Abstract
Midlife women commonly experience changes in their cognitive function as they transition through menopause and express concern about whether these changes represent the initial stages of a more serious cognitive disorder. Health-care practitioners play an important role in counseling women on cognitive changes at midlife and normalizing women’s experience. The aim of this commissioned International Menopause Society White Paper on cognition is to provide practitioners with an overview of data informing the clinical care of menopausal women and a framework for clinical counseling and decision-making. Among the topics presented are the specific cognitive changes occurring in menopause, the duration of such changes and their severity. The role of estrogen and menopause symptoms is reviewed. We present talking points for clinical counseling on the effects of hormone therapy on cognition and dementia risk in women, including discussion of absolute risk. Lastly, a brief review of modifiable risk factors for age-related cognitive decline and dementia is presented, with guidance for counseling patients on optimizing their brain health at midlife and beyond.
摘要
中年女性在进入更年期后通常会经历认知功能的变化, 并担心这些变化是否代表了更严重的认知障碍的初始阶段。卫生保健从业人员在咨询中年妇女认知变化和规范妇女治疗的经验方面发挥着重要作用。《国际绝经学会认知白皮书》的目的旨在为从业者提供绝经女性临床保健数据概述以及临床咨询和决策框架。其中的主题包括绝经期发生的特定认知变化、这种变化的持续时间及其严重程度, 对雌激素和绝经症状的作用进行综述。我们提出了关于激素治疗对女性认知和痴呆风险的影响的临床咨询要点, 包括绝经的风险讨论。最后, 简要回顾了与年龄有关的认知功能下降和痴呆的可改变的危险因素, 并指导患者在中年及以后优化大脑健康。
Introduction
Cognitive complaints are frequent in midlife women and are associated with decreased quality of life [Citation1]. These cognitive complaints are reliably validated and documented across the menopause transition (MT). Basic and clinical studies show a role for estradiol (E2) in mediating menopause-related changes in cognition [Citation2]. In addition, menopause symptoms, including vasomotor symptoms (VMS), sleep disturbances and mood changes contribute to cognitive difficulties at midlife [Citation3], but there are critical gaps in the data as to whether this period of cognitive dysfunction predicts dementia risk, and whether menopausal hormone therapy (MHT) is protective against late-onset dementia or increases the risk. The theme for the 2022 World Menopause Day is Cognition and Mood, and the goal of this International Menopause Society-commissioned White Paper on cognition is to provide menopause practitioners with an overview of data informing clinical care of menopausal women and a framework for clinical counseling and decision-making for their patients.
The key sections focus on questions commonly raised in clinical care and include the following:
What do we mean by cognitive function and brain fog?
How does cognitive function change in menopause?
What menopause-related factors appear to influence cognition?
What role does MHT play?
What other modifiable risk factors influence cognition at midlife?
What are the generally agreed upon recommendations for optimizing brain health that clinicians can share with their patients?
Defining cognition and menopause brain fog
Cognition is defined as ‘all forms of knowing and awareness, such as perceiving, conceiving, remembering, reasoning, judging, imagining, and problem solving’ [Citation4]. Cognitive complaints at menopause include difficulty recalling words and numbers, disturbances in daily life (misplacing items like keys), trouble concentrating (absent mindedness, losing a train of thought, more easily distracted) and forgetting appointments and events [Citation5]. Another manifestation of cognitive difficulties involves symptoms of attention deficit hyperactivity disorder [Citation6]. The constellation of cognitive changes at menopause is often referred to as ‘brain fog’ (see for a definition). The severity of these symptoms differs considerably across women and most often is in the mild range [Citation1].
Some research, largely based on basic science studies, suggests that cognitive problems in the MT are related to brain changes that persist into late life and may ultimately lead to dementia [Citation2,Citation7,Citation8]. But does this imply that menopause ‘causes’ dementia, and that women are on a path toward dementia once they transition through menopause? All women transition through menopause, but most women will not develop dementia. In the USA, for example, the lifetime risk of Alzheimer’s disease (AD) dementia is 19.5% at age 45 years and 21.1% at age 65 years [Citation9]. Prevalence of AD depends on biological sex and geographical location, with higher rates among women than men and higher rates in Europe and North America than in Asia, Africa and South America [Citation10]. However, women should be reassured that, unless they have a family history of early-onset AD, dementia at midlife is very rare, affecting 293.1 per 100,000 women globally [Citation10,Citation11].
Take-home messages to guide clinical counseling and decision-making
Menopause brain fog refers to the constellation of cognitive symptoms experienced by women around menopause, frequently manifesting in memory and attention difficulties.
The cognitive changes at menopause should not be confused with dementia; dementia before age 64 years is rare.
Despite some research suggesting that menopause-related cognitive problems may ultimately lead to dementia later in life, it is important to note that the problems are common and, although all women transition through menopause, the large majority of women will not develop dementia.
Cognitive domains affected by menopause
To understand which cognitive domains are affected in the MT, it is necessary to perform neuropsychological evaluations in a large cohort of women longitudinally from a premenopause baseline to postmenopause. The cognitive domains that change most reliably in the MT are verbal learning and memory, with more modest or less reliable effects on psychomotor speed and working memory/attention [Citation12–17]. Working memory refers to the ability to hold and manipulate items in short-term memory, such as keeping a new email address in mind while typing the subject of the email. Verbal learning and memory refer, respectively, to the encoding and recall of words, word pairs, short stories or other verbal material. There are reliable sex differences in verbal learning and memory in favor of women across the lifespan [Citation18,Citation19]. Midlife women’s complaints of forgetfulness are validated by studies showing that the severity of the complaint correlates with performance on tests of verbal memory [Citation20,Citation21]. In longitudinal studies, higher-order cognitive functions such as executive functions (e.g., strategic thinking, planning) do not change across the MT [Citation17].
Despite reliable evidence of menopause-related cognitive declines in these longitudinal studies, the average level of cognitive performance remained within normal limits [Citation12–16,Citation22]. The only longitudinal study to address the frequency of new-onset cognitive impairment during the MT involved low-income women of color, half of whom had human immunodeficiency virus (HIV) [Citation15]. There, 11–13% of women showed a new onset of cognitive impairment, a rate that did not differ by HIV serostatus. The factors distinguishing women who are vulnerable to a new onset of cognitive impairment in the MT are unknown. Some individuals are vulnerable to cognitive impairment and dementia due to life experiences such as a low level of education and occupations and leisure activities that put limited demands on cognition [Citation23]. These individuals are said to have low cognitive reserve [Citation23]. It may also be that persistent menopausal symptoms, particularly sleep disturbance, contribute to this vulnerability, among other factors including genetic vulnerability, physical health, mental health and life stressors.
Another key clinical question is whether these cognitive changes resolve. Findings from the Study of Women Across the Nation (SWAN) suggest that any cognitive change is limited to perimenopause [Citation12]. Findings from the Penn Ovarian Aging study indicate that difficulties in verbal learning persist in postmenopause while difficulties in verbal memory resolve in postmenopause [Citation13]. Critically, these participants were not followed well into postmenopause. Low-income women of color showed changes in verbal learning and memory, as well as more subtle changes in attention/working memory that persisted into postmenopause [Citation15]. Together, these findings suggest that memory difficulties resolve for many women but persist for some into postmenopause. Difficulties in verbal learning generally might persist into postmenopause.
More broadly, these findings help alleviate women’s concerns that their cognitive difficulties are the sign of an imminent cognitive disorder like AD. These changes begin in perimenopause and commonly normalize postmenopause. Overall, the natural history of cognitive changes at menopause suggests an etiology related to changes in sex steroid hormones and the onset of menopause symptoms, and not an early phase of a dementing disorder.
Take-home messages to guide clinical counseling and decision-making
Research studies validate patients’ cognitive complaints at menopause.
Difficulties in learning and verbal memory are especially common.
These difficulties emerge in perimenopause when menstrual cycles become irregular and cycles are skipped.
While these complaints are troublesome to women, normal range of function is typically maintained; about 11–13% of women show clinically significant impairment.
The timing of these changes suggests an etiology linked to hormones and menopause symptoms rather than AD, which is rare at this time.
What menopause-related factors appear to influence cognition?
Estrogen receptors are replete in brain areas subserving memory and other cognitive functions, including the hippocampus and prefrontal cortex [Citation24]. A causal role of E2 in menopause-related changes in memory is shown in studies where removal of the ovaries or suppression of E2 with a gonadotropin releasing hormone analog leads to declines in verbal learning and memory that are reversed with estrogen treatment [Citation25–27]. Thus, E2 declines likely contribute to the changes in verbal memory and working memory observed in longitudinal studies.
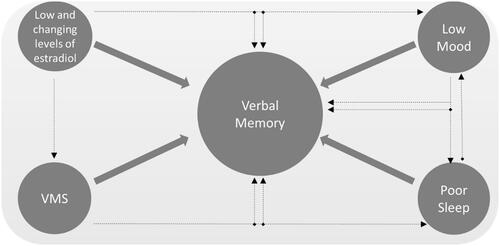
Menopause symptoms, particularly when measured objectively with wearable devices, also appear to contribute to memory difficulties at midlife. In such studies, frequent VMS strongly relate to memory difficulties that persist when controlling for self-reported sleep difficulties and objective sleep [Citation28,Citation29]. Brain imaging studies link VMS to adverse changes in brain structure and function [Citation30–32]. Initial evidence suggests that these changes may be reversed even if the intervention is not estrogen-based [Citation33]. Sleep difficulties and low mood are also associated with cognitive difficulties at menopause [Citation16,Citation20]. Although a causal role of sleep disturbance in cognitive difficulties at menopause is yet to be established, there is robust evidence from sleep deprivation studies of a causal role of sleep disturbance in verbal learning and memory difficulties [Citation34]. Depressive and anxiety symptoms are also linked to cognitive symptoms at menopause, although it is not yet known whether treating those symptoms causes a rebound in memory [Citation20]. shows a general schema for understanding the role of E2 and menopause symptoms in memory difficulties at midlife.
Figure 2. Model linking estradiol and menopausal factors to verbal memory dysfunction. VMS, vasomotor symptoms.
Take-home messages to guide clinical counseling and decision-making
Cognitive difficulties at midlife are linked to changes in E2, VMS, sleep and mood.
Treating these symptoms may benefit cognition.
What role does role does MHT play?
MHT and cognition
It may be apparent from the aforementioned evidence that MHT might confer benefit in perimenopause when cognitive difficulties emerge, and in women with bothersome VMS. Unfortunately, the effect of MHT in those two settings is unknown, as there are no randomized clinical trials of MHT or oral contraceptives on cognition in perimenopausal women, and no clinical trials of MHT on cognition in women with moderate-to-severe VMS [Citation3]. What has been studied is the effect of MHT on cognition in early and late postmenopausal women. In four large clinical trials, MHT had neutral effects on cognition in early postmenopausal women [Citation35–38]. The studies yielded similar findings across a variety of MHT regimens – oral E2, transdermal E2, conjugated equine estrogens plus medroxyprogesterone acetate (CEE/MPA) and CEE alone. The effects of combined MHT on cognition in late postmenopausal women may depend on the regimen; CEE/MPA appears to have negative effects in women aged 65 years and older [Citation39], while oral E2 plus vaginal progesterone has neutral effects in women more than 10 years beyond natural or surgical menopause [Citation37]. Small trials in surgically menopausal women suggest that estrogen therapy (ET) benefits memory [Citation25]. In older women, ET has neutral effects on cognition [Citation40–44].
MHT and dementia
Many women are fearful that, if they take MHT, they will increase their personal risk of dementia. The 5-year data from the Women’s Health Initiative (WHI) established a two-fold increased risk of all-cause dementia with CEE/MPA [Citation45]. CEE alone had no effect on risk of all-cause dementia [Citation46]. Contrasting findings were reported in 18-year follow-up data from the WHI where CEE led to a 26% decreased risk of death from AD and CEE/MPA led to no effect on death from AD [Citation47]. The reasons for the discrepancy in findings from the 5-year and 18-year follow-up data from the WHI are not fully understood. If both findings represent truth, they may suggest an early risk of HT on dementia for vulnerable women – perhaps those with low baseline cognitive performance [Citation48] or those with diabetes [Citation49,Citation50] – followed by long-term benefit.
There is no large-scale study to inform the choice of MHT formulation for cognitive end points. We therefore must rely on large, population-based studies, and those studies, like the WHI, present conflicting findings. Two such studies highlight the conflict. The first, a large Finnish case–control study (>84,000 women), found that systemic use of MHT (ET alone or estrogen plus progestin therapy (EPT)) was related to a greater risk of AD regardless of the particular formulation; even CEE was associated with an increased risk of AD [Citation51]. In contrast, the second study, a nested case–control study from clinical practices across the UK (118,501 women aged 55 years and older), found that, overall, MHT was associated with no increased or decreased risk of dementia or AD [Citation52]. Long-term use of ET for 10 years or more was associated with a reduced odds of developing dementia, whereas use of MHT for 5–9 years was associated with a 10% increased risk of dementia and use for more than 10 years was associated with a 20% increase [Citation52]. Regarding formulation, E2 for 1–5 years but not for longer was associated with a reduced risk of AD [Citation52]. In general, progesterone formulation did not influence findings, although the risk of dementia with dydrogesterone was slightly lower compared with other progestogens. Thus, there is no consistent finding in the literature on estrogen formulation and dementia risk.
For oophorectomized women, treatment with ET at least to the typical age at menopause may be advised. Oophorectomized women not treated with ET had a greater risk of cognitive decline or dementia 30 years post surgery compared to women treated with ET immediately post surgery and who remained on ET until at least age 50 years when natural menopause would have occurred [Citation53]. This study and others provide compelling evidence that bilateral oophorectomy should be undertaken with caution, the benefits and long-term risks considered, and adequate ongoing treatment and monitoring strategies should be in place [Citation54,Citation55].
Counseling on MHT and dementia: risk and potential benefit
In counseling patients about MHT and dementia, it can be helpful to convey the risk of all-cause dementia observed in the Women’s Health Initiative Memory Study (WHIMS) in terms of absolute risk and the number needed to harm. presents the risk of all-cause dementia based on the findings from the CEE/MPA arm of the WHIMS [Citation45]. In that scenario, the number of women needed to treat with CEE/MPA to cause one case of all-cause dementia is 436 women. These data may reassure women who want to use MHT for VMS relief. Women may be aware of the conflicting data on dementia risk and MHT and may express interest in taking MHT for prevention of dementia. Clinicians play an important role in contextualizing any perceived benefit from using MHT for prevention of dementia. Using the 18-year follow-up data from the WHI, which found a 26% decreased risk of death from dementia with CEE, as a best-case scenario [Citation47], the number of women needed to treat to prevent one death from AD is 2004. In other words, in that best-case scenario, only 1 in 2004 women treated with MHT would decrease her risk of death from AD. Such statistics can be helpful in conveying that an individual woman is not likely to decrease her personal risk of death from AD by using MHT. Instead, other interventions described below are recommended to lower AD risk.
Table 1. Absolute risk of incident dementia and death from Alzheimer’s disease with menopausal hormone therapy based on findings from the Women’s Health Initiative (WHI).
Take-home messages to guide clinical counseling and decision-making
Based on current guidelines, MHT is not recommended at any age to treat cognitive concerns at menopause or prevent cognitive decline or dementia.
There are two large, clinically relevant gaps in the scientific literature – whether MHT improves cognition in women with bothersome VMS and whether MHT or oral contraceptives improve cognition in perimenopause.
Use of MHT early in postmenopause appears safe for cognitive function.
Use of ET in women with early menopause may be helpful in maintaining cognitive function and lowering risk of dementia.
Use of ET even late in postmenopause appears to be safe for cognitive function.
Use of MHT late in postmenopause is risky if the formulation is CEE/MPA but appears to be neutral if the formulation is oral E2 plus vaginal progesterone.
The magnitude of the effect of MHT on dementia, whether beneficial or adverse, in the literature is small.
There is no reliable finding in the literature to guide treatment decisions about MHT formulation or duration of use on dementia risk.
Modifiable risk factors for dementia
Patients with cognitive complaints at menopause are often concerned about their risk of dementia later in life. An important message to convey to these patients is that dementia can be postponed or prevented by addressing certain health issues. Certain risk factors for dementia such as age and gender are not modifiable, but it is estimated that about 40% of dementias worldwide are due to modifiable risk factors [Citation56]. Guidelines from the World Health Organization (WHO) and the 2020 Lancet Commission [Citation56,Citation57] concur on specific modifiable risk factors, including physical activity, smoking, cognitive activity, social interaction, obesity, hypertension, diabetes, hearing impairment and depression. The Lancet Commission also included traumatic brain injury and air pollution as potentially modifiable risk factors [Citation56].
Midlife is an ideal time to intervene on modifiable risk factors for dementia, as a recent meta-analysis revealed five factors at midlife that increased dementia risk by 41–78%, including obesity, diabetes mellitus, current smoking, hypercholesterolemia and hypertension (borderline blood pressure [BP]) [Citation58]. A companion systematic review found that another three factors – hyperhomocysteinemia, psychological stress and heavy drinking – were associated with elevated dementia risk [Citation58]. provides clinicians with advice for patients on strategies to optimize cognitive health based on modifiable risk factors for dementia.
Figure 3. Patient tips to optimize brain health based on modifiable risk factors for dementia. From modifiable risk factors for dementia prevention from the World Health Organization (WHO) 2019 guidelines and the 2020 Lancet Commission [Citation56,Citation57]. BMI, body mass index; BP, blood pressure.
![Figure 3. Patient tips to optimize brain health based on modifiable risk factors for dementia. From modifiable risk factors for dementia prevention from the World Health Organization (WHO) 2019 guidelines and the 2020 Lancet Commission [Citation56,Citation57]. BMI, body mass index; BP, blood pressure.](/cms/asset/d8452fc9-e747-45cb-b448-9be293adaeef/icmt_a_2122792_f0003_b.jpg)
A multi-pronged approach to lowering dementia risk is recommended, as dementia risk increased by 20% with one risk factor, 65% with two factors and 200% with three risk factors [Citation59]. A large randomized clinical trial in individuals at risk for dementia found improved cognition with a multidomain lifestyle intervention, including diet, exercise, cognitive training and vascular risk monitoring [Citation60].
It is especially important to intervene on hypertension in midlife as a risk factor for dementia [Citation61]. Evidence from a clinical trial in adults aged 50 years and older showed that lowering blood pressure (BP) to a goal of 120 mmHg prevented mild cognitive impairment, the preclinical stage of dementia [Citation62]. A systolic BP of greater than 130 mmHg at midlife is related to a 34% increased risk of cognitive dysfunction and dementia, while the relationship between diastolic BP and dementia risk was U-shaped, with a diastolic BP between 90 and 100 mmHg associated with decreased risk for AD [Citation61].
Engaging midlife patients in physical activity and weight management strategies is important to cognitive health. Women especially appear to be motivated to reduce dementia risk through physical activity [Citation63]. In a longitudinal population-based study of midlife women, high levels of cardiovascular fitness were related to a lower dementia risk [Citation64]. The WHO recommends that older adults engage in a minimum of a 150 min of moderate-intensity aerobic physical activity a week, or 75 min of comparable vigorous-intensity aerobic physical activity throughout the week, or an equivalent mix of both [Citation57].
Social relationships and engagements are essential determinants of well-being throughout life, while social isolation, loneliness, low social activity and poor social support increase the risk of cognitive decline and dementia in older adults [Citation65]. Social engagement and connectedness have been identified as helpful interventions in preventing cognitive degeneration, particularly since there is an added risk of dementia if depression is combined with inadequate social engagement [Citation56,Citation57,Citation65].
Take-home messages to guide clinical counseling and decision-making
Some modifiable risk factors are linked to better cognitive health, including obesity, hypertension, diabetes, physical activity, smoking, cognitive activity, social interaction, hearing impairment and depression.
A multi-pronged approach to dementia prevention is recommended, as modifiable risk factors for dementia are additive.
Heart health is brain health. Assess and treat hypertension, dyslipidemia and diabetes, aiming for a BP level of ≥120/80 mmHg to optimize brain health.
Counsel patients on the importance of weight management and physical exercise to lower dementia risk. Women should maintain an exercise regimen of at least 150 min moderate-intensity aerobic physical activity weekly.
Advise patients to maintain social engagement, especially if they have a history of depression.
Advise patients to avoid excessive alcohol intake and to quit smoking to optimize brain health.
Cultural and ethnic differences
Of the six million women worldwide who become menopausal annually, 76% will live in developing countries [Citation66]. Menopause studies in high-income countries cannot be uncritically applied to women in low and middle-income countries (LMICs) [Citation56,Citation59]. Low-income American women of color, who have similarities to women in LMICs, showed greater vulnerability to menopause-related cognitive changes than higher-income American women, including enduring declines in working memory/attention and verbal recall [Citation15]. There is a paucity of data on the effect of menopause or modifiable risk factors on cognitive test performance in women from LMICs [Citation56,Citation59]. Their cognitive performance across the MT may differ from that of women in higher-income countries due to cognitive vulnerabilities related to low education level, early childhood trauma, poor nutrition, mental health challenges, physical health disorders, stressful life events and other factors that differ substantially across countries. Communicable diseases such as HIV are also more common in LMICs and findings from HIV studies in the USA cannot be generalized to those in LMICs where use of antiretroviral therapy is not as widespread. Cognitive performance in menopause depends on factors including severity of VMS, sleep disturbance, psychological symptoms, age at menopause and surgical menopause, which can differ across cultures. Lastly, the lack of pharmacological treatment options for menopausal symptoms in many countries necessitates a lifestyle-focused approach for maintaining brain health, recognizing that options for improving lifestyle can be limited [Citation67,Citation68].
Take-home messages to guide clinical counseling and decision-making
Generally, evidence on cognition and menopause is based on findings in Western societies and may not be generalized to women in other societies.
Cognitive performance depends on many factors that differ across cultures such as education level, nutrition, early childhood trauma, mental health, physical health, stressful life events, severity of menopausal symptoms, age at menopause and surgical menopause.
In LMICs, women experiencing cognitive changes at menopause can be guided to consider lifestyle interventions focused on cardiometabolic health, diet, social relationships and physical activity.
Long COVID, brain fog, memory problems and poor concentration
Persistent cognitive difficulties are seen following infection with the SARS-CoV-2, a condition that appears to disproportionately affect women [Citation69]. While deficits in memory and attention have been reported following SARS-CoV-2 infection [Citation70,Citation71], perhaps the strongest association is with executive dysfunction, as evidenced by a pre/post-SARS-CoV-2 infection study of participants in the UK biobank study [Citation72]. Although executive dysfunction can be a symptom of the MT, longitudinal studies do not show a reliable change in that cognitive domain across the MT. Unfortunately, consideration of other symptoms and perhaps even menstrual cycle irregularity may not be particularly helpful in distinguishing cognitive difficulties due to menopause from those due to SARS-CoV-2 infection. The most frequent symptoms of long-haul COVID include shortness of breath, fatigue or exhaustion and sleep disorders or insomnia [Citation73]. Night sweats and temperature dysregulation have also been reported. Menstrual cycle irregularities are associated with acute SARS-CoV-2 infection and with vaccination [Citation74], which may be explained by a short-term interruption of sex steroid hormone function, and which in turn may acutely worsen perimenopausal and postmenopausal symptoms [Citation75]. There are insufficient data to guide identification of menopause-related versus SARS-CoV-2-related cognitive issues and interventions.
Take-home messages to guide clinical counseling and decision-making
Symptoms of menopause and long COVID are very similar and may lead to misdiagnosis.
There are insufficient data to distinguish cognitive issues due to menopause from cognitive issues due to SARS-CoV-2, although executive dysfunction appears to be a prominent characteristic of SARS-CoV-2 and not menopause.
Conclusion
Cognitive complaints in menopause are common and are associated with anxiety in many women, who fear these changes predict later life dementia. Menopause practitioners play an important role in normalizing those complaints and providing evidence-based guidance for optimizing the cognitive health of their patients. The cognitive abilities most affected by menopause include the learning and recall of verbal material, and, to a lesser extent, working memory and attention. Cognitive performance remains within normal limits during the MT for the large majority of women. The memory difficulties resolve for many women postmenopause but may continue in women with cognitive vulnerabilities due to low education, social disparities and other factors. Declines in E2 and menopause symptoms – VMS, sleep disturbance and mood issues – influence cognition in midlife women. Treating those issues may help enhance cognition, although clinical trial data are not yet available to definitively recommend that approach. Memory issues at menopause should not be confused with dementia, which is rare before age 64 years. Some research suggests that perimenopausal cognitive problems may influence the risk of dementia later in life, but that work is in its early stages. MHT is not recommended at any age to treat cognitive issues at menopause or to prevent cognitive decline or dementia later in life. MHT package inserts note the increased risk of dementia found in the WHIMS; this risk translates to 436 women needing to be treated to cause one new case of dementia. Long-term follow-up data from the WHI conflict with the WHIMS data and suggest a reduced risk of death from AD. However, even in that best-case scenario, the beneficial effects of estrogen on death from AD translates to 2004 women needing to be treated to lower the risk of death from AD, a rate that argues against women using MHT to prevent dementia. There are no reliable findings in the literature to guide treatment decisions regarding formulation or duration of treatment. Clinical counseling should focus on a multi-pronged approach to reducing dementia through such modifiable risk factors as obesity, hypertension, diabetes, physical activity, smoking, cognitive activity, social interaction, hearing impairment and depression.
Potential conflict of interest
P.M. Maki has received consulting honoraria from AbbVie, Astellas, Bayer, Johnson & Johnson, Pfizer and Mithra, and has stock options in Alloy, MidiHealth and Estrigenix. N.G. Jaff has no conflict of interest. The authors alone are responsible for the content and writing of the article.
Source of funding
Nil.
References
- Greendale G, Karlamangla AS, Maki PM. The menopause transition and cognition. JAMA Insights. 2020;323(15):1495.
- Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35(1):8–30.
- Maki P, Thurston R. Menopause and brain health: hormonal changes are only part of the story. Front Neurol. 2020;11:562275.
- APA Dictionary of Psychology. APA dictionary of psychology. 2nd ed. Washington (DC): American Psychological Association; 2022. Available from: https://dictionary.apa.org/cognition
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10(4):351–362.
- Epperson C, Shanmugan S, Kim D, et al. New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology (Berl). 2015;232(16):3091–3100.
- Jett S, Malviya N, Schelbaum E, et al. Endogenous and exogenous estrogen exposures: how women’s reproductive health can drive brain aging and inform Alzheimer’s prevention. Front Aging Neurosci. 2022;16(83):1807.
- Scheyer O, Rahman A, Hristov H, et al. Female sex and Alzheimer’s risk: the menopause connection. J Prev Alzheimers Dis. 2018;5(4):225–230.
- Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310–320.
- Cao Q, Tan C, Xu W, et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2020;73(3):1157–1166.
- Hendriks S, Peetoom K, Bakker C, et al. Global prevalence of young-onset dementia: a systematic review and meta-analysis. JAMA Neurol. 2021;78(9):1080–1090.
- Greendale GA, Huang MH, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72(21):1850–1857.
- Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98(9):3829–3838.
- Kilpi F, Soares ALG, Fraser A, et al. Changes in six domains of cognitive function with reproductive and chronological ageing and sex hormones: a longitudinal study in 2411 UK mid-life women. BMC Womens Health. 2020;20(1):177.
- Maki PM, Springer G, Anastos K, et al. Cognitive changes during the menopausal transition: a longitudinal study in women with and without HIV. Menopause. 2021;28(4):360–368.
- Weber MT, Rubin LH, Schroeder R, et al. Cognitive profiles in perimenopause: hormonal and menopausal symptom correlates. Climacteric. 2021;24(4):401–407.
- Maki P, Weber M. A research primer for studies of cognitive changes across the menopause transition. Climacteric. 2021;24(4):382–388.
- Kramer J, Yaffe K, Lengenfelder J, et al. Age and gender interactions on verbal memory performance. J Int Neuropsychol Soc. 2003;9(1):97–102.
- Kramer J, Delis D, Daniel M. Sex differences in verbal learning. J Clin Psychol. 1988;44(6):907–915.
- Drogos LL, Rubin LH, Geller SE, et al. Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause. 2013;20(12):1236–1242.
- Weber M, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16(4):694–700.
- Fuh JL, Wang SJ, Lee SJ, et al. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53(4):447–453.
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028.
- Osterlund M, Keller E, Hurd Y. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–342.
- Sherwin B. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–357.
- Georgakis M, Beskou-Kontou T, Theodoridis I, et al. Surgical menopause in association with cognitive function and risk of dementia: a systematic review and Meta-analysis. Psychoneuroendocrinology. 2019;106:9–19.
- Grigorova M, Sherwin B. No differences in performance on test of working memory and executive functioning between healthy elderly postmenopausal women using or not using hormone therapy. Climacteric. 2006;9(3):181–194.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15(5):848–856.
- Fogel J, Rubin LH, Kilic E, et al. Physiologic vasomotor symptoms are associated with verbal memory dysfunction in breast cancer survivors. Menopause. 2020;27(11):1209–1219.
- Maki PM, Wu M, Rubin LH, et al. Hot flashes are associated with altered brain function during a memory task. Menopause. 2020;27(3):269–277.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23(1):27–32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103(6):1572–1578 e1571.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123–129.
- Newbury CR, Crowley R, Rastle K, et al. Sleep deprivation and memory: meta-analytic reviews of studies on sleep deprivation before and after learning. Psychol Bull. 2021;147(11):1215–1240.
- Maki PM, Gast MJ, Vieweg AJ, et al. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69(13):1322–1330.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12(6):e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810.
- Yaffe K, Sawaya G, Lieberburg I, et al. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279(9):688–695.
- Viscoli CM, Brass LM, Kernan WN, et al. Estrogen therapy and risk of cognitive decline: results from the Women’s Estrogen for Stroke Trial (WEST). Am J Obstet Gynecol. 2005;192(2):387–393.
- Almeida OP, Lautenschlager NT, Vasikaran S, et al. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27(1):141–149.
- Pefanco MA, Kenny AM, Kaplan RF, et al. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc. 2007;55(3):426–431.
- Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94(11):4152–4161.
- Shumaker S, Legault C, Rapp S, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662.
- Shumaker S, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative Randomized Trials. JAMA. 2017;318(10):927–938.
- Espeland M, Rapp S, Shumaker S, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959–2968.
- Resnick S, Espeland M, Jaramillo S, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–142.
- Espeland M, Brinton R, Hugenschmidt C, et al. Impact of type 2 diabetes and postmenopausal hormone therapy on incidence of cognitive impairment in older women. Diabetes Care. 2015;38(12):2316–2324.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364(1665):l665.
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. (
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Georgakis M, Petridou E. Long-term risk of cognitive impairment and dementia following bilateral oophorectomy in premenopausal women-time to rethink policies? JAMA Netw Open. 2021;4(11):e2133016.
- Rocca W, Mielke M, Gazzuola RL, et al. Premature or early bilateral oophorectomy: a 2021 update. Climacteric. 2021;24(5):466–473.
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446.
- Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019.
- Li X, Zhang M, Xu W, et al. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr Alzheimer Res. 2019;16(14):1254–1268.
- Peters R, Booth A, Rockwood K, et al. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022846.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263.
- Ou Y, Tan C, Shen X, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and Meta-analysis of 209 prospective studies. Hypertension. 2020;76(1):217–225.
- , Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553–561.
- Oliveira D, Knight H, Jones K, et al. Motivation and willingness to increase physical activity for dementia risk reduction: Cross-sectional UK survey with people aged 50 and over. Aging Ment Health. 2022;26(9):1899–1908.
- Hörder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: a 44-year longitudinal population study in women. Neurology. 2018;90(15):e1298–e1305.
- Penninkilampi R, Casey A, Singh M, et al. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2018;66(4):1619–1633.
- OlaOlorun F, Shen W. Menopause. In: Oxford research encyclopedia of global public health. 2020 Nov 19 [cited 2022 Sep 21]. Available from: https://oxfordre.com/publichealth/view/10.1093/acrefore/9780190632366.001.0001/acrefore-9780190632366-e-176.
- Drew S, Khutsoane K, Buwu N, et al. Improving experiences of the menopause for women in Zimbabwe and South Africa: co-producing an information resource. Soc Sci. 2022;11(4):143.
- Jaff N, Crowther N. The association of reproductive aging with cognitive function in Sub-Saharan African women. Methods Mol Biol. 2022;2343:71–91.
- Thompson E, Williams D, Walker A, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):3528.
- Vanderlind W, Rabinovitz B, Miao I, et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr Opin Psychiatry. 2021;34(4):420–433.
- Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of long Covid: findings from an online survey. PLoS ONE. 2022;17(3):e0264331.
- Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707.
- Nasserie T, Hittle M, Goodman S. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417.
- Muhaidat N, Alshrouf M, Azzam M, et al. Menstrual symptoms after COVID-19 vaccine: a cross-sectional investigation in the MENA region. IJWH. 2022;14:395–404.
- Stewart S, Newson L, Briggs T, et al. Long COVID risk – a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242.

