Abstract
Objectives: This study aimed to assess the efficacy and safety of hyaluronic acid-based vaginal pessaries (Hydeal-D) in the treatment of vulvovaginal atrophy (VVA).
Study design: The study was a prospective, multicenter clinical investigation of VVA topical treatment in 40 postmenopausal women. Patients applied one Hydeal-D pessary every 3 days for 3 months.
Main outcome measures: The primary endpoint was the amelioration of VVA signs after treatment, evaluated by measuring the change from baseline of the Vaginal Health Index (VHI) score. Secondary endpoints included the evaluation of other VVA-related signs and symptoms, safety, and patient-reported and clinician-reported satisfaction and treatment tolerability.
Results: The 3-month treatment with Hydeal-D vaginal pessaries showed efficacy for all analyzed endpoints. Improvement exceeded threshold values of VVA diagnosis, sexual dysfunction, and distress, confirming clinically relevant amelioration of VVA symptoms. Changes from baseline conditions confirmed significant improvement of all parameters including the VHI, vaginal pH, patients’ perception of VVA symptoms, sexual function, and vaginal maturation. Patients’ overall satisfaction was very high after 1 month of treatment and increased further after 3 months. No severe adverse events were reported.
Conclusions: Significant amelioration of VVA-related signs indicates that Hydeal-D vaginal pessaries are an effective, safe, and well-tolerated non-hormonal therapeutic option for VVA in postmenopausal women.
摘要
目的: 本研究旨在评估透明质酸阴道栓(Hydeal-D)治疗外阴阴道萎缩(VVA)的疗效和安全性。
研究设计: 本研究对40名进行VVA局部治疗的绝经后妇女进行了前瞻性、多中心的临床研究。患者每三天应用一次Hydeal-D , 共用3个月。
主要结局测量:主要终点是治疗后VVA体征的改善, 通过测量与基线水平相比的阴道健康指数(VHI)评分变化进行评估。次要终点包括评估其他VVA相关体征和症状、安全性、患者报告和临床医生报告的满意度和治疗耐受性。
结果: 应用透明质酸阴道栓治疗三个月对所有分析的终点指标都有效。改善超过了VVA诊断、性功能障碍和痛苦的阈值, 证实了VVA症状的临床相关改善。相比于基线状况的改变证实了所有指标的显著改善, 包括VHI、阴道pH值、患者对VVA症状的感知、性功能和阴道成熟指数。治疗1个月后患者总体满意度很高, 且3个月后进一步提高。没有严重不良事件的报道。
结论:VVA相关体征的显著改善表明透明质酸阴道栓是治疗绝经后妇女VVA的一种有效、安全、耐受性良好的非激素治疗方案。
Introduction
Vulvovaginal atrophy (VVA) is a distressing medical condition which is an integral part of the genitourinary syndrome of menopauseCitation1. VVA occurs in about 50% of postmenopausal women because of ovarian exhaustion and aging phenomenaCitation2,Citation3. Other hypoestrogenism states associated with postpartum, oophorectomy, chemotherapy, and use of antiestrogen medications may lead to VVA symptomsCitation4. Some risk factors such as obesity, diabetes mellitus, smoking, no vaginal delivery, and decreased sexual activity have also been associated with VVACitation5. The most common symptoms include vaginal dryness and dyspareunia followed by irritation, burning, itching, discharge, urinary discomfort, and bleeding with intercourseCitation6. The burden of symptoms on women’s daily living is associated with typical signs detected during a physical examinationCitation7 and with laboratory tests, such as pH and the maturation indexCitation1,Citation8. Despite the increasing number of elderly women carrying the VVA condition, the rate of effective treatment is still unsatisfactoryCitation9 and recent data indicate a delay in the clinical management of VVA symptomsCitation10. Health-care providers should be proactive, given the plethora of options, including lifestyle modification and hormonal treatments as well as non-hormonal approaches, such as lubricants and moisturizers, and non-drug therapiesCitation11. Contraindications to estrogen use, fears of hormones, potential side effects of treatments, and attitudes of postmenopausal women to VVACitation9 explain the wide use of lubricants and moisturizers alone or in association with other optionsCitation12. Both osmolality and pH are important characteristics for lubricants and moisturizers giving temporary relief to vaginal dryness and rehydrating vaginal tissues over time, respectivelyCitation13.
A subgroup of vaginal moisturizers containing hyaluronic acid (HA), a high-molecular-weight polysaccharide, have been listed as one of the most effective strategies to help women with VVA symptoms who cannot or do not want to use hormonesCitation14. Indeed, HA represents a major component of the extracellular matrix that can bind large amounts of water, because of its particular molecular structure and propertiesCitation15. The properties of HA preparations are determined by their molecular weight, concentration, and molecular modifications, which largely determine their density, permeability, and mechanical properties. Due to the ubiquitous expression of HA in the human body and its biocompatibility, HA formulations have been widely used in a variety of medical applicationsCitation16.
Recently, vaginal pessaries containing Hydeal-D (Fidia Farmaceutici, Abano Terme, Italy), a HA ester characterized by strong hydrating properties, have been developed and registered as Hyalo Gyn/Hyalofemme vaginal ovules (Class 2A medical device). Hydeal-D vaginal pessaries are non-sterile, glyceride-based, solid preparations for single-dose vaginal administration, containing 0.2% Hydeal-D, indicated for the treatment of vaginal dryness of varying origin and in the natural healing process of friction-induced microlesions in the vaginal mucosa. The principal component, Hydeal-D, has been successfully employed in a gel formulation to reduce vaginal drynessCitation17 and to prevent sexual dysfunction in postmenopausal breast cancer survivors with VVACitation18. The Hyalo Gyn/Hyalofemme vaginal ovules are made from 90% vegetal glycerides, which create a wax-like texture and ensure low friction when inserting and removing the ovules through the VVA-affected vaginal mucosa.
The overall aim of this prospective, multicenter clinical investigation was to assess the efficacy and safety of Hydeal-D vaginal pessaries in the treatment of VVA in postmenopausal women. The primary objective was to evaluate the efficacy on vaginal health measured by the Vaginal Health Index (VHI) (vaginal elasticity, fluid volume, pH, mucosa epithelial integrity, and moisture)Citation19 after 3 months of treatment. Secondary aims included the evaluation of other VVA-related signs and symptoms, safety, and patient-reported and clinician-reported satisfaction and tolerability of the treatment.
Materials and methods
Study design
The present study was a prospective, multicenter, single-arm, open-label, 3-month clinical investigation exploring the efficacy and safety of a topical treatment on postmenopausal women with VVA attending two centers in Slovakia; the investigation lasted from November 2017 to April 2018 (first patient, first visit to last patient, last visit). The study was carried out in compliance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practices International Conference on Harmonization Guidelines, and the ISO14155 Clinical investigation of medical devices for human subjects – Good Clinical Practice. The study was approved by the local ethics committees (Approval no. CIV-SK-17-09-021684) and registered on www.clinicaltrials.gov (identifier: NCT03557398). All subjects provided signed informed consent.
Patients
During a screening visit, the following inclusion and exclusion criteria were addressed. Inclusion criteria were: postmenopausal state of the patients (≥12 months since last spontaneous menstrual period, or 6 months of spontaneous amenorrhea with serum FSH levels >40 IU/l, or surgically postmenopausal for >6 months), age from 45 to 75 years, vaginal pH ≥ 5, VHI ≤ 15Citation20, and at least one of the symptoms of VVA (vaginal dryness, vaginal and/or vulvar irritation/itching, dysuria, vaginal pain associated with sexual activity), assessed as moderate to severe.
Patients were excluded if they were treated with any kind of non-hormonal product for VVA in the week preceding the screening visit or with oral or topical hormonal products within 1 month before the screening visit. Other exclusion criteria were the presence of clinical signs of vaginal infections or a history of vulvovaginal contact allergy. Patients who were not sexually active or presented acute hepatopathy, embolic disorders, severe primary disease of the kidney and hematopoietic system, history of malignant tumors, or a hypersensitivity to any component of the Hydeal-D vaginal pessaries were also excluded from the study.
Patients enrolled in the study were asked not to apply any other vaginal topical hormonal product within 1 month and/or a non-hormonal product within 1 week, prior to starting the application of Hydeal-D pessaries.
Intervention
Hydeal-D vaginal pessaries were applied once every 3 days to a total of 12 consecutive weeks (3 months) and patients were visited at baseline (V1), 4 weeks post treatment (V2), and 12 weeks post treatment (V3).
At each visit, the following clinical evaluations were performed: VHI, vaginal pH, patient perception of vulvovaginal symptoms, and assessment of sexual function. V1 and V3 furthermore included the collection of a vaginal smear for the cytological evaluation of the Vaginal Maturation Index (VMI). V2 and V3 additionally recorded the patient’s overall satisfaction and local tolerability of the product at the application site, as well as the occurrence of any adverse events (AEs). Concomitant medications taken by the patient were recorded at all visits.
Endpoint assessments
The primary endpoint of the clinical investigation was the amelioration of the vaginal signs associated with VVA, evaluated by measuring the change from baseline of the average VHI scoreCitation19 after 3 months of treatment. During the visit, the investigator performed a quantitative assessment of vaginal health evaluating vaginal elasticity, fluid volume, pH, epithelial mucosa integrity, and moisture, on a scale ranging from 1 (none) to 5 (excellent), with a total cut-off score ≤ 15. The VHI assessment was performed, as a secondary endpoint, also after 1 month of treatment. Other secondary endpoints, evaluated as changes from baseline to 1 and 3 months, included the following: vaginal pH measured by inserting a pH test strip (pH 4.0–7.0; Merck KGaA, Darmstadt, Germany) in the upper wall of the vagina (patients were scored in subgroups according to their pH, considering that pH 5–5.49 indicates mild atrophy, pH 5.5–6.49 moderate atrophy, and pH > 6.5 severe atrophyCitation20); total score of patient’s perception of VVA-associated symptoms (dryness, irritation/itching, soreness, dysuria, dyspareunia), individually reported on a 4-point scale according to the severity of perceived symptoms (0 = absent, 1 = mild, 2 = moderate, 3 = severe), ranging from 0 to 15, as previously publishedCitation21,Citation22; sexual function assessed through the Female Sexual Function Index (FSFI) questionnaire, a mean weighed score based on questions related to patients’ sexual sensations focused on six domains (desire, arousal, lubrication, orgasm, satisfaction, and pain) and scored on a 5-point scale (0 = no sexual activity, 1 = never/very low, 5 = always/very high) (the cut-off score for the diagnosis of female sexual dysfunction is ≤26.55Citation23); and personal distress associated with sexual dysfunction assessed through the Female Sexual Distress Scale – Revised (FSDS-R), a 13-item questionnaire where the threshold for female sexual distress corresponds to an overall score ≥ 11Citation24. A further secondary endpoint included the change of the VMI from baseline to 3 months of treatment. The VMI was obtained from the cellular count of vaginal smears, and quantified as the relative proportion of three types of vaginal epithelium cells (parabasal, intermediate, and superficial) indicating the degree of tissue estrogenizationCitation25. The samples were fixed, stored, and sent to a centralized laboratory (Cytophatos, spol. s.r.o., Bratislava, Slovakia) for staining (Papanicolau technique) and analysis. The VMI was calculated according to the following equation: VMI = [1 (% superficial cells)] + [0.6 (% intermediate cells)] + [0.2 (% parabasal cells)]. Other endpoints were: the patient’s global assessment of overall satisfaction (PTGA) scored on a 4-point scale (0 = dissatisfied or very dissatisfied, 1 = moderately satisfied or satisfied, 2 = very satisfied, and 3 = greatly satisfied); local tolerability of the product at the application site, evaluated after 1 and 3 months of treatment by both the clinician and the patient using a 5-point scale (1 = excellent [no reaction], 2 = good [small reaction that spontaneously resolved], 3 = moderate [reaction tolerated with difficulty by the subject], 4 = poor [reaction needing interruption of treatment], 5 = bad [serious reaction]); and safety of the treatment, evaluated by collecting all AEs during the studyCitation26.
Statistical analysis
The null hypothesis was that the lower limit of the 97.5% confidence interval of the VHI total score change from baseline to 3 months of follow-up was less than 20%. Under the formulated hypotheses, the study required a sample size of 37 patients to achieve a power of 90% and a level of significance of 5% (one-sided), assuming the standard deviation of the differences to be 40%. Assuming a dropout of no more than three subjects, it was estimated to recruit 40 postmenopausal women with VVA.
Subjects’ demographics and baseline characteristics were summarized using descriptive statistics. Frequencies and percentages were used for qualitative variables; mean ± deviation, minimum and maximum value (range), median, and first and third quartiles (interquartile range) were used for quantitative variables.
Differences in scores before and after treatment were calculated and the Shapiro–Wilk test was used to verify the normality of their distribution. A paired Student’s t-test was used to test differences in scores with a normal distribution, and the Wilcoxon signed-rank test used for non-normal distributions and for non-parametric values. All statistical tests were considered significant if inferior to 0.05 (p < 0.05).
Results
Forty postmenopausal women (mean age, 57.1 ± 5.9 years) diagnosed with VVA were enrolled in the study, and all patients completed the study. Demographics and baseline characteristics are summarized in .
Table 1. Patients’ demographics and baseline characteristics (N = 40).
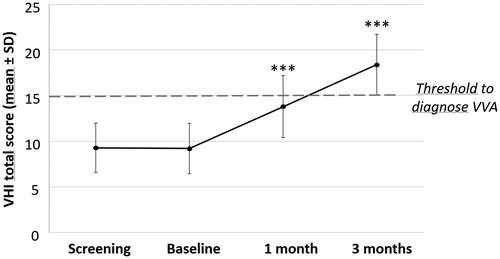
Scores and percentage changes in overall VHI, the primary endpoint of this study, are shown in and Supplementary Table 1. The mean VHI total score increased significantly (p < 0.0001) from 9.2 ± 2.74 at baseline to 18.4 ± 3.32 at 12 weeks of treatment (). The percentage change from baseline (111.9 ± 55.24% after 3 months) by far exceeded the expected mean VHI increase of 20% set for the sample size calculation. Significance of the VHI percentage change from baseline to month 3 was confirmed by repeated-measures analysis of variance (p < 0.0001) considering the baseline score as a covariate and thus confirming the efficacy of Hydeal-D over time. The single VHI item category changes from baseline considerably increased during 1 and 3 months of treatment (p < 0.0001) and at least 90% of patients improved in ≥1 item category after 3 months (Supplementary Table 1). The number of patients with an overall VHI score ≤ 15, the threshold value for VVA diagnosis, decreased from 100% at screening and baseline visits to 50% and 20% at the 4-week and 12-week follow-up visits, respectively.
Figure 1. Effect of Hydeal-D vaginal pessaries on the Vaginal Health Index (VHI) of postmenopausal women. VHI scores, expressed as mean ± standard deviation (SD), evaluated in 40 patients at screening, at baseline, and at 1 and 3 months of treatment are presented. Significance levels were calculated at 1 and 3 months of treatment compared to baseline (***p < 0.0001, signed-rank test) and confirmed by repeated-measures analysis of variance. VVA, vulvovaginal atrophy.
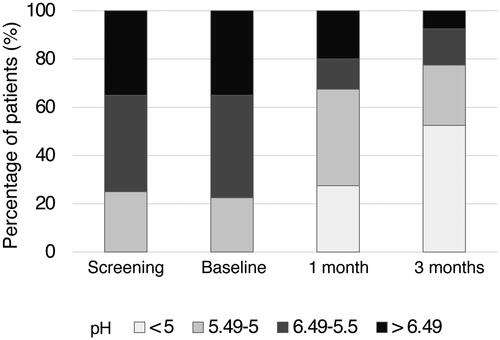
The analysis of the secondary endpoints showed that vaginal pH, which is an indicator of VVA, significantly improved both after 1 and 3 months of Hydeal-D treatment (p < 0.0001, signed-rank test) The subgroup of patients with vaginal pH < 5 increased from no patient at baseline to 11 patients (27.5%) and 21 patients (52.7%) after 1 and 3 months of treatment, respectively. Accordingly, the number of patients in the subgroup with pH > 6.49 decreased from 14 patients (35%) at baseline to eight patients (20%) at month 1 and three patients (7.5%) at month 3 ( and Supplementary Table 2). Patients with a pH score improvement of at least one grade numbered 27 (67.5%) after 1 month and 36 (90%) after 3 months of treatment ( and Supplementary Table 2). Only three patients with pH > 6.49 showed no decrease in pH after 3 months but displayed improvement in the other signs and symptoms of VVA.
Figure 2. Significant decrease in vaginal pH in postmenopausal women treated with Hydeal-D vaginal pessaries. Vaginal pH changes were assessed in 40 postmenopausal women at screening, at baseline, and after 1 or 3 months of treatment with Hydeal-D. The percentage of patients in four different pH subgroups at all visits is reported. Significance levels were calculated by signed-rank test at 1 and 3 months compared to baseline. Differences were considered significant with p < 0.05. ***p < 0.0001.
The mean total score for patient’s VVA symptom perception improved significantly (p < 0.0001), decreasing from 7.0 ± 2.45 at baseline to 2.5 ± 2.09 and 0.5 ± 0.85 after 1 and 3 months, respectively (). The analysis of single items showed a significant reduction (p < 0.0001) during both 1 and 3 months of treatment. Patient’s perception of vaginal dryness improved in at least one score in 97.5% of patients ().
Table 2. Summary statistics for the total score of patients’ perception of vulvovaginal symptoms (N = 40).
Table 3. Single symptom score change of patients' perception of vulvovaginal symptoms (N = 40).
Results from the FSFI questionnaire showed that the patients’ perception of sexual function improved significantly after 1 month (p = 0.0142) and 3 months (p < 0.0001) of using the Hydeal-D vaginal pessaries. The FSFI mean full scale score value of 22.5 at baseline increased to 24.7 at 1 month and to 28.5 at 3 months. A mean full scale value lower than 26.55, the female sexual dysfunction cut-off value, was reported by 67.5% of patients at baseline and decreased to 47.5% and 30% after 1 month and 3 months, respectively. Female sexual distress was reduced from 14.8 at baseline to 13.6 (p = 0.0963) after 1 month and to 8.6 (p < 0.0001) after 3 months of treatment, with an increase in the number of patients reporting values lower than the cut-off value for personal distress of 11, from 47.5% at baseline to 55% and 70% of at 1 month and 3 months, respectively (Supplementary Table 3).
Furthermore, the mean VMI increased significantly (p = 0.0022) from 54.6 at baseline to 62.8 at month 3. Interestingly, the increase in the percentage of superficial cells was not significant during 3 months of treatment, whereas a significant improvement was observed in parabasal cells (decrease from 31.3% at baseline to 14.8% at month 3; p = 0.0004). For intermediate cells, the increase from 51.8% at baseline to 63.5% at month 3 was also significant (p = 0.0187) (Supplementary Table 4).
Patients’ overall satisfaction was very high, as 25 patients (62.5%) were greatly satisfied and 14 patients (35.0%) were satisfied to very satisfied with the treatment after 3 months. Only one patient (2.5%) was dissatisfied or very dissatisfied after 3 months of treatment. Thirty-seven patients (92.5%) positively appreciated study treatment already after 1 month and the number of patients with higher satisfaction grade further increased to month 3 (Supplementary Table 4).
Local tolerability of the product at the application site was evaluated as excellent (no reaction) by 100% of the investigators in all patients and at all time points.
Excellent local tolerability was reported by all patients at both follow-up visits, except for two patients presenting vulvovaginal erythema reported as an AE and who evaluated local tolerability as good – one patient at month 1 and the second patient at month 3. During the study, three AEs were reported in two patients; two AEs were mild vulvovaginal erythema, the first was related and the second possibly related to the study device, according to the investigators. The third AE was reported as mild, and not related to the study device in one of the two patients who experienced vulvovaginal erythema. No severe AE was reported and no patient discontinued her participation due to an AE.
Discussion
The present prospective, multicenter clinical investigation demonstrated that 3-month treatment with the HA-based Hydeal-D vaginal pessaries was safe and effective in ameliorating postmenopausal VVA signs and symptoms. Improvement exceeded, in some patients, the thresholds for diagnosis of VVA and sexual dysfunction. Efficacy was confirmed by significant changes in all evaluated parameters: VHI, vaginal pH, patient perception of vaginal dryness, dyspareunia, irritation/itching, soreness, dysuria, sexual function and distress, and VMI. Interestingly, a statistically relevant improvement was observed already after 1 month of treatment, but an even more relevant clinical improvement was evident for some parameters at 3 months of treatment. Patients’ overall satisfaction with the treatment was very high, and tolerability and safety were also high.
In spite of the severe impact on quality of life and sexual well-being of postmenopausal women, VVA is still greatly underdiagnosed and undertreatedCitation3. Due to the concerns related to hormonal-based topical treatments, non-hormonal vaginal strategies are widely usedCitation27, with moisturizers showing some therapeutic effectCitation28. Since the water-retaining capacity of vaginal tissues is decreased during menopause and HA is naturally excreted by vaginal tissues, HA-based preparations represent a promising strategy to correct hydration and improve clinical symptoms and signs related to VVACitation17,Citation18. A previous prospective observational studyCitation29 described an improvement of the mean VHI from 9.65 at baseline to 19.96 after 8 weeks of topical vaginal treatment with an HA-based liquid preparation, a finding similar to our present results. A placebo-controlled, short-term studyCitation30 showed that daily use of Hydeal-D vaginal gel was more effective in reducing symptoms of VVA, whereas the safety and tolerability were similar in both treatment arms. When HA-based preparations were compared with local estrogen treatments, the results were mixedCitation17,Citation31,Citation32, but indicated that both treatments provided relief of vaginal symptoms, improved epithelial atrophy, decreased vaginal pH, and increased VMI. Interestingly enough, even though no head-to-head trials have been performed, the short-term trophic effect of HA-based Hydeal-D vaginal pessaries seems to be similar to that observed with other non-hormonal approaches, such as laser therapiesCitation33,Citation34 and even the use of serotonin reuptake inhibitorsCitation35. Thus, it seems likely that any strategies ameliorating discrete parameters of the functional anatomy of the vagina, such as vasodilation, collagenesis, and cellularity of the extracellular matrix, may be effective in treating VVA signs and symptoms. However, we are still in need of long-lasting and comparative data; most importantly, we lack a real decision-making algorithm to establish a tailored approach to women’s expectations and to the type and severity of specific complaints associated with VVACitation36.
Even the restoration of sexual function and the decrease of sexual distress with HA-based Hydeal-D vaginal pessaries is of note, given the high rate of sexual dysfunction associated with VVACitation37.
The present study provides an overall evaluation of the multifactorial signs and symptoms of VVA ranging from objective measures of the vaginal morphology to subjective patient-reported symptoms and perception of sexual function. In addition, the global improvement of these numerous primary and secondary endpoints with a follow-up of 3 months further corroborates the study results. Limitations of this study include the absence of randomization and a control arm, as well as the fact that treatment was not blinded either for the investigator or for the patient. Indeed, the lack of a control arm and the lack of blinding do not allow one to exclude a placebo effect or the effect of other confounding factors from the observed improvements in VVA-associated symptoms.
In conclusion, this clinical investigation suggests that Hydeal-D vaginal pessaries could be a safe, well-tolerated, and effective non-hormonal therapeutic option for treatment of VVA in postmenopausal women, thus offering a further topical application choice for the treatment of this common condition in clinical practice. Future randomized controlled studies should be carried out to further confirm the results reported in the present study.
Potential conflict of interest
No potential conflict of interest was reported by the authors.
Source of funding
This study was funded by Fidia Farmaceutici SpA.
Supplemental Material
Download MS Word (44.4 KB)References
- Portman DJ, Gass MLS; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Climacteric 2014;17:557–63
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013;5:437–47
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric 2014;17:3–9
- Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000;61:3090–6
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Julia MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005;52:S46–S52
- Palacios S, Nappi RE, Bruyniks N, Particco M, Panay N; EVES Study Investigators. The European Vulvovaginal Epidemiological Survey (EVES): prevalence, symptoms and impact of vulvovaginal atrophy of menopause. Climacteric 2018;21:286–91
- Nappi RE, Palacios S, Bruyniks N, Particco M, Panay N; EVES Study Investigators. The burden of vulvovaginal atrophy on women’s daily living: implications on quality of life from a face-to-face real-life survey. Menopause 2019;26:485–91
- Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010;13:509–22
- Krychman M, Graham S, Bernick B, Mirkin S, Kingsberg SA. The Women’s EMPOWER Survey: women’s knowledge and awareness of treatment options for vulvar and vaginal atrophy remains inadequate. J Sex Med 2017;14:425–33
- Panay N, Palacios S, Bruyniks N, Particco M, Nappi RE; EVES Study investigators. Symptom severity and quality of life in the management of vulvovaginal atrophy in postmenopausal women. Maturitas 2019;124:55–61
- Naumova I, Castelo-Branco C. Current treatment options for postmenopausal vaginal atrophy. Int J Womens Health 2018;10:387–95
- Sinha A, Ewies AA. Non-hormonal topical treatment of vulvovaginal atrophy: an up-to-date overview. Climacteric 2013;16:305–12
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric 2016;19:151–61
- Donders GGG, Ruban K, Bellen G, Grinceviciene S. Pharmacotherapy for the treatment of vaginal atrophy. Expert Opin Pharmacother 2019;20:821–35
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997;242:27–33
- Salwowska NM, Bebenek KA, Żądło DA, Wcisło-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol 2016;15:520–6
- Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med 2013;10:1575–84
- Advani P, Brewster AM, Baum GP, Schover LR. A pilot randomized trial to prevent sexual dysfunction in postmenopausal breast cancer survivors starting adjuvant aromatase inhibitor therapy. J Cancer Surviv 2017;11:477–85
- Bachmann GA, Notelovitz M, Kelly SJ, Thompson C, Owens A. Long-term nonhormonal treatment of vaginal dryness. Clin Pract Sex 1992;8:12–17
- Weber MA, Limpens J, Roovers JP. Assessment of vaginal atrophy: a review. Int Urogynecol J 2015;26:15–28
- Nappi RE, Cagnacci A, Becorpi AM, et al. Monurelle Biogel(®) vaginal gel in the treatment of vaginal dryness in postmenopausal women. Climacteric 2017;20:467–75
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause 2008;15:885–9
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208
- Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med 2008;5:357–64
- Van der Laak JA, Schijf CP, Kerstens HM, Heijnen-Wijnen TH, de Wilde PC, Hanselaar GJ. Development and validation of a computerized cytomorphometric method to assess the maturation of vaginal epithelial cells. Cytometry 1999; 35:196–202
- Nappi RE, Benedetto C, Campolo F, et al. Efficacy, tolerability and safety of a new medical device, Monurelle Biogel(®) vaginal gel, in the treatment of vaginal dryness: a randomized clinical trial in women of reproductive age. Eur J Obstet Gynecol Reprod Biol 2016; 203:82–8
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) – results from an international survey. Climacteric 2012;15:36–44
- Shifren JL. Genito-urinary syndrome of menopause. Clin Obstet Gynecol 2018;61:508–16
- Origoni M, Cimmino C, Carminati G, et al. Postmenopausal vulvovaginal atrophy (VVA) is positively improved by topical hyaluronic acid application. A prospective, observational study. Eur Rev Med Pharmacol Sci 2016;20:4190–5
- Grimaldi EF, Restaino S, Inglese S, et al. Role of high molecular weight hyaluronic acid in postmenopausal vaginal discomfort. Minerva Ginecol 2012;64:321–9
- Ekin M, Yaşar L, Savan K, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet 2011;283:539–43
- Jokar A, Davari T, Asadi N, Ahmadi F, Foruhari S. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery 2016;4:69–78
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric 2014;17:363–9
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015;18:757–63
- Shea AK, Meschino D, Wolfman W. The effect of serotonin reuptake inhibitors on the vaginal epithelium in postmenopausal women. Climacteric 2019;22:507–10
- Nappi RE, Martini E, Cucinella L, et al. Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for healthy aging in women. Front Endocrinol (Lausanne) 2019;10:561
- Nappi RE, Cucinella L, Martella S, Rossi M, Tiranini L, Martini E. Female sexual dysfunction (FSD): prevalence and impact on quality of life (QoL). Maturitas 2016;94:87–91

