors in the rat vagina. J Steroid Biochem Mol Biol 2005;96:201–15
- Sourla A, Flamand M, Belanger A, et al. Effect of dehydroepiandrosterone on vaginal and uterine histomorphology in the rat. J Steroid Biochem Mol Biol 1998;66:137–49
- Berger L, El-Alfy M, Labrie F. Effects of intravaginal dehydroepiandrosterone on vaginal histomorphology, sex steroid receptor expression and cell proliferation in the rat. J Steroid Biochem Mol Biol 2008;109:67–80
- Mayo Clinic: Mayo Medical Laboratories. Test Definition: ESTF: Estrogens, Estrone (E1) and Estradiol (E2), Fractionated, Serum https://www.mayomedicallaboratories.com/test-catalog/Clinical%-C2%B1and%C2%B1Interpretive/84230 [last accessed 13 Mar 2018]; 2017
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008;111:178–94
- Nakamoto J. Endocrine testing. In: Jameson JL, De Groot LJ, de Kretser DM, et al, eds. Endocrinology: Adult and Pediatric (Seventh Edition) [Internet]. Philadelphia: Elsevier Saunders; 2016: 2655–2688e1. Available from: https://www.sciencedirect.com/science/article/pii/B9780323189071010015
- Labrie F, Martel C, Bérube R, et al. Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol 2013;138:359–67
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009;16:897–906
- Bouchard C, Labrie F, Archer DF, et al. Decreased efficacy of twice-weekly intravaginal dehydroepiandrosterone on vulvovaginal atrophy. Climacteric 2015;18:590–607
- Archer DF, Labrie F, Bouchard C, et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone). Menopause 2015;22:950–63
- Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2016;23:243–56
- Archer DF, Labrie F, Montesino M, et al. Comparison of intravaginal 6.5mg (0.50%) prasterone, 0.3mg conjugated estrogens and 10μg estradiol on symptoms of vulvovaginal atrophy. J Steroid Biochem Mol Biol 2017;174:1–8
- Labrie F, Archer DF, Bouchard C, et al. Prasterone has parallel beneficial effects on the main symptoms of vulvovaginal atrophy: 52-week open-label study. Maturitas 2015;81:46–56
- Ke Y, Gonthier R, Simard JN, et al. Serum steroids remain within the same normal postmenopausal values during 12-month intravaginal 0.50% DHEA. Horm Mol Biol Clin Investig 2015;24:117–29
- Nakamoto J, editor. Part 16 Endocrine Testing. Philadelphia, PA: Elsevier Saunders; 2016
- Portman DJ, Labrie F, Archer DF, et al. Lack of effect of intravaginal dehydroepiandrosterone (DHEA, prasterone) on the endometrium in postmenopausal women. Menopause 2015;22:1289–95
- Krychman M, Graham S, Bernick B, et al. The women’s EMPOWER survey: women’s knowledge and awareness of treatment options for vulvar and vaginal atrophy remains inadequate. J Sex Med 2017;14:425–33
- Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause 2018;25:837–47
