Abstract
Ospemifene is a selective estrogen-receptor modulator approved for treating menopause-related moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy (VVA), in the United States, and for treating menopause-related, symptomatic VVA in women not appropriate for local estrogen therapy in Europe. This review summarizes the effects of ospemifene on bone, including bone biomarker data from a phase 3 vaginal dryness study. Early-phase studies of postmenopausal women showed that ospemifene dose-dependently decreased bone turnover markers versus placebo, similar to raloxifene. A 12-week, phase 3 study of ospemifene 60 mg/day in postmenopausal women showed improvements in all VVA parameters and significantly greater decreases in seven of nine bone biomarkers versus placebo. Lower bone resorption markers with ospemifene were observed regardless of time since menopause (≤5 years or >5 years) or baseline bone mineral density (BMD) (normal [n = 18], osteopenia [n = 164], or osteoporosis [n = 21]). Biomarker studies (n = 565 who took ospemifene) therefore support a potential role for ospemifene in maintaining bone health (and possibly reducing fracture risk) in postmenopausal women taking it for VVA; however, caution is warranted because data are limited to biochemical markers, rather than fracture and BMD. Although studies show that bone turnover predicts BMD and fractures, any hypothesis about a bone-sparing effect of ospemifene needs testing in rigorous, long-term, phase 3 studies monitoring fractures and BMD.
Chinese abstract
Ospemifene是一种选择性雌激素受体调节剂, 在美国被批准用于治疗与更年期相关的中度至重度性交困难和阴道干涩, 外阴阴道萎缩 (VVA) 的症状, 在欧洲用于治疗不适合局部雌激素治疗的绝经相关的、有症状的VVA。这篇综述总结了ospemifene对骨骼的影响, 包括来自3期阴道干燥研究的骨生物标志物数据。对绝经后妇女的早期研究表明, 与安慰剂相比, ospemifene剂量依赖性地降低骨转换标志物, 类似于雷洛昔芬。一项为期12周的3期研究显示, 与安慰剂相比, 绝经后妇女每天服用60毫克Ospemifene, 所有VVA参数均有改善, 9种骨生物标志物中7种显著降低。无论绝经后的时间 (≤5年或> 5年) 或基线骨密度 (BMD) (正常[n = 18], 骨质减少[n = 164]或骨质疏松症[n = 21]) 如何, 均观察到ospemifene降低骨吸收标志物。因此, 生物标志物研究 (n=565人服用ospemifen) 支持了ospemifene在绝经后妇女中治疗VVA时维持骨骼健康 (并可能降低骨折风险) 方面的潜在作用;然而, 谨慎是必要的, 因为数据仅限于生化标记物, 而不是骨折和BMD。虽然研究表明骨转换可以预测骨密度和骨折, 但任何关于ospemifene的节骨作用的假设都需要在监测骨折和骨密度的严格的长期3期研究中进行检验。
Introduction
Osteoporosis, a ‘silent’ progressive weakening in bone strength due to declining bone mass and bone quality, predisposes to fracture riskCitation1. Both osteoporosis and low bone mass (osteopenia) are associated with significantly elevated risk of fractureCitation2, and are highly prevalent in Europe and the United States with the incidence of both expected to rise over timeCitation3,Citation4. Osteoporotic fracture, which occurs in one in three women aged >50 years worldwideCitation5, is a serious consequence of osteoporosis; it negatively affects quality of life and is associated with increased mortality and health-care costsCitation5–7.
Selective estrogen receptor modulators (SERMs) work through a mixed agonist/antagonist effect on estrogen receptors, mostly having varying agonist effects on boneCitation8. Raloxifene is a SERM approved worldwide for treating and preventing osteoporosis in postmenopausal women and for reducing risk of invasive breast cancer in postmenopausal women with osteoporosis or at high risk of breast cancerCitation9. Bazedoxifene is approved in Europe, Japan, and South Korea for the treatment of osteoporosis in postmenopausal women at high risk of fractureCitation10.
Ospemifene is a SERM approved at 60 mg/day orally in the United States for the treatment of moderate to menopause-related severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy (VVA), and in Europe for menopause-related, moderate to severe symptomatic VVA in women who are not candidates for local vaginal estrogen therapyCitation11,Citation12. Because estrogen deficiency predisposes to both VVA and osteoporosis, there is great interest in studying the effects of ospemifene on bone health in postmenopausal women. The primary objective of this review was to summarize the effects of ospemifene on bone parameters and present new bone biomarker data from a recent phase 3 studyCitation13.
Methodology
PubMed was searched since its inception for articles pertaining to the effects of ospemifene on bone. Keywords in the search strategy included ospemifene and bone. Search results were reviewed for articles reporting primary clinical data on the effects of ospemifene on bone in postmenopausal women. Bibliographies of the included studies and reviews were also scanned for additional, relevant clinical studies.
Clinical trials
Phase 1 and 2 studies
Both phase 1 and phase 2 studies have evaluated the effects of ospemifene on bone, mostly by examining its effects on biochemical bone markers. Ospemifene doses of 25, 50, 100, and 200 mg/day were evaluated in 40 healthy postmenopausal women in a 12-week randomized, double-blind phase 1 clinical trialCitation14. Ospemifene dose-dependently decreased procollagen type I N-terminal and C-terminal propeptides (PINP and PICP) and osteocalcin, but only the dose of 200 mg significantly (p = 0.01) decreased bone-specific alkaline phosphatase (ALP) versus placeboCitation14.
A double-blind, phase 2 study tested the safety and efficacy of ospemifene 30, 60, and 90 mg versus placebo in 159 healthy postmenopausal women (n = 30–40 in each group) for 12 weeksCitation15. Ospemifene induced a dose-dependent decrease from baseline relative to placebo in four of five bone markers tested (serum PINP, bone-specific ALP, and urinary C-terminal and N-terminal cross-linking telopeptides of type I collagen [CTX and NTX], but not PICP; all tests for linearity, p ≤ 0.03)Citation15. History of menopausal hormone therapy use was also associated with greater increases in NTX and CTX levelsCitation15. Thus, the effects on bone resorption suggested that ospemifene acted as an estrogen agonist, and that these effects were more prominent in women who had previously used menopausal hormone therapyCitation15. Beneficial effects on bone markers declined 2–4 weeks after stopping ospemifene treatmentCitation15.
Ospemifene (30, 60, and 90 mg) was compared with raloxifene (60 mg) for bone resorption and bone formation markers in a 3-month, double-blind, phase 2 study of 118 healthy postmenopausal women (n = 29–30 in each group)Citation16. Bone resorption was assessed with urinary NTX and CTX, while bone formation was evaluated with serum PINP, PICP, bone-specific ALP, and osteocalcinCitation16. Overall effects on bone markers were largely similar between the ospemifene and raloxifene groupsCitation16. Most treatment groups had a decline in bone markers, which were largest with ospemifene 60 mg and 90 mg, and raloxifene 60 mgCitation16. There were no significant differences between ospemifene and raloxifene for CTX, PICP, ALP, or osteocalcinCitation16.
Overall, phase 1 and 2 data on bone biomarkers suggest that ospemifene may have positive effects on bone, comparable to those exerted by raloxifene in postmenopausal women.
Phase 3 study
Study design and methodology
A 12-week multicenter, double-blind, randomized, placebo-controlled trial was conducted to evaluate the efficacy and safety of once-daily oral ospemifene 60 mg for the treatment of moderate to severe vaginal dryness as the most bothersome symptom of VVA due to menopauseCitation13. Eligible participants were healthy postmenopausal women aged 40–80 years who had moderate or severe vaginal dryness (rated as 0 = none; 1 = mild; 2 = moderate; and 3 = severe on a validated questionnaire), ≤5% superficial cells on vaginal wall smear, and vaginal pH > 5.0 at baselineCitation13.
Women were randomized 1:1 to ospemifene 60 mg or matching placebo for treatment up to 12 weeksCitation13. Randomization was stratified by moderate or severe vaginal dryness and the presence or absence of a uterus (limited to 60% of participants without a uterus in each group)Citation13. The four co-primary efficacy endpoints were changes from baseline to week 12 in the percentages of vaginal parabasal cells and superficial cells, vaginal pH, and severity of self-reported vaginal dryness with ospemifene versus placeboCitation13. Safety was assessed by treatment-emergent adverse events up to 14 days after the last doseCitation13. Secondary endpoints included changes in five markers of bone resorption (bone sialoprotein, CTX, deoxypyridinoline, NTX, and tartrate-resistant acid phosphatase 5b [TRACP-5b]) and four markers of bone formation (serum total ALP, bone-specific ALP, serum osteocalcin, and PINP) from baseline to week 12.
Results
A total of 316 women were randomized to ospemifene and 315 women to placeboCitation13. Demographics and baseline characteristics were similar between treatment groupsCitation13. The mean age was approximately 60 years, mean body mass index was 27.2 kg/m2, and mean duration of VVA was 8–9 yearsCitation13.
Serum levels of the nine bone biomarkers were similar between groups at baseline. When markers were measured as least-squares mean changes from baseline to week 12, the differences between treatment groups were negative for all bone resorption markers, with significantly greater decreases for ospemifene versus placebo for CTX, NTX, and TRACP-5b (all three comparisons, p ≤ 0.02) (; ). Likewise, ospemifene was associated with greater least-squares mean decreases at week 12 for all bone formation markers compared with placebo (all comparisons, p ≤ 0.008) (; ).
Figure 1. LS mean changes from baseline for bone resorption markers with ospemifene versus placebo at week 12. ap < 0.05; bp < 0.001; cp < 0.0001. CTX, C-terminal cross-linking telopeptides of type I collagen; DPD, deoxypyridinoline; LS, least-squares; NTX, N-terminal cross-linking telopeptides of type I collagen; TRACP-5b, tartrate-resistant acid phosphatase 5b.
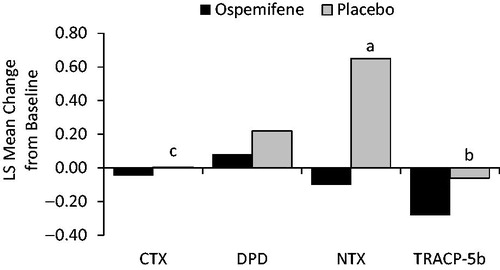
Figure 2. LS mean changes from baseline for bone formation markers with ospemifene versus placebo at week 12. ap < 0.01; bp < 0.0001. ALP, serum total alkaline phosphatase; BS-ALP, bone specific serum total alkaline phosphatase; LS, least-squares; OLC, osteocalcin; PINP, procollagen type I N-terminal propeptide.
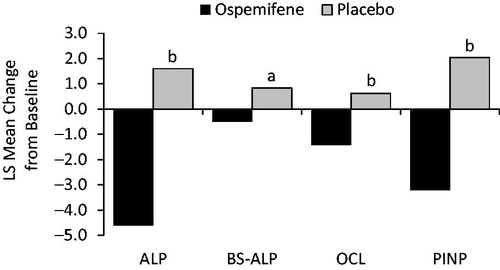
Table 1. Changes at week 12 in markers of bone resorption with ospemifene versus placeboTable Footnotea.
Table 2. Changes at week 12 in markers of bone formation with ospemifene versus placeboTable Footnotea.
In a subgroup having baseline dual-energy X-ray absorptiometry, 118 women had normal bone parameters (≤5 years since menopause, n = 53; >5 years since menopause, n = 65), 164 had osteopenia (n = 60 and n = 104, respectively), and 21 had osteoporosis (n = 7 and n = 14). Definitions of osteoporosis (2.5 standard deviations below the young adult mean) and osteopenia (>1 but <2.5 standard deviations below the young adult mean) were used according to the World Health Organization criteriaCitation17. Biomarker data varied by baseline dual-energy X-ray absorptiometry/bone mineral density (BMD) and by years since menopause. Women in early menopause (≤5 years) and later menopause (>5 years) had numerically lower values for bone resorption markers with ospemifene versus placebo, and the benefit appeared to apply regardless of baseline BMD (normal, osteopenia, or osteoporosis), although the osteoporosis group was too small to allow definitive conclusions. It is further assumed that women in early menopause, who have an accelerated rate of bone resorption, may trend toward a larger treatment effect with ospemifene than would women in later menopause. Most biochemical bone markers numerically support the hypothesis that women taking ospemifene had an attenuation of bone loss when compared with women assigned to placebo.
Discussion
Clinical studies support a potential role for ospemifene in maintaining bone health in postmenopausal women while they are taking it for the treatment of moderate to severe VVA. Serum levels of bone biomarkers in 565 women who received ospemifene in phase 1, phase 2, and phase 3 studies had significantly greater mean decreases from baseline to week 12 relative to placebo for most of the bone resorption and bone formation markers studied.
Clinical studies and animal models indicate that ospemifene has effects on bone markers significantly better than those of placebo and similar to those exerted by oral estrogens and other SERMs (i.e. raloxifene and bazedoxifene) approved for osteoporosis treatment and prevention. As described earlier, clinical studies in postmenopausal women showed that ospemifene administration induced a dose-dependent decrease of bone turnover markers relative to placeboCitation15, with an effect similar to that of raloxifeneCitation16.
In animal studies, the effects of ospemifene on bone were similar to those with raloxifene or estradiol, or in sham-operated (versus ovariectomized [OVX]) animalsCitation18,Citation19. An OVX rat model showed that both ospemifene and raloxifene exert bone protective effects, decreasing bone turnover and maintaining bone volumeCitation18, although varying effects on osteoclast expressionCitation19 suggest that ospemifene and raloxifene have different mechanisms of action, which warrants further evaluation. Animal data further support positive effects of ospemifene on bone mineral content, BMD, bone histology, and bone strengthCitation18,Citation19. When given to OVX rats at 1, 5, or 25 mg/kg for 1 year, ospemifene prevented decreases in bone mineral content and BMD in the femur and tibia in a dose-dependent manner relative to an untreated OVX group (all p ≤ 0.01)Citation19. Ospemifene 10 mg/kg for 4 weeks significantly prevented loss of trabecular bone volume and preserved bone strength in the femur and lumbar vertebrae in OVX rats (all p < 0.05 versus OVX controls), with effects comparable to those of 40 or 50 µg/kg estradiolCitation18,Citation19.
These findings with ospemifene suggest similar effects on bone as reported in phase 3 randomized trials showing significant decreases in osteocalcin and CTX with bazedoxifene (10, 20, or 40 mg) and raloxifene (60 mg) versus placebo at all time points from 3 to 24 months (all doses versus placebo, p < 0.001)Citation20. Decreases in these bone markers were sustained with 60 mg raloxifene versus placebo after 24 months (p < 0.05)Citation21 and 36 months (p < 0.001)Citation22 of treatment in postmenopausal women at risk for osteoporosis.
Clinical implications
Phase 3 trials suggest that ospemifene may be beneficial to the bone health of women who are taking it to treat VVA. Animal and clinical studies demonstrate that ospemifene is superior to placebo in reducing bone turnover. In addition, improvements in CTX and osteocalcin similar to those reported with raloxifene and bazedoxifene suggest that ospemifene may be hypothesized to improve BMD (and possibly reduce fracture risk) in postmenopausal women while they are taking it to treat VVA.
Caution is warranted, however, because current clinical data for the effects of ospemifene on bone are limited to changes in biochemical markers. On the other hand, several studies have demonstrated a correlation between biochemical markers of bone turnover and both BMD and fractures. A literature review from the International Osteoporosis Foundation concluded that increased levels of bone resorption markers (above the upper limit of the premenopausal range) elevate the risk of fractures by approximately two-fold, and that bone markers may be used to assess fracture risk if BMD data are not availableCitation23. The Malmö Osteoporosis Prospective Risk Assessment (OPRA) study found that elevations of urine osteocalcin, serum CTX, and serum TRACP-5b were associated with any type of fracture in women (all p < 0.05 for clinical vertebral fractures)Citation24. Urine osteocalcin and serum TRACP-5b were also associated with hip fractures, at least one fracture, and multiple fractures (all p < 0.05) in these women. The Os des Femmes de Lyon (OFELY) study of healthy, untreated postmenopausal women reported a two-fold increase in relative risk for osteoporotic fractures in women with the highest baseline urinary CTX and serum CTX levels (relative risk 2.3 and 2.1, respectively; both p = 0.01), and this risk was maintained even after adjusting for body weight, height, and prevalent fracturesCitation25.
Data from the Multiple Outcomes of Raloxifene Evaluation (MORE) study also suggest a correlation between bone biomarkers and fracture riskCitation26. A subgroup analysis of 2722 women treated with raloxifene for 12 months found a greater decrease in vertebral fracture risk in those women who had significant reductions in serum osteocalcin (p = 0.003) and bone ALP (p = 0.005) from baselineCitation26. In another MORE report of raloxifene treatment for 3 years, the percent decrease in osteocalcin from baseline to 1 year was a better predictor of reduced vertebral fracture risk at 3 years than was the percent increase in femoral neck BMD at 1 year, regardless of whether women had a prevalent fracture at baseline; in fact, the 2-year percentage decrease in osteocalcin could account for 34% of the decrease in vertebral fracture riskCitation27.
If ospemifene has effects on bone biomarkers similar to those of estrogens and other approved SERMs in postmenopausal women, and given that changes in bone markers may predict protection from fracture, then these same beneficial effects of preventing bone loss and fracture risk may be seen in women taking ospemifene for relief of VVA. However, such a hypothesis would need to be tested and proven in rigorous, long-term phase 3 clinical studies monitoring fractures and BMD, similar to several studies that documented significant increases in BMD and decreases in fractures with raloxifene and bazedoxifene treatment versus placebo in postmenopausal women with osteoporosisCitation22,Citation28,Citation29. Preliminary subgroup analyses also suggest that ospemifene decreases values of bone resorption markers with numerically better effects than placebo, particularly in early menopause (≤5 years), a time of accelerated bone loss.
Conclusions
Bone biomarker data in conjunction with animal bone quality data suggest that ospemifene does have bone-protective properties, and, thus, may provide a potential additive bone benefit for women who take ospemifene to treat VVA. These data support further studies to evaluate bone protection with ospemifene in postmenopausal women.
Potential conflict of interest
T. J. de Villiers has been a speaker or advisory board member for Abbott, Adcock Ingram, Aspen, MSD, and Pfizer. C. Altomare is an employee of Shionogi, USA. M. Particco is an employee of Shionogi Europe. M. Gambacciani has received research support, grants, and/or occasional honoraria as a symposium speaker or advisory board member from Bayer, MSD, Gedeon Richter, Pfizer, Shionogi, and Theramex.
Source of funding
Shionogi sponsored the phase 3 study and funded the medical writing support provided by Laura Ninger, ELS and Kathleen Ohleth, PhD, CMPP (Precise Publications, LLC).
Acknowledgements
The authors would like to thank Laura Ninger, ELS and Kathleen Ohleth, PhD, CMPP for medical writing assistance.
References
- National Institute of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement 2000;17:1–45
- Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815–22
- Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014;29:2520–6
- Kanis JA, Johnell O, Oden A, et al. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 2000;27:585–90
- International Osteoporosis Foundation. Broken bones, broken lives: a roadmap to solve the fragility fracture crisis in Europe. 2018 [October 18, 2018]. Available from: http://share.iofbonehealth.org/EU-6-Material/Reports/IOF%20Report_EU.pdf
- Klop C, van Staa TP, Cooper C, Harvey NC, de Vries F. The epidemiology of mortality after fracture in England: variation by age, sex, time, geographic location, and ethnicity. Osteoporos Int 2017;28:161–8
- Zaheer S, LeBoff MS, Osteoporosis: Prevention and Treatment. November 2018 https://www.ncbi.nlm.nih.gov/books/NBK279073/.
- Evista® (raloxifene hydrochloride) Prescribing Information. Lilly S.A. Madrid, Spain. 2011
- Conbriza (bazedoxifene) Summary of Product Characteristics. Pfizer Pharmaceuticals. Ireland
- Osphena® [(ospemifene) tablets, for oral use] Prescribing Information. Shionogi Inc. Florham Park, NJ. January 2019
- Archer DF, Altomare C, Jiang W, Cort S. Effects of ospemifene on lipid and coagulation parameters in postmenopausal women. Menopause 2015;22:1375
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: a phase 3, randamized, double-blind placebo-controlled, multicenter trial. Menopause 2019;26:611–21
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause 2016;23:638–44
- Komi J, Heikkinen J, Rutanen EM, et al. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecol Endocrinol 2004;18:152–8
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006;24:314–8
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group (meeting held in Rome from Jun 22-25, 1992). 1994 [August 6, 2015]. Available from: https://apps.who.int/iris/handle/10665/39142
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology 2000;141:809–20
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids 2013;78:1273–80
- Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2007;23:525–35
- Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641–7
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637–45
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 2000;11:S2–17
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 2004;19:386–93
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000;15:1526–36
- Bjarnason NH, Sarkar S, Duong T, et al. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 2001;12:922–30
- Sarkar S, Reginster JY, Crans GG, et al. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res 2003;19:394–401
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res 2008;23:1923–34
- Palacios S, Silverman SL, de Villiers TJ, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause 2015;22:806–13
