Abstract
Objective
This article reports the first live birth after cryopreserved ovarian tissue transplantation to prevent premature ovarian insufficiency in China.
Methods
A patient with myelodysplastic syndrome received ovarian tissue cryopreservation before hematopoietic stem cell transplantation, and six ovarian cortex strips were thawed and transplanted into her peritoneal pocket 2 years later.
Results
Pregnancy occurred spontaneously 27 months after grafting, and a healthy girl was born at 38 weeks gestation. Until now, the child has developed normally without any major diseases.
Conclusions
We report the first live birth resulting from ovarian tissue cryopreservation and transplantation in China.
摘要
目的:本文报道应用冻存卵巢组织移植技术防治早发性卵巢功能不全中国首例活产。
方法:1例骨髓增生异常综合征患者在造血干细胞移植前接受卵巢组织冻存, 2年后, 其中6片卵巢皮质片复苏后移植到患者盆腹膜袋中。
结果:移植后27个月自然妊娠, 孕38周时分娩一名健康女婴。到目前为止, 婴儿发育正常, 无任何明显疾病。
结论:我们报道了卵巢组织冻存移植后中国首例活产。
Introduction
The gonadal toxicity of anticancer treatments causes severe damage to survivors’ ovarian function, especially those for hematologic diseases after hematopoietic stem cell transplantation [Citation1,Citation2]. To safeguard the fertility of young patients at high risk of premature ovarian insufficiency, ovarian tissue cryopreservation and transplantation (OTCT) have been offered as a routine clinical technique [Citation3]. Until 2020, the cumulative number of live births after OTCT exceeded 200 worldwide [Citation4]. The first ovarian tissue cryobank in China was established in 2012 by our center, in which nearly 400 cases of cryopreservation and 10 cases of transplantation have been successfully performed [Citation5,Citation6]. One of these patients diagnosed with myelodysplastic syndrome (MDS) became pregnant spontaneously and delivered recently, and we report on this first live birth resulting from OTCT in China.
Materials and methods
The case refers to a 29-year-old unmarried, nulliparous female patient diagnosed with MDS (RAEB-I SPSS 2.0 int-2) in 2016. After being treated with thalidomide, prednisone acetate and retinoic acid for 5 months and achieving remission, chemotherapy with high-dose cytarabine, busulfan and cyclophosphamide was scheduled for subsequent hematopoietic stem cell transplantation. Before the onset of chemotherapy, she was referred to our clinic for fertility preservation counseling due to the high gonadotoxicity of the alkylating agents.
Clinical application of OTCT was approved by the Ethics Committee, the New Technology Committee and the Expert Committee of our hospital. Informed written consent was obtained from this patient.
In September 2016, part of the patient’s bilateral ovary was removed by laparoscopy and transported to our cryobank at a temperature of 4–8 °C. Pelvic endometriotic nodules were found during surgery and were coagulated by bipolar coagulation. The ovarian cortex was cryopreserved in 23 pieces of size 4 mm × 8 mm according to previously reported protocols [Citation7,Citation8]. Ovarian biopsies showed a density of 15 follicles per 3 mm with good viability. The patient developed complete ovarian failure following hematopoietic stem cell transplantation.
The patient married 2 years later, and expressed her wish for a pregnancy. Her blood tests and bone marrow aspirate reflected complete remission of MDS. Serum gonadotropin levels were still in the postmenopausal range, and ultrasound showed atrophy of the uterus and ovaries without any antral follicles inside. The decision to perform frozen–thawed ovarian tissue transplantation was made after a multidisciplinary consultation.
In September 2018, after testing the follicle density and viability of frozen-thawed biopsies (33 follicles per 3 mm biopsy), six ovarian cortex strips were thawed and introduced into the peritoneal pocket in the right ovarian fossa using laparoscopy. Three months later, the patient resumed spontaneous menses, the gonadotropins returned to premenopausal levels and antral follicles were detected in the graft site using ultrasound.
The patient underwent three natural cycles of oocyte retrieval, but none of the embryos implanted after the transfer. In June 2020, a hysterosalpingogram showed bilateral fallopian tubal obstruction. The patient therefore underwent tubal dilation and recanalization, and the obstruction was successfully overcome.
Results
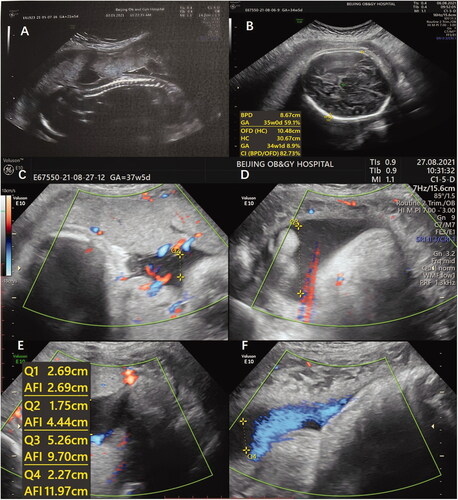
The patient maintained regular menses until December 2020, 27 months after ovarian tissue transplantation, and became pregnant spontaneously. In January 2021, ultrasound confirmed a viable intrauterine pregnancy with a fetal heartbeat. An ultrasound assessment showed that the nuchal translucency thickness was 1.7 mm at 12 + 3 weeks gestation. A fetal anatomical scan at 22 weeks revealed a healthy fetus (). The uneventful pregnancy progressed to term without any complications. Ultrasound at 38 weeks gestation indicated that the fetus was developing normally, and was in a breech position.
Figure 1. (A–F) Ultrasound indicating that the fetus was developing normally during the whole gestation.
On 31 August 2021, a cesarean section at 38 weeks gestation was performed because the fetus was still in the breech position. A healthy girl weighing 3345 g and 49 cm long was born with Apgar scores of 10/10/10. The patient has been breastfeeding after delivery. Until now, the child has developed normally without any major diseases (). We will continue to measure the physical and neurological development of the baby in the long future.
Discussion
The health of children born after frozen–thawed ovarian tissue transplantation remains poorly studied, so every case of successful OTCT, live birth and follow-up information of the offspring is worth reporting. According to current data, no diseases related to OTCT occurred in reported live births [Citation9].
Ovarian tissue cryopreservation (OTC), the only way for prepubertal girls and patients who cannot delay anticancer treatments to preserve fertility and ovarian endocrine function [Citation10], has been more and more widely used especially in hematological patients. For example, hematologic disease was the most common indication for OTC in major European OTC centers according to a recent report [Citation11]. Safety issues surrounding OTCT have always been a focus of concern. Several recent studies have shown that cryopreservation of ovarian tissue after the patient has achieved remission can significantly reduce the risk of relapse [Citation12,Citation13]. On the other hand, when using low gonadotoxic agents, pretreatment before OTC does not compromise success rates in ovarian function restoration, pregnancy and live birth after transplantation [Citation11]. To date, among all hematological patients including 12 leukemia patients who underwent OTCT, no relapses related to ovarian tissue grafting occurred [Citation14]. MDS involves clonal hematopoietic stem cell disorders, which may progress to acute myeloid leukemia in some high-risk patients. In this current case, the patient achieved remission following 5 months of pretreatment before OTC, and multidisciplinary consultation and systematic evaluation were conducted before transplantation. Until now, no recurrence has occurred over 3 years since ovarian tissue grafting.
Frozen–thawed ovarian tissue can be transplanted to orthotopic or heterotopic sites. Of all live births reported worldwide, most cases underwent orthotopic transplantation, namely grafting onto the remaining ovary or the created peritoneal pocket. In the current case, the latter method was adopted for transplantation and spontaneous pregnancy was achieved, indicating that the oocyte can be ovulated through the peritoneum and then be picked up by the tubal fimbria. Recent data demonstrate that the live birth rates of each orthotopic surgical technique are equivalent [Citation11]. Interestingly, it seems that OTCT patients are more likely to conceive spontaneously rather than by in vitro fertilization. In some centers, the natural pregnancy rate in OTCT patients can reach 91% [Citation15]. This may be explained by insufficient corpus luteum function. However, further research is needed.
Here, we report the first case of live birth in a patient with MDS after OTCT in China. We will carry out a close long-term follow-up of this newborn.
Potential conflict of interest
Nil.
Source of funding
Supported by Beijing Natural Science Foundation [7202047]; Capital’s Funds for Health Improvement and Research [2020-2-2112]; Beijing Hospitals Authority’ Ascent Plan [DFL20181401]; Capital’s Funds for Health Improvement and Research [2016-2-2113]; Beijing Municipal Science & Technology Commission [Z161100000516143]; Beijing Hospitals Authority Clinical medicine Development of special funding support [XMLX201710].
Additional information
Funding
References
- Spears N, Lopes F, Stefansdottir A, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. 2019;25(6):673–693.
- Kim S, Kim SW, Han SJ, et al. Molecular mechanism and prevention strategy of chemotherapy- and radiotherapy-induced ovarian damage. Int J Mol Sci. 2021;22(14):7484.
- Dolmans MM, Falcone T, Patrizio P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil Steril. 2020;114(2):279–280.
- Dittrich R, Kliesch S, Schuring A, et al. Fertility preservation for patients with malignant disease. Guideline of the DGGG, DGU and DGRM (S2k-Level, AWMF registry no. 015/082, november 2017) – recommendations and statements for girls and women. Geburtshilfe Frauenheilkd. 2018;78(6):567–584.
- Ruan X, Cui Y, Du J, et al. Randomized study to prove the quality of human ovarian tissue cryopreservation by xenotransplantation into mice. J Ovarian Res. 2019;12(1):46.
- Ruan X, Cheng J, Korell M, et al. Ovarian tissue cryopreservation and transplantation prevents iatrogenic premature ovarian insufficiency: first 10 cases in China. Climacteric. 2020;23(6):574–580.
- Ruan X, Du J, Korell M, et al. Case report of the first successful cryopreserved ovarian tissue retransplantation in China. Climacteric. 2018;21(6):613–616.
- Ruan X. Chinese society of gynecological endocrinology affiliated to the international society of gynecological endocrinology guideline for ovarian tissue cryopreservation and transplantation. Gynecol Endocrinol. 2018;34(12):1005–1010.
- Tammiste T, Kask K, Padrik P, et al. A case report and follow-up of the first live birth after heterotopic transplantation of cryopreserved ovarian tissue in Eastern Europe. BMC Womens Health. 2019;19(1):65.
- Practice committee of American society for reproductive medicine. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101:1237–1243.
- Dolmans MM, von Wolff M, Poirot C, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril. 2021;115(5):1102–1115.
- Rodriguez-Wallberg KA, Milenkovic M, Papaikonomou K, et al. Successful pregnancies after transplantation of ovarian tissue retrieved and cryopreserved at time of childhood acute lymphoblastic leukemia – a case report. Haematologica. 2021;106(10):2783–2787.
- Poirot C, Fortin A, Dhédin N, et al. Post-transplant outcome of ovarian tissue cryopreserved after chemotherapy in hematologic malignancies. Haematologica. 2019;104(8):e360–e363.
- Kristensen SG, Wakimoto Y, Colmorn LB, et al. Use of cryopreserved ovarian tissue in the danish fertility preservation cohort. Fertil Steril. 2021;116(4):1098–1106.
- Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–2041.