Abstract
Endocrine therapy in breast cancer survivors can cause severe ‘climacteric’ symptoms, which may compromise therapy adherence. To determine whether such symptoms can be treated with herbal medication containing black cohosh in the form of isopropanolic Cimicifuga racemosa extract (iCR) alone or in fixed combination with St John’s wort (Hypericum perforatum [HP]) (iCR + HP), a systematic literature search was conducted. Results were viewed in relation to experimental data and metabolism of endocrine therapies. Most breast cancer survivors receiving endocrine therapy experienced reductions in climacteric symptoms under iCR/iCR + HP. Tamoxifen’s interference potential may be countered by using higher iCR doses or iCR + HP. No estrogen-like effects at the breast or on hormones were seen. After breast cancer, even if receiving tamoxifen, patients using iCR/iCR + HP had significantly increased recurrence-free survival rates compared to non-users. These results are substantiated by experimental data demonstrating antiproliferative and anti-invasive effects of iCR in breast cancer cells and enhancement of the antineoplastic effects of tamoxifen. There are no known clinical interactions for iCR and HP with endocrine therapies. The HP extract used in iCR + HP did not exhibit any clinically relevant interaction potential. In conclusion, with its positive benefit–risk profile, iCR/iCR + HP may offer a safe non-hormonal therapeutic option for breast cancer survivors receiving endocrine therapy.
摘要
乳腺癌幸存者的内分泌治疗可导致严重的“更年期”症状, 这可能会影响其治疗的依从性。为了明确这些症状是否可以被黑升麻-异丙醇提取的美类叶生麻提取物(iCR)单独应用或联合圣约翰草(贯叶连翘(HP)(iCRþHP)缓解, 我们进行了系统的文献检索。主要对内分泌治疗的实验数据和代谢结果进行搜集。大多数乳腺癌幸存者接受内分泌治疗后应用iCR / iCRþHP能够减轻更年期症状。他莫西芬对iCR或iCRþHP减轻更年期症状的潜在干扰可以通过应用更大剂量的iCR或iCRþHP抵消。该治疗方案没有在乳房或激素水平上发现雌激素样作用。接受他莫昔芬治疗的乳腺癌患者, 应用iCR / iCRþHP的存活率显著增加。这些结果通过实验数据证实了iCR在乳腺癌细胞中的抗增殖和抗侵袭作用, 而且其能增强他莫西芬的抗肿瘤作用。目前尚未发现iCR和HP与内分泌治疗存在相互作用。用于iCRþHP的HP提取液未表现出任何临床相关的干扰作用。总之, iCR / iCRþHP可能为接受内分泌治疗的乳腺癌幸存者提供一个安全的非激素治疗选择。
Introduction
Breast cancer patients receiving endocrine therapy (e.g. tamoxifen) often suffer from adverse reactions like hot flushes, sleep problems, depressive symptoms, irritability, and mood swingsCitation1. With increasing severity, these complaints can force patients to discontinue their antihormonal therapyCitation2,Citation3. Hormone therapy (HT) is generally not recommended for breast cancer survivorsCitation4. Consequently, non-hormonal treatment options are highly appreciated.
Among these options, herbal medicinal products with Cimicifuga racemosa (CR; Actaea racemosa or black cohosh) are widely used and are also recommended by the European Medicines Agency (EMA) for the treatment of climacteric complaintsCitation5. Oxford Level 1 evidence for ameliorating natural neurovegetative and psychic menopausal symptoms is available for the special isopropanolic C. racemosa extract (iCR), used as standard-dose monotherapy or higher dosed in a fixed combination with St John’s wort (Hypericum perforatum [HP]) (iCR + HP)Citation6,Citation7. In randomized controlled trials (RCTs), iCR and iCR + HP significantly improved climacteric symptoms compared to placeboCitation8–12. RCTs showed the comparable efficacy of iCR to low-dose transdermal hormone therapy or tiboloneCitation13,Citation14. In patients with more intense symptoms and/or pronounced psychic components, iCR + HP was superior to iCR monotherapyCitation15.
In clinical practice, it is often asked whether breast cancer patients suffering from side effects of endocrine therapy can be treated with iCR or iCR + HP: what about safety at the breast and interactions commonly attributed to HP? Answers to this question could have consequences for the treatment schedule of thousands of patients as breast cancer has become the most common cancer in women worldwide, comprising 16% of all female cancers and 22.9% of female invasive cancersCitation16,Citation17. Like in western countries, the breast cancer incidence is increasing in AsiaCitation18. In China, breast cancer constitutes 12.2% of all newly diagnosed breast cancers in the world, accounting for 9.6% of the global breast cancer deaths. In 2015, of the 4.292 million newly diagnosed cancer cases in China, 268,600 were breast cancer, accounting for 15% of new female cancersCitation19. The incidence rate among those aged 45–59 years was 23%Citation19.
This review intends to offer orientation for health care providers by giving an overview of the experimental and clinical data on iCR/iCR + HP safety at the breast, its use in breast cancer patients, and putative interactions with endocrine treatment.
Methods
MEDLINE, EMBASE, EMBASE Alert, BIOSIS, and PubMed were searched for clinical studies with iCR/iCR + HP (Remifemin/Remifemin plus) and iCR + HP’s specific HP component. All clinical data regarding safety at the breast, efficacy and safety in breast cancer patients, and interactions published from 1997 (when the EU Guideline on Good Clinical Practice E6 came into effect) until April 2018 were included. The search was complemented by a manual search in the authors’ libraries.
Inclusion criteria were the medical use of iCR/iCR + HP or iCR + HP’s specific HP component, their efficacy and safety in breast cancer patients, influences on the breast, and safety with regard to interactions. Patient-relevant endpoints were the reduction of climacteric complaints (e.g. frequency and severity of hot flushes, climacteric symptom scales), recurrence-free survival, findings with possible impact on breast cancer, and frequency and severity of adverse events (AEs) including interactions.
Additionally, a narrative overview is given on experimental data in breast tissue, breast cancer cells (BCCs), and animal models. Potential clinical interactions of HP and iCR/iCR + HP, which are officially recorded in monographs or standard summaries of product characteristics, are viewed in relation to metabolism pathways of endocrine therapies.
Results
Clinical studies with iCR/iCR + HP in breast cancer patients
The searches revealed six studies in which breast cancer patients were treated with iCR or iCR + HPCitation20–24,Citation26. In a double-blind RCT, 85 breast cancer patients, who were mainly medicated with tamoxifen (dosage not reported), received iCR (n = 42 with n = 29 tamoxifen users; 1 tablet twice daily [b.i.d.]) or placebo (n = 43 with n = 30 tamoxifen users) for 2 monthsCitation20. A comparable decrease in number (–27%) and intensity of hot flushes was reported without significant group differences. However, iCR-treated patients experienced significantly less sweating compared to the placebo group (p = 0.04). Gonadotropin levels did not change significantly under treatment. AEs were mostly minor and related to tamoxifen. A few serious AEs occurred; they were not related to iCR.
Fifty breast cancer patients receiving 10–40 mg tamoxifen after primary therapy (100% surgery, 87% radiation, 50% chemotherapy) were treated with iCR for 6 monthsCitation21,Citation25. Treatment started with 1 tablet b.i.d. and could be adjusted according to patients’ requirements after 4 weeks, resulting in dosages of 1–4 tablets iCR/day or a switch to iCR + HP. The total Menopause Rating Scale II decreased significantly from severe (17.6) to moderate (13.6), with the best improvements in hot flushes, sweating, sleep problems, and anxiety. The responder rate, defined as satisfying/good/very good efficacy by patients’ self-assessment, was 70.2% at last observation. Sixty percent of the patients wanted to continue iCR medication after study termination. Twenty-two patients reported AEs, which were not linked to iCR/iCR + HP. Ninety percent judged the tolerability as good or very good, none as bad. No recurrences occurred during the observation period.
Patients with (n = 13, n = 6 using tamoxifen or raloxifene) and without (n = 8) breast cancer, who suffered severely from an average of 8.3 hot flushes/day, were treated with iCR (1 tablet b.i.d.) in a pilot studyCitation22. They experienced significant reductions in daily hot flush frequency (–50%), weekly hot flush score (–56%), hot flush severity (–22%), and sweating (–80%) after 4 weeks. Fifty-two percent of the patients reported a 50% or greater decrease in hot flush frequency. Large improvements were also noted for sleeping problems and fatigue.
Another open, uncontrolled study in 23 Asian patients with gynecological cancers, including two breast cancer cases, reported significant reduction of the Kupperman Menopause Index, hot flushes, sweating, and depressive moods after 3 months of iCR treatment (1 tablet b.i.d.)Citation23. Estradiol and gonadotropin values remained uninfluenced.
A study in 15 Asian breast cancer patients after surgery and chemotherapy (12 receiving endocrine therapy) found significant improvements in the Kupperman Menopause Index, hot flushes, sweating, Traditional Chinese Medicine syndrome scores, and quality of life after 4 weeks of iCR use (1 tablet b.i.d.)Citation26. All patients reported good or very good tolerability.
A pharmacoepidemiological cohort study based on the IMS Disease Analyser Mediplus database included 18,861 breast cancer survivors, of whom 1102 were treated with iCR or iCR + HPCitation24. The mean overall observation time was 3.6 years (6 months to >9 years). Compared to non-iCR users, patients taking iCR or iCR + HP had a lower recurrence rate. This was also the case for patients receiving tamoxifen, regardless of whether they had used iCR monotherapy or iCR + HPCitation24,Citation27. Patients treated with iCR/iCR + HP had an average 4.5-year increase of recurrence-free survival.
Clinical data relevant for breast safety
Mammographic breast density (visually assessed) and proliferation of breast epithelial cells (fine-needle aspiration biopsies) did not increase after 6 months of treatment with iCR (n = 74)Citation28. Digitized assessment of the mammograms confirmed these findingsCitation29. Breast ultrasound did not reveal changes after 6 months of iCR treatment (n = 54)Citation11,Citation30. Three to 6-month treatment with iCR (1–3 tablets b.i.d.) did not significantly change estradiol, follicle stimulating hormone, luteinizing hormone, prolactin, sex hormone-binding globulin, or testosterone levels (n = 523)Citation10,Citation11,Citation13,Citation20,Citation23,Citation30–36. Ultrasound did not reveal any increase in endometrial thickness after 3 to 6-month iCR use (n = 451)Citation10,Citation11,Citation13,Citation14,Citation28,Citation30,Citation32,Citation34,Citation35. Two case–control studies found that taking iCR/iCR + HP was associated with a reduced risk for breast cancerCitation37,Citation38. The larger study (6646 controls with 320 iCR/iCR + HP users; 3257 breast cancer cases with 112 iCR/iCR + HP users) demonstrated that lifestyle factors, tumor histology, and receptor status did not influence the resultsCitation37. Treatment duration was positively associated with risk reduction.
Experimental data in breast cancer cells, animal models of breast cancer, and human breast tissue
No increase of proliferation in estrogen receptor-positive MCF-7 BCC occurred under iCRCitation39–41. Instead, iCR dose-dependently inhibited MCF-7 BCC proliferationCitation42–45 and enhanced the antineoplastic effects of tamoxifenCitation42. iCR suppressed tumor cell invasion in estrogen receptor-negative and highly invasive MDA-MB 231 BCCCitation46. Antiproliferative effects of iCR on MCF-7 and MDA-MB 231 BCC were caused by activation of caspases and induction of apoptosisCitation44.
The well-established dimethylbenz(a)-anthracene-induced estrogen receptor-positive mammary tumor model in Sprague Dawley rats was used in most in-vivo studiesCitation47–50. In contrast to mestranol-treated rats, no stimulation of tumor growth was found in animals treated with iCR in doses up to 100-fold the human therapeutic doseCitation47. No significant differences to vehicle control were observed, but a trend toward reduced tumor growth was seen. Prolactin, follicle stimulating hormone, luteinizing hormone, organ weight, and endometrial proliferation remained unaffected by iCRCitation47. Compared to vehicle control, iCR treatment, starting from prepubertal age, resulted in marked retardation of tumor growth and a significantly prolonged life spanCitation48. Combination of iCR with tamoxifen increased the incidence of tumor-free rats from 20 to 50% with a pronounced retardation of neoplastic growthCitation49. Necropsy found the individual tumor burden to be reduced by 50%. Concomitant iCR application neither affected serum estradiol nor the antineoplastic effects of the aromatase inhibitor (AI) formestaneCitation50. One study used a transgenic MMTV-neu mouse model, in which breast cancer is promoted by mouse mammary tumor virus (MMTV)Citation51. Mice bear the rat oncogene neu, the rodent homolog of human epidermal growth factor receptor 2 (HER2); thus, this artificial model generates spontaneous, highly progressive breast cancer with rapidly developing lung metastases. No differences were detected in the incidence and onset of breast cancer between iCR and control. Incidence of lung metastases was increased; however, neu transgene was not upregulated in the treatment group. Estrus cycling and hormone levels were not modified. No uterotrophic activity occurred.
In benign breast tissue from premenopausal and postmenopausal women, iCR dose-dependently inhibited steroid sulfatase activity and reduced local estrone and estradiol productionCitation52.
Clinical data on interactions of iCR, iCR + HP, or the HP component
Since 1997, 27 clinical studies, including 12,318 patients treated with iCR/iCR + HP, have monitored AEs and allowed co-medicationCitation9–15,Citation20–24,Citation26,Citation28–35,Citation53–58. Apart from case–control studies, two further studies, including 128 patients taking iCR, were excluded due to missing AE reporting in the corresponding publicationsCitation36,Citation59. None of the clinical studies with iCR/iCR + HP revealed any AEs in terms of adverse interactions.
Two double-blind RCTs versus placebo were conducted with 28 healthy volunteers to examine the pharmacokinetic interaction potential of the HP extract used to produce iCR + HPCitation60. The daily HP dose equaled the HP intake of the maximum recommended iCR + HP dosage (2 tablets b.i.d.) and corresponded to 1.0 mg total hypericin and 3.5 mg hyperforin. The substrates used in study A were single doses of alprazolam (1 mg, for cytochrome P [CYP] 3A4) and caffeine (100 mg, for CYP1A2) on days 1 and 11. In study B, single doses of tolbutamide (500 mg, for CYP2C9) on days 1 and 11 and multiple doses of digoxin (0.75 mg on days –2 and –1, 0.25 mg on days 2–11, for p-glycoprotein) were used. No statistically significant differences between HP and placebo were found for the area under the curve (AUC0–24) for alprazolam, caffeine (AUC0–12), paraxanthine, tolbutamide, 4-hydroxytolbutamide, and digoxin. HP-induced AUC change was less than 12% of the initial median AUC and, thus, clinically irrelevant.
Discussion
Efficacy of iCR/iCR + HP in breast cancer
In natural climacteric complaints, efficacy data gained with iCR/iCR + HP are consistently positive (Oxford Level 1 evidence)Citation6, yet the efficacy data we analyzed for breast cancer patients with tamoxifen-induced symptoms are mixed. In practice-oriented, uncontrolled studies, iCR ameliorated neurovegetative and psychic symptoms caused by antihormonal treatmentCitation21–23,Citation26. In contrast, one RCT found that decreases in hot flushes after 8 weeks were not significantly different from placeboCitation20. However, the iCR group reported significantly less sweating, which is remarkable considering the small sample sizeCitation20. In the pilot study, reduction of sweating (–80%) was also greater than for hot flushes (–50%)Citation22. A relevant shortcoming of these two studies is their short duration, which does not meet the EMA’s and Food and Drug Administration’s requirements of at least 12 weeks of treatmentCitation61,Citation62. CR exhibits the first effects after 2–4 weeks but efficacy increases with longer treatment duration and should not be definitively assessed before 12 weeksCitation63. In the longer observational study (24 weeks), the reduction of tamoxifen-induced neurovegetative and psychic complaints by iCR was significant and clinically relevant after 3 and 6 monthsCitation21,Citation25. An increase in iCR dose or a switch to iCR + HP was allowed, which may have contributed to better treatment effects. In natural climacteric complaints, iCR + HP, with a higher iCR dose, demonstrated superior efficacy compared to standard-dose iCRCitation15. Practical experience suggests that patients with tamoxifen-induced complaints benefit from higher CR doses than the standard dose used for natural menopausal symptomsCitation64. A responder rate of 70% shows that most, although not all, patients with tamoxifen-induced complaints benefit from iCRCitation25. This is consistent with hot flush reductions observed in an open, controlled, 1-year studyCitation65. Over several years, comprehensive everyday experience has been gained by two of the authors at the Beijing Obstetrics and Gynecology Hospital, Capital Medical University, its Menopause Clinic, and its Centre for Ovarian Tissue Retransplantation. These institutions, the first official ones of their kind in China, are highly frequented by breast cancer patients needing treatment for severe climacteric complaints. The Gynecological Endocrinology Department treats approximately 100,000 patients every year (96,908 in 2016); the approximately 1% of patients diagnosed with hormone-dependent breast cancer receive a standard dose of iCR for 6–48 months. Recently, within this department the first official International Fertility Protections Center using Ovarian Tissue Cryopreservation in China has been established, a method increasingly used for breast cancer patients. So the need to treat climacteric symptoms of these patients is strongly increasing, and because hormone therapy mostly is contraindicated, often black cohosh (iCR) is now used. Efficacy and tolerability of this treatment is judged in this department as good. For iCR, good efficacy on iatrogenic climacteric symptoms has also been demonstrated after endometrial cancer surgery, in hysterectomized premenopausal women, or in endometriosis patients with goserelin-induced complaintsCitation30,Citation35,Citation66.
Safety of iCR/iCR + HP after breast cancer – experimental data
In vitro, iCR inhibited MCF-7 BCC proliferation and enhanced the anti-estrogenic effects of tamoxifenCitation39–45. In vivo, iCR did not stimulate but, rather, retarded tumor growth and increased the life spanCitation47,Citation48. Concomitant iCR treatment enhanced tamoxifen efficacy and had no influence on the effects of the AI formestaneCitation49,Citation50. In the MMTV-neu mouse model, iCR did not promote tumor growth. However, an increase in lung metastases was seen that could not be explained by neu upregulation and has never been reproducedCitation51. Relevance of this artificial model (with its viral promoter and development of lung metastases prior to primary tumors) and treatment conditions (almost lifelong therapy with dosages far above the human therapeutic dose) for human HER2-positive breast cancer is doubtful. CR actually inhibited the growth of HER2-overexpressing human MDA-MB435 cells, and the CR ingredient actein induced apoptosis in MCF-7 cells transfected for HER2Citation67.
The anti-invasive effects of iCR in MDA-MB 231 BCC demonstrate that iCR inhibits at least the first step of the metastasis cascadeCitation46. Long-term iCR treatment led to a higher rate of carcinoma-free animals and did not affect metastasis in the DA/Han rat endometrial carcinomaCitation68. In the RUCA-I rat endometrial carcinoma model, a slight decrease in lung metastases was seen under iCR treatmentCitation69. Embryonic stem cell-expressed RAS was identified as a potent oncogenic driver in the HER2-positive MMTV model, acting via hyperactivation of the phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, which stimulates tumor cell proliferation and metastasis and induces therapy resistanceCitation70,Citation71. Recently, actein was identified as an effective inhibitor of the PI3K/AKT/mTOR pathway, showed anti-angiogenic effects in vitro, and decreased breast tumor size and metastasis in vivoCitation72,Citation73. CR activated AMP-activated protein kinase, which is known to inhibit mTORCitation74. Tamoxifen upregulates HER2 expression in the dimethylbenz(a)-anthracene tumor model, leading to tamoxifen resistance. The increase of tumor-free animals and the retardation of tumor growth, observed when iCR was combined with tamoxifen, may indicate a potential of iCR to counter therapy resistancesCitation49,Citation75.
Regarding the effect of iCR in benign breast tissue, the inhibition of steroid sulfatase may suggest a reduced primary risk of breast cancer using iCRCitation52: the decrease of local estrone and estradiol production lowers the rapid proliferation potential that could lead to replication mistakes generating breast cancer cells. In addition, direct anti-estrogenic effects of iCR were detected in reporter gene assaysCitation39.
Safety of iCR/iCR + HP after breast cancer – clinical data
Breast cancer patients tolerated iCR treatment well; this was also reported for naturally climacteric women in whom AE frequency and intensity were comparable to placeboCitation6,Citation7,Citation20–23,Citation26. It was previously believed that CR, due to its putative ingredient formononetin, may exert estrogenic effects. However, iCR does not contain formononetin and did not exhibit ER activityCitation76,Citation77. Consistent clinical data show that iCR does not influence hormone levels, breast density, breast cell proliferation, or endometrial thicknessCitation10,Citation11,Citation13,Citation14,Citation20,Citation23,Citation28–31,Citation33–35.
The association of iCR/iCR + HP intake with a reduced risk to develop breast cancer was independent from tumor receptor status, with 20.3% HER2-positive patientsCitation37. Breast cancer survivors treated with iCR/iCR + HP benefitted from an additional 4.5 years of recurrence-free survival compared to iCR non-usersCitation24. A re-evaluation of the study data also demonstrated a decreased rate of distant (e.g. lung) metastases in iCR/iCR + HP users compared to non-usersCitation78. The clinical data are promising and justify further prospective, controlled trials focusing on the potential protective effects of iCR on breast cancer initiation and progression.
HP interactions and relevance for breast cancer patients receiving endocrine therapy
In contrast to CR, for which no interactions are known, HP is noted for various interactions, which are listed in the HP monograph of the Herbal Medicinal Products Committee of the EMACitation79. However, interactions with tamoxifen or AIs do not belong to these known HP interactions and no such clinical data have been describedCitation79,Citation80.
HP interactions, which arise mainly via induction of CYP3A4 and p-glycoprotein, are especially known for high-dose preparations and extracts rich in hyperforinCitation81,Citation82. Hyperforin, an agonist at the pregnane X receptor, upregulates genes for the metabolism of xenobioticsCitation83. Therefore, HP’s compatibility with concomitant medication depends on the dosage and hyperforin content. The hyperforin content of German HP herbal medicinal products varies greatlyCitation84. While high-dose hyperforin extracts induced CYP3A, no clinically relevant influence on CYP3A or p-glycoprotein was found for HP preparations low in hyperforinCitation85–87.This is in line with the lack of clinically relevant interactions of the HP extract used to produce iCR + HPCitation60. Due to synergistic effects with CR, iCR + HP does not need a high HP dose; thus, the extract does not contain much hyperforin.
Tamoxifen
Concomitant medication with CYP2D6 inhibitors (e.g. paroxetine, fluoxetine) should be avoided in patients using tamoxifen since it leads to reductions of endoxifen levelsCitation88. Tamoxifen is metabolized into its active metabolite endoxifen mainly via CYP2D6 and CYP3A4 ()Citation89,Citation90. Therefore, inhibition of CYP2D6 (and, as the case may be, inhibition of CYP3A4) reduces the generation of endoxifen and possibly the efficacy of tamoxifen. The pharmacokinetics of tamoxifen is complex, involving additional CYP isoenzymes and the inactivation of tamoxifen and its active metabolites via phase II reactionsCitation91. Tamoxifen’s metabolism and the impact of endoxifen levels on tamoxifen’s efficacy are controversially discussedCitation92,Citation93. A recently published prospective multicenter study showed that the objective response rate (using RECIST criteria 1.0), clinical benefit, progression-free survival, and tolerability were not associated with endoxifen levelsCitation93.
Figure 1. Main metabolism pathways of tamoxifen into its active metabolite endoxifen. CYP, cytochrome P.
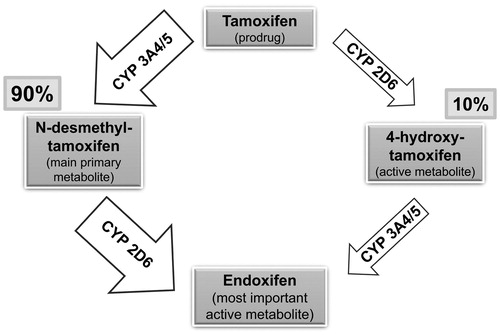
HP does not influence CYP2D6 but induces CYP3A4Citation83. Theoretically, a CYP3A4 induction could – via increased metabolism of tamoxifen into its active metabolite endoxifen – even lead to an enhancement of tamoxifen efficacy. A reduction of tamoxifen plasma levels of unclear clinical relevance has been described during the application of strong CYP3A4 inducers like rifampicinCitation88. Such a reduction may suggest increased metabolism into the active metabolite endoxifen. Because rifampicin’s induction effect on CYP3A4 substrates is 25 times that of HP and rifampicin also induces tamoxifen glucuronidation, it is arguable whether HP would have rifampicin-like effects on tamoxifen plasma levelsCitation83,Citation94. In contrast to CYP2D6 inhibitors, it is not deemed necessary to abstain from the application of CYP3A4 inducers during tamoxifen therapyCitation88. This seems appropriate for iCR + HP regarding the reduced recurrence risk in tamoxifen-treated patients, which was not only apparent in patients using iCR monotherapy but also in patients using iCR + HPCitation24,Citation27. If the HP component in iCR + HP had attenuated tamoxifen’s efficacy, a decrease but not actual prolongation of recurrence-free survival could have been expected.
Aromatase inhibitors
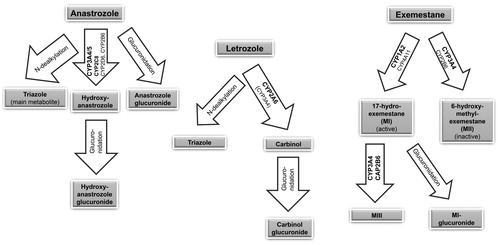
Anastrozole metabolites (triazole, hydroxyanastrozole, hydroxyanastrozole glucuronide, and anastrozole glucuronide) result from N-dealkylation, hydroxylation, and glucuronidation processes ()Citation95,Citation96. The main metabolite, triazole, is inactiveCitation95. Enzymes involved in anastrozole metabolism have not been completely identifiedCitation95. In vitro, the formation of hydroxyanastrozole was mainly catalyzed by CYP3A4/5 and, to a lesser extent, by CYP2C8, CYP2D6, and CYP2B6 ()Citation95,Citation97. CYP3A4 inducers could possibly increase the production of one of the metabolites (hydroxyanastrozole), but it should be taken into account that anastrozole itself inhibits CYP3A4Citation95. No influence of potent CYP inducers on anastrozole metabolism is known, and safety data analyses from clinical studies did not show significant interactions with commonly prescribed drugsCitation95.
Letrozole elimination resulting from metabolic clearance (Clm = 2.1 l/h) is rather slow compared to liver perfusion (ca. 90 l/h)Citation98. In vitro, CYP2A6 and CYP3A4 were active in transforming letrozole to its main, inactive carbinol metabolite (4,4′-methanol-bisbenzonitrile), which is further glucuronidized ()Citation98,Citation99. In vivo, only CYP2A6 genotypes showed significant associations with plasma letrozole concentrations, suggesting that CYP2A6 is the principal clearance mechanismCitation100. Therefore, significant interactions of HP with letrozole are not to be expected.
Exemestane is metabolized via oxidation into inactive 6-hydroxymethylexemestane (MII), primarily by CYP3A4 and CYP2B6, and reduced by aldoketoreductases, CYP4A11, and CYP1A2 to 17-hydroexemestane (MI), the majorly active but less potent metaboliteCitation101,Citation102. The metabolites are further glucuronidized, but MI is predominantly converted into MIII (major metabolite of 17-hydroexemestane) by CYP3A4/5 and CYP2B6 ()Citation101,Citation103. The potent CYP3A4 inducer rifampicin led to an AUC reduction of exemestaneCitation103. To date, the clinical relevance has not been clarified, and it cannot be excluded that CYP3A4 inducers like HP could reduce exemestane’s efficacyCitation103. Exemestane plasma concentrations depend on various other factors (liver and kidney function, ethnicity, body mass index, previous chemotherapy, genetic CYP polymorphisms)Citation104. While the German summary of product characteristics does not deem dosage adjustments necessary, the US prescribing information recommends exemestane updosing for patients using strong CYP3A4 inducers like rifampicin or phenytoin. However, for HP no clinical interactions with exemestane are reported and for the HP in iCR + HP no clinically relevant interactions could be detectedCitation60,Citation79. Therefore, such updosing in patients on iCR + HP therapy may increase exemestane’s side effects due to overdosing. When uncertain or in patients prone to lower exemestane levels (e.g. black ethnicity, high body mass index, prior chemotherapy), monitoring estradiol values may be helpful to determine the necessity of updosing exemestane.
Conclusion and practical consequences
Most breast cancer patients experience beneficial effects of iCR on tamoxifen-induced neurovegetative and psychic complaints. Still, RCTs with sufficient treatment duration should be conducted in breast cancer patients to confirm these results. Considering the consistently convincing effects of iCR for natural climacteric complaints, tamoxifen seems to affect treatment efficacy; this may be countered by applying higher iCR doses or iCR + HP, respectively. Clinical and experimental data have consistently demonstrated that iCR lacks estrogen-like effects at the breast or uterus. Therefore, the use of iCR in breast cancer patients can be regarded as safe. Epidemiological data and increasing knowledge on CR’s mechanisms of action suggest protective effects of iCR in terms of prolonged recurrence-free survival, warranting future prospective trials. No interactions with endocrine therapy are known for iCR or have been reported for HP. The HP extract used in iCR + HP did not exhibit clinically relevant interactions. Therefore, iCR/iCR + HP can also be used in breast cancer survivors receiving endocrine therapy. The overall benefit–risk profile of iCR/iCR + HP for breast cancer patients is positive, and treatment adherence of patients suffering from side effects of antihormonal treatment could be increased by offering iCR/iCR + HP.
Conflict of interest
A. O. Mueck, X. Ruan, and A.-M. Beer report no conflict of interest. B. Naser and S. Pickartz are employees of Schaper & Brümmer, the manufacturer of iCR/iCR + HP. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Lorizio W, Wu AHB, Beattie MS, Rugo H, Tchu S, Kerlikowske K. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat 2012;132:1107–18
- Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol 2010;73:156–66
- Fallowfield L. Acceptance of adjuvant therapy and quality of life issues. Breast 2005;14:612–16
- NAMS. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause 2017;24:1–26
- European Union herbal monograph on Cimicifuga racemosa (L.). Nutt., Rhizoma [Internet]. 2018; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2017/08/WC500233056.pdf
- Beer A-M, Neff A. Differentiated evaluation of extract-specific evidence on Cimicifuga racemosa's efficacy and safety for climacteric complaints. Evid Based Complement Alternat Med 2013;2013:860602
- Beer A-M. Cimicifuga racemosa bei klimakterischen Beschwerden – Aktuelle Daten bestätigen Wirksamkeit und Sicherheit. Z Phytother 2015;36:10–17
- Stoll W. Phytotherapeutikum beeinflußt atrophisches Vaginalepithel: Doppelblindversuch Cimicifuga vs. Oestrogenpräparat. Therapeutikon 1987;1:23–31
- Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke-von Zepelin HH. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol 2005;105:1074–83
- Li Y, Cui M, Gao S. Efficacy of remifemin for control of climacteric symptoms. Prog Obstet Gynecol 2011;20:462–5
- Jiang K, Jin Y, Huang L, Feng S, Hou X, Du B. Black cohosh improves objective sleep in postmenopausal women with sleep disturbance. Climacteric 2015;18:559–67
- Uebelhack R, Blohmer JU, Graubaum HJ, Busch R, Gruenwald J, Wernecke KD. Black cohosh and St. John's wort for climacteric complaints: a randomized trial. Obstet Gynecol 2006;107:247–55
- Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol 2005;20:30–5
- Bai W, Henneicke-von Zepelin HH, Wang S, Zheng S, Liu J, Zhang Z. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel-controlled study versus tibolone. Maturitas 2007;58:31–41
- Briese V, Stammwitz U, Friede M, Henneicke-von Zepelin HH. Black cohosh with or without St. John's wort for symptom-specific climacteric treatment-results of a large-scale, controlled, observational study. Maturitas 2007;57:405–14
- Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med 2015;13:195
- Schneider AP, 2nd, Zainer CM, Kubat CK, Mullen NK, Windisch AK. The breast cancer epidemic: 10 facts. Linacre Q 2014;81:244–77
- Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308–24
- Fan L, Strasser-Weippl K, Li J-J, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–e89
- Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739–45
- Rostock M, Fischer J, Mumm A, Stammwitz U, Saller R, Bartsch HH. Black cohosh (Cimicifuga racemosa) in tamoxifen-treated breast cancer patients with climacteric complaints – a prospective observational study. Gyneco Endocrinol 2011;27:844–8
- Pockaj BA, Loprinzi CL, Sloan JA, et al. Pilot evaluation of black cohosh for the treatment of hot flashes in women. Cancer Invest 2004;22:515–21
- Wu X, Remifemin improve gynecological malignant tumor postoperative patients of menopause syndrome for the clinical research. Dalian Medical University: Master Degree Thesis; 2011.
- Henneicke-von Zepelin HH, Meden H, Kostev K, Schröder-Bernhardi D, Stammwitz U, Becher H. Isopropanolic black cohosh extract and recurrence-free survival after breast cancer. Int J Clin Pharmacol Ther 2007;45:143–54
- Fischer J. Cimicifuga racemosa Extrakt (Remifemin®) bei Mamma-Ca-Patientinnen mit klimakterischen Beschwerden unter hormontherapeutischer Behandlung mit Tamoxifen – eine Anwendungsbeobachtung: Albert-Ludwigs-Universität; 2006.
- Huang X. The clinical research of Black cohosh extract in breast cancer patients with climacteric complaints 2011.
- Henneicke-von Zepelin H-H, Meden H, Kostev K, Schröder-Bernhardi D, Dietlein G, Friede M. Pharmakoepidemiologische Kohortenstudie zur Anwendung von Remifemin/Remifemin plus bei Patientinnen mit Mammakarzinom, einschließlich Hormonrezeptor-positiver Tumore. Interner Bericht Studiencode SB-FEM 1400. 2005.
- Hirschberg AL, Edlund M, Svane G, Azavedo E, Skoog L, von SB. An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women. Menopause 2007;14:89–96
- Lundström E, Hirschberg AL, Soderqvist G. Digitized assessment of mammographic breast density – effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas 2011;70:361–4
- Li W, Sun N-X, Chen X, Lang D-F, Jin Z-J. Cimicifuga racemosa for treatment of menopausal symptoms in patients with early endometrial cancer after operation. Acad J Second Mil Med Univ 2012;33:562–4
- Liske E, Hänggi W, Henneicke-von Zepelin H-H, Boblitz N, Wüstenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med 2002;11:163–74
- Sun N-x, Jin Z-j, Jia X-f, Li W. Black cohosh improves vaginal atrophy in postmenopausal women. Acad J Second Mil Med Univ 2012;32:339–41
- Garcia-Perez MA, Pineda B, Hermenegildo C, Tarin JJ, Cano A. Isopropanolic Cimicifuga racemosa is favorable on bone markers but neutral on an osteoblastic cell line. Fertil Steril 2009;91:1347–50
- Nesselhut T, Liske E, editors. Pharmacological measures in postmenopausal women with an isopropanolic aqueous extract of Cimicifugae racemosae rhizoma. 10th Annual Meeting of the North American Menopause Society (NAMS); 1999: Menopause.
- Chen J, Gao H, Li Q, Cong J, Wu J, Pu D. Efficacy and safety of remifemin on peri-menopausal symptoms induced by post-operative GnRH-a therapy for endometriosis: a randomized study versus tibolone. Med Sci Monit 2014;20:1950–7
- Reame NE, Lukacs JL, Padmanabhan V, Eyvazzadeh AD, Smith YR, Zubieta JK. Black cohosh has central opioid activity in postmenopausal women: evidence from naloxone blockade and positron emission tomography neuroimaging. Menopause 2008;15:832–40
- Obi N, Chang-Claude J, Berger J, et al. The use of herbal preparations to alleviate climacteric disorders and risk of postmenopausal breast cancer in a German case-control study. Cancer Epidemiol Biomarkers Prev 2009;18:2207–13
- Rebbeck TR, Troxel AB, Norman S, et al. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer 2007;120:1523–8
- Zierau O, Bodinet C, Kolba S, Wulf M, Vollmer G. Antiestrogenic activities of Cimicifuga racemosa extracts. J Steroid Biochem Mol Biol 2002;80:125–30
- Bodinet C, Freudenstein J. Influence of marketed herbal menopause preparations on MCF-7 cell proliferation. Menopause 2004;11:281–9
- Omer-Adam MA, Zieg H, Bodinet C, Liske E. Quantification of characteristic marker substances and evaluation of their effects on MCF-7 cell proliferation. 7th Joint Meeting of GA, AFERP, ASP. 2008.
- Nesselhut T, Bodinet C, Schneider P, Freudenstein J. Inventors use of extract of Cimicifuga racemose; 2001.
- Bodinet C, Freudenstein J. Influence of Cimicifuga racemosa on the proliferation of estrogen receptor-positive human breast cancer cells. Breast Cancer Res Treat 2002;76:1–10
- Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Evaluation of cell death caused by triterpene glycosides and phenolic substances from Cimicifuga racemosa extract in human MCF-7 breast cancer cells. Biol Pharm Bull 2004;27:1970–5
- Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Cimicifuga racemosa extract inhibits proliferation of estrogen receptor-positive and negative human breast carcinoma cell lines by induction of apoptosis. Breast Cancer Res Treat 2004;84:151–60
- Hostanska K, Nisslein T, Freudenstein J, Reichling J, Saller R. Inhibitory effect of an isopropanolic extract of black cohosh on the invasiveness of MDA-MB 231 human breast cancer cells. In Vivo 2007;21:349–55
- Freudenstein J, Dasenbrock C, Nisslein T. Lack of promotion of estrogen-dependent mammary gland tumors in vivo by an isopropanolic Cimicifuga racemosa extract. Cancer Res 2002;62:3448–52
- Nisslein T, Freudenstein J, editors. Prepubertal treatment with black cohosh attenuates pathogenicity in the DMBA rat model of mammary carcinoma. 10th world congress on the menopause; 2002 P06-08; Berlin: Climacteric.
- Nisslein T, Freudenstein J, editors. Synergistic effects of Black Cohosh and Tamoxifen in an animal model of mammary carcinoma. 6th European Congress on Menopause; 2003: Maturitas.
- Nißlein T, Freudenstein J. Coadministration of the aromatase inhibitor formestane and an isopropanolic extract of black cohosh in a rat model of chemically induced mammary carcinoma. Planta Med 2007;73:318–22
- Davis VL, Jayo MJ, Ho A, et al. Black cohosh increases metastatic mammary cancer in transgenic mice expressing c-erbB2. Cancer Res 2008;68:8377–83
- Stute P, Nisslein T, Gotte M, Kamischke A, Kiesel L, Klockenbusch W. Effects of black cohosh on estrogen biosynthesis in normal breast tissue in vitro. Maturitas 2007;57:382–91
- Huang YX, Song L, Zhang X, Lun WW, Pan C, Huang YS. Clinical study of combined treatment of remifemin and paroxetine for perimenopausal depression. Zhonghua Yi Xue Za Zhi 2013;93:600–2
- Xi S, Liske E, Wang S, et al. Effect of isopropanolic Cimicifuga racemosa extract on uterine fibroids in comparison with tibolone among patients of a recent randomized, double blind, parallel-controlled study in Chinese women with menopausal symptoms. Evid Based Complement Alternat Med 2014;2014:1.
- Liske E. Phytokombination lindert psychovegetative Leiden. [Phytocombination alleviates psychovegatative disorders]. TW Gynäkologie 1997;10:172–5
- Schmidt M, Polasek W, Käufeler R. Wirksamkeit und Sicherheit von Traubensilberkerze (Cimicifuga racemosa, Cimifemin®) bei Menopausebeschwerden: Therapiebeobachtung unter Praxisbedingungen. J Für Menopause 2005;1:27–32
- Vermes G, Banhidy F, Acs N. The effects of remifemin on subjective symptoms of menopause. Adv Ther 2005;22:148–54
- Arriaza Peso E, del Carmen Arévalo Páez M, Ángeles Grandas Alonso M, Olleros Izard T. Eficacia de Cimicifuga racemosa para el tratamiento de la clínica vasomotora y psíquica en pacientes menopáusicas. [Efficacy of Cimicifuga racemosa in the treatment of vasomotor and psychic symptoms in menopausal patients]. Progresos de Obstetricia y Ginecología 2008;51:20–7
- Julia Molla MD, Garcia-Sanchez Y, Romeu Sarri A, Perez-Lopez FR. Cimicifuga racemosa treatment and health related quality of life in post-menopausal Spanish women. Gynecol Endocrinol 2009;25:21–6
- Arold G, Donath F, Maurer A, et al. No relevant interaction with alprazolam, caffeine, tolbutamide, and digoxin by treatment with a low-hyperforin St John's wort extract. Planta Med 2005;71:331–7
- European Medicines Agency (EMA), Committee for medicinal products for human use (CHMP). Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. 2005.
- FDA. Guidance for Industry Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms — Recommendations for Clinical Evaluation. 2003.
- Beer A-M. Update Cimicifuga racemosa – neue Erkenntnisse aus Wissenschaft und Forschung: Differenzierte Evidenz für Wirksamkeit und Sicherheit von Traubensilberkerzen-Arzneimitteln zur Behandlung klimakterischer Beschwerden. J Gynäkol Endokrinol 2014;24:6–10
- Beer A-M. Cimicifuga in den Wechseljahren: Gibt es Risiken? MMW – Fortschritte Der Medizin 2013;155:22
- Hernández Muñoz G, Pluchino S. Cimicifuga racemosa for the treatment of hot flushes in women surviving breast cancer. Maturitas 2003;44:S59–S65
- Lehmann-Willenbrock E, Riedel HH. Klinische und endokrinologische Untersuchungen zur Therapie ovarieller Ausfallerscheinungen nach Hysterektomie unter Belassung der Adnexe. Zent bl Gynäkol 1988;110:611–18
- Einbond LS, Wen-Cai Y, He K, et al. Growth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cells. Phytomedicine 2008;15:504–11
- Nisslein T, Freudenstein J, editors. Long term treatment of uterine prone Dark Agouti rats with a standardized isopropanolic extract of Cimicifuga racemosa. 51st Annual Congress of the Society for Medicinal Plant Research; 2003; Kiel, Germany.
- Nisslein T, Freudenstein J. Concomitant administration of an isopropanolic extract of black cohosh and tamoxifen in the in vivo tumor model of implanted RUCA-I rat endometrial adenocarcinoma cells. Toxicol Lett 2004;150:271–5
- Ikink GJ, Boer M, Bakker ERM, et al. Insertional mutagenesis in a HER2-positive breast cancer model reveals ERAS as a driver of cancer and therapy resistance. Oncogene 2018;37:1594–609
- Paplomata EO´, Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol 2014;6:154–66
- Ji L, Zhong B, Jiang X, et al. Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways. Oncotarget 2017;8:112498–515
- Yue GG, Xie S, Lee JK, et al. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci Rep 2016;6:35263
- Moser C, Vickers SP, Brammer R, Cheetham SC, Drewe J. Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine 2014;21:1382–9
- Moi LL, Flageng MH, Gjerde J, et al. Steroid receptor coactivators, HER-2 and HER-3 expression is stimulated by tamoxifen treatment in DMBA-induced breast cancer. BMC Cancer 2012;12:247
- Kennelly EJ, Baggett S, Nuntanakorn P, et al. Analysis of thirteen populations of black cohosh for formononetin. Phytomedicine 2002;9:461–7
- Beck V, Unterrieder E, Krenn L, Kubelka W, Jungbauer A. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol 2003;84:259–68
- Kostev K. Re-evaluations regarding the REMIFEMIN-study (2004) "study code: SB-FEM1400". IMS® Disease Analyzer 2009.
- Community Herbal Monograph on Hypericum perforatum L., Herba. Well-established medicinal use [Internet]. 2009. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2010/01/WC500059145.pdf
- Fachinformation (Summary of Product Characteristics). Remifemin plus Johanniskraut. Aug. 2015.
- Mueller SC, Majcher-Peszynska J, Uehleke B, et al. The extent of induction of CYP3A by St. John's wort varies among products and is linked to hyperforin dose. Eur J Clin Pharmacol 2006;62:29–36
- Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V. Hyperforin in St. John's wort drug interactions. Eur J Clin Pharmacol 2006;62:225–33
- Stargrove MB, Treasure J, McKee DL, Herb, Nutrient and Drug Interactions: Clinical Implications and Therapeutic Strategies. Mosby Elsevier: St. Louis, Missouri, United States of America 2008:140–59.
- Wurglics M, Westerhoff K, Kaunzinger A, et al. Comparison of German St. John's wort products according to hyperforin and total hypericin content. J Am Pharm Assoc (Wash) 2001;41:560–6
- Mueller SC, Uehleke B, Woehling H, et al. Effect of St John's wort dose and preparations on the pharmacokinetics of digoxin. Clin Pharmacol Ther 2004;75:546–57
- Mueller SC, Majcher-Peszynska J, Mundkowski RG, et al. No clinically relevant CYP3A induction after St. John´s wort with low hyperforin content in healthy volunteers. Eur J Clin Pharmacol 2009;65:81–7
- Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H. The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol 2006;62:512–26
- Musterfachinformation Tamoxifencitrat [Internet]. 2015 [cited 04.07.2017]. Available from: https://sunset-clause.dimdi.de/muster/O9b59fc22f0a34cf7a8fb53b59eee5096.pdf
- ter Heine R, Binkhorst L, de Graan AJM, et al. Population pharmacokinetic modelling to assess the impact of CYP2D6 and CYP3A metabolic phenotypes on the pharmacokinetics of tamoxifen and endoxifen. Br J Clin Pharmacol 2014;78:572–86
- Tan SH, Lee S-C, Goh B-C, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res 2008;14:8027–41
- Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE. PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics 2013;23:643–7
- Cronin-Fenton DP, Damkier P, Lash TL. Metabolism and transport of tamoxifen in relation to its effectiveness: new perspectives on an ongoing controversy. Future Oncol 2014;10:107–22
- Neven P, Jongen L, Lintermans A, et al. Tamoxifen metabolism and efficacy in breast cancer: a prospective multicenter trial. Clin Cancer Res 2018;24:2312–18
- Kuypers DR, Verleden G, Naesens M, Vanrenterghem Y. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate-glucuronosyltransferase. Clin Pharmacol Ther 2005;78:81–8
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Musterfachinformation Anastrozol. 18.04.2018 ed2018.
- Linardi A, Damiani D, Longui CA. The use of aromatase inhibitors in boys with short stature: what to know before prescribing? Arch Endocrinol Metab 2017;61:391–7
- Kamdem LK, Liu Y, Stearns V, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol 2010;70:854–69
- Fachinformation (Summary of Product Characteristics). Femara 2.5 mg. Oct. 2017.
- Murai K, Yamazaki H, Nakagawa K, Kawai R, Kamataki T. Deactivation of anti-cancer drug letrozole to a carbinol metabolite by polymorphic cytochrome P450 2A6 in human liver microsomes. Xenobiotica 2009;39:795–802
- Desta Z, Kreutz Y, Nguyen AT, et al. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 2011;90:693–700
- Landry KK, David FA, Zeruesenay D. 17-Hydroexemestane: a potent inhibitor of CYP19 (aromatase) and substrate of CYP3A. J Drug Metabolism Toxicol 2014;5:2.
- Kamdem LK, Flockhart DA, Desta Z. In vitro cytochrome P450-mediated metabolism of exemestane. Drug Metab Dispos 2011;39:98–105
- Fachinformation (Summary of Product Characteristics). Aromasin Dec. 2017.
- Hertz DL, Kidwell KM, Seewald NJ, et al. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J 2017;17:521–7