Abstract
Uterine bleeding is a common reason why women discontinue menopausal hormone therapy (HT). This systematic review compared bleeding profiles reported in studies for continuous-combined HT approved in North America and Europe for moderate to severe vasomotor symptoms in postmenopausal women with a uterus. Non-head-to-head studies showed that uterine bleeding varies by formulation and administration route, with oral having a better bleeding profile than transdermal formulations. Cumulative amenorrhea over a year ranged from 18 to 61% with oral HT and from 9 to 27% with transdermal HT, as reported for continuous-combined HT containing 17β-estradiol (E2)/progesterone (P4) (56%), E2/norethisterone acetate (NETA) (49%), E2/drospirenone (45%), conjugated equine estrogens/medroxyprogesterone acetate (18–54%), ethinyl estradiol/NETA (31–61%), E2/levonorgestrel patch (16%), and E2/NETA patch (9–27%). Amenorrhea rates and the mean number of bleeding/spotting days improved over time. The oral E2/P4 combination was amongst those with lower bleeding rates and may be an appropriate alternative for millions of women seeking bioidentical HT and/or those who have bleeding concerns with other HT.
摘要
子宫出血是妇女中断绝经激素治疗(HT)的常见原因。本系统性综述比较了北美和欧洲批准的有子宫的绝经后女性中重度血管舒缩症状连续联合HT治疗药物在研究中报道的出血情况。非面对面的研究表明, 子宫出血因制剂和给药途径的不同而不同, 口服比经皮制剂更容易出血。闭经超过一年的人中, 大约为18%-61%采用口服HT, 9%-27%采用经皮HT治疗。据报道采用的连续联合HT治疗有17β-雌二醇(E2)/孕酮(P4)(56%), E2/醋酸炔诺酮(NETA)(49%)、E2/屈螺酮(45%)、马结合雌激素/醋酸甲羟孕酮(18-54%)、炔雌醇/NETA(31%-61%)、E2/左炔诺孕酮贴片(16%)和E2/NETA贴片(9-27%)。闭经率和和平均出血/点滴出血天数随时间而改善。口服E2/P4组合是出血率较低的一组药物之一, 可能对于追求HT的女性和/或其他HT引起出血的女性的合适的选择。
Introduction
Menopausal hormone therapy (HT) is used to treat postmenopausal hot flushes, to treat symptoms associated with vulvovaginal atrophy, and to prevent bone loss. While therapy with estrogen alone is effective in relieving these symptoms, addition of a progestogen is necessary for women with an intact uterus to counteract estrogen-induced endometrial stimulation, protecting the endometriumCitation1. However, endometrial breakthrough bleeding with HT often occurs in postmenopausal women due to the addition of a progestogenCitation2.
Undesired uterine bleeding can interfere with women’s daily activities and negatively impact their physiological well-being, reducing their quality of lifeCitation3. Uterine bleeding is also one of the major contributors to the discontinuation of HTCitation4. In a large, 1-year, international study assessing the effects of combined HT on health-related quality of life, uterine bleeding was the most common reason for discontinuation, accounting for 32% of women who prematurely withdrew from the studyCitation5. Postmenopausal women presenting with uterine bleeding ≥6 months after initiating combined HT should also be assessedCitation1. However, this often necessitates endometrial evaluation, as well as other clinical assessmentsCitation6, increasing the use of health-care resources and causing anxiety in women. Tolerability of an HT with regard to uterine bleeding is thus important, as adherence to the treatment is crucial for taking full advantage of HT benefitsCitation7.
A variety of continuous-combined HT preparations with different formulations, progestogens, and dosages has been developed to treat postmenopausal symptoms with acceptable tolerability to improve compliance. This systematic review evaluated uterine bleeding profiles of oral and transdermal continuous-combined HT products that are available in North America and Europe for treating moderate to severe vasomotor symptoms in postmenopausal women with a uterus.
Methods
A list of continuous-combined, estrogen-progestogen HT products approved in North America and in Europe with indications for the treatment of moderate to severe vasomotor symptoms or estrogen deficiency symptoms in postmenopausal women with an intact uterus was compiled. Prescribing information or summaries of product characteristics for these approved products were obtained. A systematic literature search generally following the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was performed (). English-language studies were identified using the Medline database (PubMed) with the keywords of menopause and bleeding combined with conjugated estrogen or estradiol. Interventional studies, including randomized controlled and non-comparative trials, of approved formulations were included in this review.
Bleeding data collected over 1 year (≥48 weeks, or 12–13 cycles of 28–30 days) from comparative or separate studies of continuous-combined oral or transdermal HT (estrogen plus progestogen) with at least 25 women per treatment group were summarized. Cumulative amenorrhea and other bleeding data were also collected from relevant prescribing information or summaries of product characteristics. Amenorrhea was defined as no bleeding or spotting. Spotting was defined as not requiring sanitary protection, while bleeding required sanitary protection. The primary goal of this review was to compare cumulative amenorrhea rates (proportions of women remaining amenorrheic) from cycle 1 to cycle 12/13 and during the last cycle or quarter of 1 year as reported in comparative studies or separate studies for different HT products. Other data reviewed included the mean number of bleeding/spotting days and discontinuation rates due to irregular bleeding.
Results
A list of continuous-combined HT products approved for treatment of postmenopausal symptoms in North America and Europe is summarized in . Estrogens in these products include 17β-estradiol (E2), conjugated equine estrogens (CEE), ethinyl estradiol (EE), and estradiol valerate (E2V), while progestogens include progesterone (P4), norethindrone acetate or norethisterone acetate (NETA), dydrogesterone (DYD), drospirenone, medroxyprogesterone acetate (MPA), levonorgestrel, and dienogest. The majority of the products are oral HT formulations (). All continuous-combined formulations reviewed contain synthetic progestogens (progestins) except for one product containing E2/P4.
Table 1. Approved, continuous-combined hormone therapy products for the treatment of vasomotor symptoms in North America and Europe.
Forty-five studies met the inclusion criteria and were included in the review ()Citation8–52. Most of them were randomized trials with an active control (n = 25)Citation8–32, followed by placebo-controlled studies (n = 14)Citation33–46 and non-comparative studies (n = 6)Citation47–52. The majority were not head-to-head studies of the HT combinations compared in this review. Bleeding data reported in the included studies and product prescribing information/summaries of product characteristics ()Citation8–63 were reviewed.
Table 2. Summary of bleeding data with approved continuous-combined hormone therapy.
Cumulative amenorrhea rates from cycle 1 to cycle 12/13
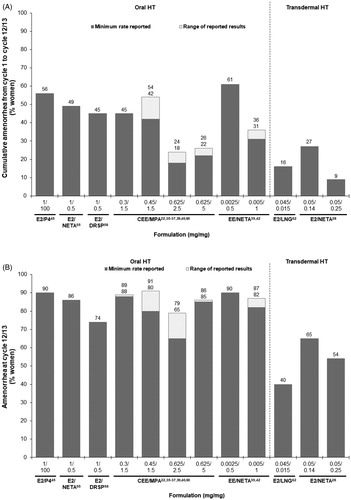
Cumulative amenorrhea rates over 1 year were reported for 12 HT combinations ()Citation22,Citation28,Citation35–40,Citation42,Citation45,Citation55,Citation58,Citation60,Citation62. The rates were generally lower with transdermal HT (9−27%) than oral HT (18−61%) in non-head-to-head studies. The highest cumulative amenorrhea rates were reported for oral 1 mg/100 mg E2/P4Citation45 and 0.0025 mg/0.5 mg EE/NETACitation42 (). In addition, women using lower doses of hormones had higher rates of cumulative amenorrhea (), as observed in studies comparing different doses of CEE/MPACitation35,Citation60, EE/NETACitation42, and transdermal E2/NETACitation28. Cumulative amenorrhea rates with continuous-combined HT started to improve as early as cycle 2 or 3, and continued to increase over time, with the highest rates observed in the last cycle or quarterCitation22,Citation28,Citation35–37,Citation39,Citation40,Citation42,Citation45,Citation55,Citation58,Citation60,Citation62.
Figure 2. Non-head-to-head trial comparison of reported (A) cumulative amenorrhea rates from cycle 1 to cycle 12/13 and (B) amenorrhea rates at cycle 12/13 with continuous-combined hormone therapy (HT). Numbers labeled above bars indicate the minimum (lower) and maximum (upper) reported by different studies, with a single number indicating only one percentage being reported. CEE, conjugated equine estrogens; DRSP, drospirenone; E2, 17β-estradiol; EE, ethinyl estradiol; LNG, levonorgestrel; MPA, medroxyprogesterone acetate; NETA, norethindrone acetate or norethisterone acetate; P4, progesterone.
Amenorrhea rates at cycle 12/13
Oral HT showed a better bleeding profile than most of the transdermal HT formulations. In studies reporting cumulative amenorrhea, the observed amenorrhea rates at cycle 12/13 ranged from 65 to 91% for oral HT and from 40 to 65% for transdermal HT (). High amenorrhea rates (≥90%) at cycle 12/13 were achieved with oral HT of 1 mg/100 mg E2/P4Citation45, 0.45 mg/1.5 mg CEE/MPACitation37, and 0.0025 mg/0.5 mg EE/NETACitation42, close to the rates observed with placebo in the same studies ().
Overall amenorrhea
Several studies reported percentages of women who had no bleeding or spotting over a year (overall amenorrhea); however, overall, amenorrhea was not clearly defined in the studies and could not be assumed to be cumulative amenorrhea. Relatively high percentages of women reported overall amenorrhea with 0.5 mg/2.5 mg E2/DYDCitation21,Citation52, 1 mg/5 mg E2/DYDCitation21, and 0.025 mg/0.125 mg E2/NETA patchCitation15,Citation43 (). The study comparing different doses of transdermal E2/NETA also showed a higher rate of overall amenorrhea with the lower doseCitation15.
Mean number of bleeding/spotting days
The majority of studies reported a reduction in the mean number of bleeding/spotting days over time with HT (). Most of the reported numbers were ≤2 during the last cycleCitation19,Citation21,Citation25,Citation26,Citation30,Citation39,Citation49,Citation52,Citation54 or ≤5 during the last quarterCitation11,Citation34,Citation38,Citation54, while longer durations were observed for doses of both oral E2/NETACitation13 and transdermal 0.05 mg/0.17 mg E2/NETACitation29. Less than 1 day of bleeding/spotting at the end of 1 year was reported for 1 mg/100 mg E2/P4Citation54, 0.5 mg/2.5 mg E2/DYDCitation21,Citation52, 1 mg/5 mg E2/DYDCitation21, 0.005 mg/1 mg EE/NETACitation39, all three doses of E2V/MPACitation25,Citation26, and transdermal 0.05 mg/2.5 mg E2/NETACitation30.
Discontinuation due to bleeding
Study discontinuation rates due to irregular bleeding were in the range of 0.2–30% for 14 HT combinations in separate studies (). Some studies reported a <2% rate of discontinuation due to bleeding for six HT combinations, including oral 1 mg/100 mg E2/P4Citation45, 1 mg/0.5 and 2 mg/1 mg E2/NETACitation8,Citation10,Citation11,Citation15,Citation48, 0.5 mg/0.25 mg E2/drospirenoneCitation12, 0.5 mg/2.5 mg E2/DYDCitation52, and transdermal 0.025 mg/0.125 mg E2/NETACitation15,Citation43.
Discussion
This systematic review of uterine bleeding data reported over 1 year shows that bleeding improves over time with HT use, and that oral HT provides better bleeding control than transdermal HT. In non-head-to-head studies, oral 1 mg/100 mg E2/P4 and 0.0025 mg/0.5 mg EE/NETA were found to be associated with a better bleeding profile as compared with some of the other oral combined HT or transdermal HT formulations containing progestin. Uterine bleeding begins to decrease after two or three cycles of HT use and the number of women remaining amenorrheic gradually increases over time.
Non-head-to-head trial comparisons revealed that generally more women stayed amenorrheic throughout 1 year with oral HT compared with transdermal HT. While cumulative amenorrhea rates with oral HT ranged from 18 to 61%, all of the reported rates with transdermal HT were less than 30%. Higher incidence of uterine bleeding with most of the transdermal formulations would possibly limit the choices of a transdermal HT with acceptable tolerability with regard to bleeding. Even with the low-dose transdermal E2/NETA (0.025 mg/0.125 mg), a somewhat higher discontinuation rate versus oral 1 mg/0.5 mg E2/NETA was reported when compared directly in a randomized trial (3.6% vs. 1.8%)Citation11.
A combination of transdermal estrogen and oral P4, although not regulatory approved, is commonly prescribed in some countriesCitation64. However, no studies properly addressing the bleeding profile or endometrial safety of continuous-combined transdermal estrogen and oral P4 were identified in our search.
Incidence of bleeding varies by HT formulationCitation65. In non-head-to-head studies, oral E2/P4 was associated with a better bleeding profile compared with most of the other oral or transdermal HT formulations containing progestin. Although the exact mechanism remains unclear, it has been suggested that some progestogens induce changes in the structure of the microvasculature of the endometrium causing endometrial bleedingCitation2,Citation66. In vitro studies demonstrated that P4 has less impact on the delicate ratio among angiogenic/anti-angiogenic factors in the endometrial glands and stroma compared with progestinsCitation67,Citation68. Several studies included in this review also demonstrated more favorable bleeding profiles with lower doses of hormonesCitation15,Citation35,Citation42,Citation60, despite similar ratios of estrogen/progestogen being used in some studiesCitation15,Citation42.
The incidence of uterine bleeding usually decreased over time with continuous-combined HT. HT discontinuation due to bleeding also occurred more often within the first 6 months of useCitation10,Citation15,Citation25. Additionally, bleeding during the early cycles of treatment could be predictive of bleeding in subsequent cycles, as it was shown that the majority of women with bleeding earlier in studies were more likely to report bleeding during subsequent cyclesCitation29,Citation69.
Time since menopause was another factor associated with uterine bleeding incidence. Studies found that bleeding was less frequently reported in postmenopausal than perimenopausal womenCitation70,Citation71. Cumulative amenorrhea rates were generally higher in women further from menopause than in those closer to menopauseCitation35. Significant associations of amenorrhea with age and time since menopause were also found for the E2/P4 formulation, showing less bleeding in older women or for women with a longer time from menopauseCitation45.
The present review has some limitations as it is a cross-study comparison. Methodologies adopted for bleeding data analysis in each study were not consistent. Not all studies calculated a cumulative amenorrhea rate over 1 year and some of the oral HT formulations (0.5 mg/0.1 mg E2/NETA, E2V/dienogest, and all doses of E2V/MPA) were therefore excluded from the comparisons of amenorrhea rates. Amenorrhea rates were evaluated in differently defined populations, such as intent-to-treat, safety, completer, or efficacy-evaluable populations among studies. The mean number of bleeding/spotting days was analyzed in women reporting bleeding/spotting events in some studies, while this was measured in all women in other studies. Such inconsistencies between studies could limit data interpretations in this review. In addition, bleeding data with transdermal HT were limited as the majority of the approved products were oral HT and fewer studies were on transdermal than oral combinations. Strengths of this review include the fact that it provides a comprehensive overview of irregular bleeding with continuous-combined HT. Moreover, this review provides value with comparisons among non-head-to-head trials from different publications.
Conclusion
Comparison of published bleeding data with various continuous-combined HT in separate studies shows that oral HT formulations in general result in higher amenorrhea rates than most transdermal HT formulations. Additionally, amenorrhea rates were mostly higher with lower doses of hormones within the same hormone combinations. Lastly, uterine bleeding decreased over time as shown by an increase in amenorrhea rates and a decrease in the mean numbers of bleeding/spotting days over time, demonstrating improvements with persistent use of continuous-combined HT. The oral E2/P4 combination was amongst those with the lowest bleeding rates and may be an appropriate alternative for millions of women seeking bioidentical HT and/or those who have bleeding concerns with other HT.
Potential conflict of interest
J.H.P. has served as a consultant to Pfizer, Shionogi, Sojournix, and TherapeuticsMD; and has stock options with TherapeuticsMD. D.F.A. has served as a consultant to AbbVie, Actavis, Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis, InnovaGyn, Merck, Pfizer, Radius Health, Sermonix, Shionogi, Teva Women’s Healthcare, and TherapeuticsMD; and has received research support from Actavis, Bayer Healthcare, Endoceutics, Glenmark, Merck, Radius Health, Shionogi, and TherapeuticsMD. S.R.G. has served as a consultant to Cook ObGyn, Cooper Surgical, and IBSA; is on the advisory board of AbbVie, AMAG, and TherapeuticsMD; and has also served on the speaker’s bureau of AMAG, Duchesnay, and TherapeuticsMD. R.K. has served as a consultant to Amgen, Astellas, Cooper Surgical, Jazz Pharma, Lupin, Radius Health and TherapeuticsMD; and also serves on the speaker’s bureau of AMAG, Cooper Surgical, and TherapeuticsMD. B.B. and S.M. are employees of TherapeuticsMD with stock/stock options.
Acknowledgements
The authors would like to acknowledge the medical writing assistance of H. Zhang, PhD, D. Verlaan, PhD, and K. Ohleth, PhD, of Precise Publications, LLC.
Additional information
Funding
References
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24:728–53
- Archer DF. Endometrial bleeding in postmenopausal women: with and without hormone therapy. Menopause 2011;18:416–20
- Arbuckle R, Humphrey L, Abraham L, et al. Qualitative cross-cultural exploration of vaginal bleeding/spotting symptoms and impacts associated with hormone therapy in post-menopausal women to inform the development of new patient-reported measurement tools. Maturitas 2014;78:219–27
- Newton KM, Reed SD, Nekhyludov L, et al. Factors associated with successful discontinuation of hormone therapy. J Womens Health (Larchmt) 2014;23:382–8
- Welton AJ, Vickers MR, Kim J, et al. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ 2008;337:a1190–a1190
- Munro MG, Southern California Permanente Medical Group's Abnormal Uterine Bleeding Working Group. Investigation of women with postmenopausal uterine bleeding: clinical practice recommendations. Perm J 2014;18:55–70
- Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging 2007;24:37–55
- Archer DF, Dorin MH, Heine W, Nanavati N, Arce JC. Uterine bleeding in postmenopausal women on continuous therapy with estradiol and norethindrone acetate. Endometrium Study Group. Obstet Gynecol 1999;94:323–9
- Yildirim G, Tugrul S, Uslu H, Pekin O, Eren S. Effects of two different regimens of continuous hormone replacement therapy on endometrial histopathology and postmenopausal uterine bleeding. Arch Gynecol Obstet 2006;273:268–73
- Hammar ML, van de Weijer P, Franke HR, Pornel B, von Mauw EM, Nijland EA. Tibolone and low-dose continuous combined hormone treatment: vaginal bleeding pattern, efficacy and tolerability. BJOG 2007;114:1522–9
- Samsioe G, Dvorak V, Genazzani AR, et al. One-year endometrial safety evaluation of a continuous combined transdermal matrix patch delivering low-dose estradiol-norethisterone acetate in postmenopausal women. Maturitas 2007;57:171–81
- Genazzani AR, Schmelter T, Schaefers M, Gerlinger C, Gude K. One-year randomized study of the endometrial safety and bleeding pattern of 0.25 mg drospirenone/0.5 mg 17β-estradiol in postmenopausal women. Climacteric 2013;16:490–8
- Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N, Trimegestone 3, Study G. Bleeding profile and endometrial safety of continuous combined regimens 1 mg 17beta-estradiol/trimegestone versus 1 or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol 2005;21:142–8
- Hammar M, Christau S, Nathorst-Boos J, Rud T, Garre K. A double-blind, randomised trial comparing the effects of tibolone and continuous combined hormone replacement therapy in postmenopausal women with menopausal symptoms. BJOG 1998;105:904–11
- Mattsson LA, Bohnet HG, Gredmark T, Torhorst J, Hornig F, Hüls G. Continuous, combined hormone replacement: randomized comparison of transdermal and oral preparations. Obstet Gynecol 1999;94:61–5
- Doren M, Rubig A, Bennink C, Holzgreve HJ. Impact on uterine bleeding and endometrial thickness: tibolone compared with continuous combined estradiol and norethisterone acetate replacement therapy. Menopause 1999;6:299–306
- Graser T, Koytchev R, Muller A, Oettel M. Comparison of the efficacy and endometrial safety of two estradiol valerate/dienogest combinations and kliogest for continuous combined hormone replacement therapy in postmenopausal women. Climacteric 2000;3:109–18
- Rozenberg S, Caubel P, Lim PC. Constant estrogen, intermittent progestogen vs. continuous combined hormone replacement therapy: tolerability and effect on vasomotor symptoms. Int J Gynaecol Obstet 2001;72:235–43
- Odmark IS, Jonsson B, Backstrom T. Bleeding patterns in postmenopausal women using continuous combination hormone replacement therapy with conjugated estrogen and medroxyprogesterone acetate or with 17beta-estradiol and norethindrone acetate. Am J Obstet Gynecol 2001;184:1131–8
- Archer DF, Thorneycroft IH, Foegh M, et al. Long-term safety of drospirenone-estradiol for hormone therapy: a randomized, double-blind, multicenter trial. Menopause 2005;12:716–27
- Stevenson JC, Durand G, Kahler E, Pertynski T. Oral ultra-low dose continuous combined hormone replacement therapy with 0.5 mg 17beta-oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double-blind, controlled study. Maturitas 2010;67:227–32
- Pickar JH, Bottiglioni F, Archer DF. Amenorrhea frequency with continuous combined hormone replacement therapy: a retrospective analysis. Menopause Study Group. Climacteric 1998;1:130–6
- Archer DF, Pickar JH. Hormone replacement therapy: effect of progestin dose and time since menopause on endometrial bleeding. Obstet Gynecol 2000;96:899–905
- Baracat EC, Barbosa IC, Giordano MG, et al. A randomized, open-label study of conjugated equine estrogens plus medroxyprogesterone acetate versus tibolone: effects on symptom control, bleeding pattern, lipid profile and tolerability. Climacteric 2002;5:60–9
- Heikkinen J, Vaheri R, Timonen U. Long-term safety and tolerability of continuous-combined hormone therapy in postmenopausal women: results from a seven-year randomised comparison of low and standard doses. J Br Menopause Soc 2004;10:95–102
- Mattsson LA, Skouby S, Rees M, et al. Efficacy and tolerability of continuous combined hormone replacement therapy in early postmenopausal women. Menopause Int 2007;13:124–31
- Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once-a-week 17á-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause 2002;9:195–207
- Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol 1999;94:498–503
- Ylikorkala O, Rozenberg S. Efficacy and tolerability of fully transdermal hormone replacement in sequential or continuous therapy at two doses of progestogen in postmenopausal women. Maturitas 2000;37:83–93
- Oosterbaan HP, van Buuren AH, Schram JH, et al. The effects of continuous combined transdermal oestrogen-progestogen treatment on bleeding patterns and the endometrium in postmenopausal women. Maturitas 1995;21:211–19
- Stevenson JC, Teter P, Lees B. 17beta-estradiol (1 mg/day) continuously combined with dydrogesterone (5, 10 or 20 mg/day) increases bone mineral density in postmenopausal women. Maturitas 2001;38:197–203
- AinMelk Y. Comparison of two continuous combined estrogen progestogen regimens in postmenopausal women: a randomized trial. Fertil Steril 1996;66:962–8
- Archer DF, Pickar JH, Bottiglioni F. Bleeding patterns in postmenopausal women taking continuous combined or sequential regimens of conjugated estrogens with medroxyprogesterone acetate. Menopause Study Group. Obstet Gynecol 1994;83:686–92
- Jirapinyo M, Theppisai U, Manonai J, Suchartwatnachai C, Jorgensen LN. Effect of combined oral estrogen/progestogen preparation (kliogest) on bone mineral density, plasma lipids and postmenopausal symptoms in HRT-naive Thai women. Acta Obstet Gynecol Scand 2003;82:857–66
- Archer DF, Dorin M, Lewis V, Schneider DL, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertil Steril 2001;75:1080–7
- Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric 2013;16:338–46
- Pinkerton JV, Harvey JA, Lindsay R, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab 2014;99:E189–E98
- Kagan R, Abreu P, Andrews E. Vaginal bleeding/spotting with conjugated estrogens/bazedoxifene, conjugated estrogens/medroxyprogesterone acetate, and placebo. Postgrad Med 2018;130:687–93
- Simon JA, Liu JH, Speroff L, Shumel BS, Symons JP. Reduced vaginal bleeding in postmenopausal women who receive combined norethindrone acetate and low-dose ethinyl estradiol therapy versus combined conjugated equine estrogens and medroxyprogesterone acetate therapy. Am J Obstet Gynecol 2003;188:92–9
- Kazerooni T, Zolghadri J. The comparison of bleeding patterns with high-dose and low-dose hormone replacement therapy in postmenopausal women. Gynecol Endocrinol 2004;19:64–8
- Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women's Health Initiative. Obstet Gynecol 2005;105:1063–73
- Rowan JP, Simon JA, Speroff L, Ellman H. Effects of low-dose norethindrone acetate plus ethinyl estradiol (0.5 mg/2.5 microg) in women with postmenopausal symptoms: updated analysis of three randomized, controlled trials. Clin Ther 2006;28:921–32
- Brynhildsen J, Hammar M. Low dose transdermal estradiol/norethisterone acetate treatment over 2 years does not cause endometrial proliferation in postmenopausal women. Menopause 2002;9:137–44
- Limpaphayom KK, Bunyavejchevin S. Clinical effects of 17 beta-estradiol and norethisterone acetate in postmenopausal Thai women. J Med Assoc Thai 2000;83:410–17
- Mirkin S, Goldstein SR, Archer DF, Pickar JH, Graham S, Bernick B. Endometrial safety and bleeding profile of a 17β-estradiol/progesterone oral softgel capsule (TX-001HR). Menopause 2020;27:410–7
- Rozenberg S, Pornel B, Koninckx PR, Palacios S, Christiansen C. Endometrium protection and acceptability of nasally administered continuously combined hormone therapy: a multicentre, multinational, double-blind trial in post-menopausal women evaluating three regimens of 17beta-estradiol and norethisterone when compared with an orally administered 17beta-estradiol norethisterone regimen. Hum Reprod 2009;24:1739–47
- Haines CJ, Chung TK, Lau TK. Sonographic measurement of endometrial thickness as a predictor of vaginal bleeding in women using continuous combined hormone replacement therapy. Gynecol Obstet Invest 1997;44:187–90
- Bassol S, Carranza-Lira S, Celis-Gonzalez C, et al. The impact of a monophasic continuous estro-progestogenic treatment on Latin American menopausal women. Maturitas 2005;50:189–95
- Quereux C, Pornel B, Bergeron C, Ferenczy A. Continuous combined hormone replacement therapy with 1 mg 17beta-oestradiol and 5 mg dydrogesterone (Femoston-conti): endometrial safety and bleeding profile. Maturitas 2006;53:299–305
- Graser T, Romer T, Wiedey KD, Janaud A. Climodien (estradiol valerate 2 mg plus dienogest 2 mg) is safe and effective in the treatment of postmenopausal complaints. Climacteric 2001;4:332–42
- Mattsson LA, Ipsen HE, Granqvist CJ, Kokot-Kierepa M, Study G. Ultra-low-dose estradiol and norethisterone acetate: bleeding patterns and other outcomes over 52 weeks of therapy. Climacteric 2015;18:419–25
- Bergeron C, Nogales FF, Rechberger T, Tatarchjuk T, Zipfel L. Ultra low dose continuous combined hormone replacement therapy with 0.5mg 17beta-oestradiol and 2.5mg dydrogesterone: protection of the endometrium and amenorrhoea rate. Maturitas 2010;66:201–5
- Bijuva® (estradiol and progesterone) capsules, for oral use Prescribing Information. Boca Raton, FL: TherapeuticsMD; 2019
- TherapeuticsMD. Data on file; 2019
- Activella® (estradiol/norethindrone acetate) tablets. Princeton, NJ: Novo Nordisk Inc; 2006
- Kliovance (1 mg/0.5 mg film-coated tablets) summary of product characteristics. Gatwich, UK: Novo Nordisk Limited; 2016
- Kliofem (film-coated tablets) summary of product characteristics. Gatwich, UK: Novo Nordisk Limited; 2016
- Angeliq® (drospirenone and estradiol) tablets, for oral use Prescribing Information. Bayer Healthcare. Whippany, NJ; 2005
- Angeliq (film-coated tablets) summary of product characteristics. Bayer plc; 2018
- Prempro™/Premphase® (conjugated estrogens/medroxyprogesterone acetate tablets) Prescribing Information. Wyeth Pharmaceuticals Inc. Philadelphia, PA. 2008
- Climodien (2 mg/2mg coated tablets) summary of product characteristics. Berlin, Germany: Bayer Pharma; 2017
- Climara Pro® (estradiol/Levonorgestrel transdermal system) Prescribing Information. Northridge, CA; 3M Drug Delivery Systems; 2007
- Evorel conti summary of product characteristics. London, UK: Theramex UK Unlimited; 2019
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer 2005;114:448–54
- Mirkin S, Archer DF, Taylor HS, Pickar JH, Komm BS. Differential effects of menopausal therapies on the endometrium. Menopause 2014;21:899–908
- Mirkin S, Navarro F, Archer DF. Hormone therapy and endometrial angiogenesis. Climacteric 2003;6:273–7
- Mirkin S, Wong BC, Archer DF. Effect of 17 beta-estradiol, progesterone, synthetic progestins, tibolone, and tibolone metabolites on vascular endothelial growth factor mRNA in breast cancer cells. Fertil Steril 2005;84:485–91
- Mirkin S, Archer DF. Effects of levonorgestrel, medroxyprogesterone acetate, norethindrone, progesterone, and 17beta-estradiol on thrombospondin-1 mRNA in Ishikawa cells. Fertil Steril 2004;82:220–2
- Perry W, Wiseman RA, Cullen NM. Combined oral estradiol valerate-norethisterone treatment over three years in postmenopausal women. 1. Clinical aspects and endometrial histology. Gynecol Endocrinol 1998;12:109–22
- von Holst T, Lang E, Winkler U, Keil D. Bleeding patterns in peri and postmenopausal women taking a continuous combined regimen of estradiol with norethisterone acetate or a conventional sequential regimen of conjugated equine estrogens with medrogestone. Maturitas 2002;43:265–75
- Rouskova D, Mittmann K, Schumacher U, Dietrich H, Zimmermann T. Effectiveness, tolerability and acceptance of an oral estradiol/levonorgestrel formulation for the treatment of menopausal complaints: a non-interventional observational study over six cycles of 28 days. Gynecol Endocrinol 2014;30:712–16