Abstract
Objective
This study aimed to determine the effects of estetrol (E4) on hemostasis, lipids, carbohydrate metabolism and bone turnover in postmenopausal women.
Methods
This study was a multicenter, randomized, double-blind placebo-controlled phase 2 trial. Participants (n = 180, age 43–64 years) received E4 2.5 mg, 5 mg, 10 mg and 15 mg or placebo once daily for 12 weeks. Changes from baseline at week 12 were evaluated versus placebo for hemostasis parameters, sex hormone binding globulin (SHBG), lipids, carbohydrate metabolism and bone markers.
Results
Changes for hemostasis parameters were minimal with a small increase only in the normalized activated protein C sensitivity ratio in the E4 15 mg group versus placebo. SHBG increased in the E4 5 mg, 10 mg and 15 mg groups versus placebo. High-density lipoprotein cholesterol increased in all E4 groups; changes were not consistent for other lipids. Significant decreases versus placebo were seen for insulin resistance (E4 10 mg group), hemoglobin A1c (E4 15 mg group) and type 1 collagen C-terminal telopeptide (E4 10 mg and 15 mg groups). Small decreases in osteocalcin in the E4 5 mg, 10 mg and 15 mg groups were significant versus the increase observed in placebo.
Conclusion
E4 had limited impact on hemostasis and potentially beneficial effects on lipids, carbohydrate metabolism and bone turnover.
摘要
目的:研究雌甾醇(E4)对绝经后女性凝血、糖脂代谢及骨代谢的影响。
方法:本研究是一项多中心、随机、双盲、安慰剂对照的2期试验。受试者(n=180, 年龄43-64岁)服用E4 2.5 mg、5 mg、10 mg和15 mg或安慰剂, 每天1次, 持续12周。与安慰剂相比, 第12周评估凝血参数、性激素结合球蛋白(SHBG)、糖脂代谢和骨标志物的变化。
结果:与安慰剂组相比, E4 15 mg组的凝血参数变化极小, 仅活化蛋白C敏感率有小幅增加。与安慰剂组相比, E4 5 mg、10 mg和15 mg组SHBG增加。所有E4组高密度脂蛋白胆固醇均升高;其他脂类的变化并不一致。与安慰剂相比, 胰岛素抵抗(E4 10 mg组)、糖化血红蛋白(E4 15 mg组)和I型胶原C-末端交联顶端肽(E4 10 mg和15mg组)显著降低。E4 5 mg、10 mg和15 mg组的骨钙素水平显著低于安慰剂组的骨钙素水平。
结论:E4对凝血的影响有限, 对糖脂代谢和骨转化有潜在的改善作用。
Introduction
As women age after menopause, risks increase for osteoporosis, cardiovascular disease and cognitive decline. Hormone therapy (HT), used to relieve the spectrum of menopausal symptoms that include both vasomotor and genitourinary symptoms, can also reduce bone loss, bone fractures, new onset diabetes mellitus, coronary heart disease and all-cause mortality [Citation1]. Nevertheless, the risk of developing venous thromboembolism (VTE) is still a matter of concern for women around the age of the menopause and starting oral HT [Citation2]. The risk of VTE is increased especially with preparations containing oral conjugated equine estrogen (CEE) [Citation3], is less pronounced with oral estradiol (E2) and is lowest with transdermal E2 preparations [Citation3,Citation4]. Combinations containing CEE or E2 and medroxyprogesterone acetate or norethisterone have been linked to an even greater increased risk of VTE [Citation3]. The VTE risk has been explained, at least in part, by the hemostatic imbalance induced by HT [Citation5–7]. HT can also impact metabolic markers including total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDLc), triglycerides and glucose levels [Citation8–11].
Estetrol (E4) is a native estrogen with selective action in tissues [Citation12–15]. In a multiple rising-dose trial in postmenopausal women, 2–40 mg of E4 administered once daily improved vaginal cytology and vasomotor symptoms [Citation16].
The primary objective of the E4Relief trial was to select the minimum effective dose of E4 for the treatment of postmenopausal symptoms. The trial showed that E4 15 mg is the minimum effective daily dose for the treatment of vasomotor symptoms [Citation17,Citation18]. Here, we report one of the secondary objectives, which was to determine the effects of different daily doses of E4 (2.5–15 mg) on hemostasis parameters, sex hormone binding globulin (SHBG), lipid profile, carbohydrate metabolism and markers of bone turnover.
Methods
Trial design
This was a multicenter, randomized, double-blind, placebo-controlled, phase 2, dose-finding trial in hysterectomized and non-hysterectomized postmenopausal women (ClinicalTrials.gov NCT02834312, EudraCT 2015-004018-44). The trial was approved by the independent ethics committees of participating centers and conducted in accordance with the ethical principles established by the Declaration of Helsinki and the International Conference on Harmonization – Good Clinical Practice (ICH E6 [R2]) guidelines. All participants gave written informed consent and had the right to withdraw at any time.
Trial population and treatments
Enrolled were postmenopausal women (amenorrhea for at least 12 consecutive months with follicle stimulating hormone >40 IU/l; or amenorrhea for at least 6 months with follicle stimulating hormone >40 IU/l and E2 < 20 pg/ml; or at least 6 weeks after surgical bilateral oophorectomy), aged 40–65 years with a body mass index (BMI) of 18–35 kg/m2. Women with an intact uterus were eligible if transvaginal ultrasound showed a bilayer endometrial thickness ≤5 mm. More detailed selection criteria have been described previously, as well as potential washout conditions [Citation17]. Eligible participants were randomly allocated (1:1:1:1:1) to daily oral treatment with E4 2.5 mg, 5 mg, 10 mg or 15 mg (SEQENS VLG CHEM, France), or placebo, for 12 weeks. E4 and placebo were manufactured by Haupt Pharma Munster GmbH, Germany and packaged and supplied by Almax, UK. All non-hysterectomized women received 10 mg dydrogesterone (Dufaston®, manufacturers Abbott Italy and Mylan Italy, supplied by Almax, UK) once daily for 14 days, after completion of E4 or placebo treatment.
Measurements and outcome parameters
Trial visits were scheduled for screening (visit 1), randomization (visit 2, baseline), after 4 weeks of treatment (visit 3), after 12 weeks of treatment (visit 4 [W12], end of treatment) and after the dydrogesterone treatment (visit 5, follow-up). Blood samples for the assessment of hemostasis and metabolic parameters were taken at baseline and end of treatment (W12). Samples were analyzed by central laboratories (BARC, Belgium and InterLab GmbH/Synlab, Germany), and samples for the normalized activated protein C sensitivity ratio (nAPCsr) were analyzed by QUALIblood, Belgium. The following parameters were analyzed:
•Hemostasis:
○Anticoagulant factors – antithrombin, protein C, free protein S, free tissue factor pathway inhibitor;
○Procoagulant factor – factor VIII;
○Functional assay: resistance for activated protein C (APC) as assessed by the validated endogenous thrombin potential-based APC resistance assay and expressed as the nAPCsr [Citation19];
○Coagulation markers – prothrombin fragment 1 + 2 and D-dimer.
•SHBG;
•Lipid profile – triglycerides, HDLc, low-density lipoprotein cholesterol, total cholesterol and total cholesterol/HDLc ratio;
•Carbohydrate metabolism – fasting glucose, insulin, glycated hemoglobin (HbA1c, expressed as HbA1c/hemoglobin ratio) and insulin resistance (assessed by Homeostasis Model Assessment – Insulin Resistance [HOMA-IR]).
•Bone turnover: osteocalcin and type I collagen C-terminal telopeptide (CTX-1).
All analytical methods, including reference ranges, are presented in Supplemental Table S1.
Statistical analysis
Statistical analyses were performed using SAS® for Windows®. Parameters were summarized using descriptive statistics. No formal sample size calculation was performed for the secondary endpoints in this trial and no formal statistical analysis was planned. The absolute difference between results observed at baseline and W12 in each E4 treatment group was explored using a non-parametric test (Wilcoxon signed-rank test). Pairwise comparisons for each dose of E4 versus placebo were done using the Dwass–Steel–Critchlow–Fligner procedure (without adjustment for multiplicity). The p-value was set at 0.05. We included only participants in the analysis who completed 12 weeks of treatment and who did not have any changes in concomitant medication that could affect any of the hemostatic or metabolic parameters during their participation in the trial. In addition, their treatment compliance needed to be ≥80% based on self-reported medication intake.
Results
Trial population
The trial was conducted in 35 centers in Europe (Belgium, Czech Republic, Great Britain, Ireland and Poland). In total, 260 women were randomized, of which 257 women received trial treatment. A total of 180 participants were included in the analysis of the hemostasis and metabolic parameters (E4 2.5 mg, n = 44; E4 5 mg, n = 32; E4 10 mg, n = 35; E4 15 mg, n = 35; placebo, n = 34) (Supplemental Figure S1). Baseline characteristics in each of the five groups are presented in . Mean age ranged between 53.2 and 54.9 years, mean BMI ranged between 25.4 and 26.7 kg/m2 and mean time since menopause ranged between 4.2 and 5.2 years among the different treatment groups. Treatment groups were comparable for all characteristics assessed.
Table 1. Baseline characteristics by treatment group of all participants who received at least one dose of E4 or placebo.
Hemostasis and metabolic parameters
The median (minimum–maximum) values for the parameters and the change from baseline at week 12 (absolute and % change) are presented in . The outcome of the statistical analysis is presented in Supplemental Table S2. Absolute changes from baseline are shown in , and relative changes from baseline are shown in Supplemental Figure S2.
Figure 1. Absolute changes from baseline at week 12 in (A) hemostasis parameters and (B) metabolic parameters.
E4, estetrol; HDL, high-density lipoprotein; HOMA-IR, Homeostasis Model Assessment – Insulin Resistance; LDL, low-density lipoprotein; nAPCsr, normalized activated protein C sensitivity ratio; SHBG, sex hormone binding globulin; TFPI, tissue factor pathway inhibitor.
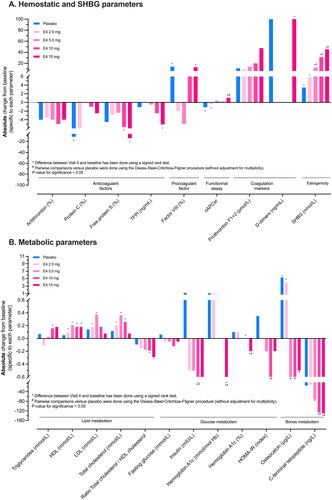
Table 2. Median (minimum, maximum) hemostasis and SHBG, lipid profile, glucose metabolism and bone markers parameters at baseline and week 12, and change from baseline at week 12 (absolute and % change).
Hemostasis parameters
Overall, absolute changes from baseline for any of the hemostasis parameters were small. For the anticoagulant factors, significant absolute median decreases from baseline were observed for protein C in the placebo group (–11.5%), for free protein S in the E4 10 mg and 15 mg groups (–5.7% and −14.2%, respectively) and for tissue factor pathway inhibitor in the E4 15 mg group (–5.1 µg/l). None of these changes from baseline in the E4 groups were significant compared to the placebo group. For factor VIII activity, a statistically significant absolute median increase from baseline was observed in the placebo group (14%). A significant absolute (relative) decrease from baseline in the nAPCsr was observed in the placebo and E4 2.5 mg groups (median −1.20 [–31.0%] and −0.41 [–15.5%], respectively), while a significant absolute (relative) increase was observed in the E4 15 mg group (median 1.09 [+41.9%]), which was statistically different from the decrease observed in the placebo group. D-Dimer increased from baseline in the E4 15 mg and placebo groups (median 100 µg/l for both), which was statistically significant compared to baseline in the E4 15 mg group but not clinically relevant. Such small variation in D-dimers may arise from multiple reasons not related to thrombotic risk, especially in the absence of any clinical suspicion of VTE [Citation20].
Sex hormone-binding globulin
A dose-dependent absolute (relative) increase in SHBG compared to baseline was observed in all E4 groups (median 5.8 [+10.3%], 12.3 [+23.3%], 31.2 [+61.8%] and 45.4 [+99.4%] nmol/l for E4 2.5 mg, 5 mg, 10 mg and 15 mg, respectively) which was statistically significant compared to placebo for the 5, 10 and 15 mg groups.
Metabolic parameters
Lipid profile
Absolute changes from baseline for triglycerides were small in all study groups; in the E4 10 mg group, a median increase of 0.16 mmol/l from baseline reached significance. None of the changes in the E4 groups were significant compared to placebo. HDLc levels significantly increased from baseline in all E4 groups (median 0.09, 0.21, 0.18, and 0.18 mmol/l for E4 2.5 mg, 5 mg, 10 mg and 15 mg, respectively) while in the placebo group no significant increase was observed. Significant increases from baseline were observed for low-density lipoprotein cholesterol in the E4 2.5 mg and 5 mg groups (median 0.18 and 0.38 mmol/l, respectively), and for total cholesterol in the E4 2.5 mg, 5 mg and 10 mg groups (median 0.21, 0.36 and 0.26 mmol/l, respectively). None of these changes were significantly different versus placebo. The total cholesterol/HDLc ratio decreased by dose and significantly changed from baseline in the E4 10 mg and 15 mg groups (median −0.19 and −0.29, respectively) but did not significantly change versus the placebo group. Altogether, these data confirm the low impact of E4 on plasma lipid levels.
Glucose metabolism
No significant changes in fasting glucose levels from baseline were observed. Insulin and HOMA-IR significantly decreased from baseline and versus placebo in the E4 10 mg group (median −2.3 mIU/l for insulin and −0.8 for HOMA-IR). No significant changes were observed for glucose and HOMA-IR in the other E4 groups. The HbA1c/hemoglobin ratio decreased and was significant from baseline in the E4 10 mg group and from baseline and versus placebo in the E4 15 mg group (median −0.0 and −0.2%, respectively).
Bone turnover
Median osteocalcin significantly increased from baseline in the placebo and E4 2.5 mg groups (median 5.4 and 3.9 µg/l respectively). The changes in the E4 5 mg, 10 mg and 15 mg groups were not significant from baseline but were significant versus placebo. CTX-1 showed a decrease which was significant from baseline in the E4 5 mg, 10 mg and 15 mg groups (median −73, −127 and −131 ng/l, respectively). The decreases were significant versus placebo in the E4 10 mg and 15 mg groups.
Discussion
In this dose-finding phase 2 trial, E4 showed minimal impact on hemostasis parameters while a potential beneficial impact was observed on lipids, carbohydrate metabolism and bone markers.
Hemostatic biomarkers and SHBG, the marker reflecting estrogenicity, have been proposed as indicative for the assessment of the hepatic impact of estrogen and progestogen therapies [Citation21]. It has been suggested that the impact on liver proteins including procoagulant and anticoagulant factors could reflect the prothrombotic tendency observed with conventional and, even low dose, HT [Citation22,Citation23]. The risk of VTE is increased in an aging population and in subjects with higher BMI, and oral HT further exacerbates this risk by a factor of 1.5–2 on average [Citation3,Citation4,Citation24–26], making the development of an oral HT with less or no impact on hemostasis of upmost importance. In this trial, the impact of E4 on hemostasis parameters was considered minimal, which confirms the known safety profile of E4 observed in other indications [Citation27–29]. Specifically, the nAPCsr increase observed with E4 15 mg in postmenopausal women (i.e. relative increase of 41.9%) is similar to the increase observed after three cycles in women of reproductive age treated with the oral contraceptive E4 15 mg in association with drospirenone 3 mg (i.e. relative increase of 39.5%) [Citation28]. This nAPCsr increase in the E4 15 mg group is statistically significant compared to placebo but this observation also has to be balanced by the decrease observed in the placebo group after 12 weeks of therapy (i.e. −31.0%). This may have influenced the result and may be explained by the higher baseline value of the placebo group compared to E4 15 mg (i.e. 3.37 versus 2.19, p-value for difference = 0.0059, Mann–Whitney test). The reason for this higher nAPCsr value at baseline in the placebo group deserves further investigations. Compared to ethinylestradiol-containing products used in contraception where the nAPCsr was increased by 165% (ethinylestradiol plus levonorgestrel) and 229% (ethinylestradiol plus drospirenone) at cycle 3 (12 weeks) [Citation28], E4 either administered alone in postmenopausal women or in combination with drospirenone in contraception seems to have a neutral profile on this global coagulation marker.
The fact that E4 has less impact on the nAPCsr than oral micronized E2 (i.e. reported increase of 103% in the trial by Post et al. [Citation30]) is reassuring. The other hemostatic factors did not differ from placebo although some were impacted after 12 weeks of treatment. However, intra-individual variations cannot be excluded, as supported by the results observed in the placebo group for protein C, factor VIII, nAPCsr and SHBG. The absence of a clinically significant impact of E4 on hemostasis compared with placebo suggests that E4 may exert a low risk of VTE in postmenopausal women. The relative changes in the nAPCsr with E4 alone are within the range observed with E4 in combination with drospirenone, a combination that has been predicted to be at lower risk of VTE according to a model based on APC resistance data [Citation29].
As expected from previous investigations with E4 in different settings [Citation27,Citation28,Citation31–33], SHBG is increased in the presence of E4. The levels of SHBG after 12 weeks of treatment with E4 15 mg in postmenopausal women (i.e. 92.7 nmol/l) is in the same range as SHBG levels observed after 3 cycles (12 weeks) of E4 15 mg in association with drospirenone 3 mg (i.e. 97.0 nmol/l) [Citation28]. Also, the magnitude of the effect of E4 on SHBG levels (99.4% increase in the E4 15 mg group) is similar to or lower than the increases observed with oral administration of E2 at doses of 4 mg (171% [Citation34]), 2 mg (133% [Citation35], 67% [Citation34] and 62% [Citation32]) and 1 mg (44% [Citation36]) or CEE at the dose of 0.625 mg (109% [Citation36], 93% [Citation37] and 113% [Citation38]). However, and importantly, while some data support a link between SHBG and global markers of the risk of VTE in the context of hormonal contraception [Citation39,Citation40], levels of SHBG do not correlate with markers of the risk of VTE in postmenopausal women [Citation21–23] and should therefore not drive one to conclusions regarding the VTE risk profile of any HT. Since only the unbound fraction of sexual hormones is biologically active, increasing SHBG decreases E2 and testosterone activity, potentially having a negative effect on energy, mood, sex drive and sexual responsiveness [Citation41], when E2 is used for hormone replacement therapy. E4 does not bind SHBG and is therefore not affected by the SHBG rise. Nevertheless, in a study comparing transdermal E2 with oral CEE and placebo, symptoms related directly to tissue effects of estrogens on the reproductive tract, such as lubrication and pain on penetration, were alleviated with the use of estrogen therapy whatever the route of administration, suggesting at least two distinctive effects of estrogen on sexual function [Citation42]. Further work is thus needed to determine the effect of E4 on sexual function and responsiveness.
Regarding the impact of E4 on the metabolic profile, HDLc levels showed an increase after 12 weeks of treatment in all E4 groups (i.e. increase from baseline ranging from 0.09 to 0.21 mmol/l). These changes were not significantly different from placebo. As the impact on total cholesterol was not consistent over the different treatment groups, this E4-related increase in HDLc translated into a decrease of the total cholesterol/HDLc ratio in the E4 10 mg and 15 mg groups only, although this was not significantly different from placebo. For triglycerides, an increase from baseline was observed with the E4 10 mg group. However, the median value at week 12 was similar to placebo (1.38 vs. 1.42 mmol/l, respectively) and remained within the reference range. Although some increases were observed in other lipid parameters, these were not consistent over the E4 groups. Overall, the impact of E4 on the lipid profile observed in this trial seems neutral and even beneficial since the increase in HDLc counteracts the known decrease due to estrogen deficiency during menopause [Citation43,Citation44].
The decrease in HbA1c observed in the E4 10 mg and 15 mg groups suggests an improved glucose tolerance, which is also supported by the decreases in HOMA-IR. Interestingly, these changes already occur after only 12 weeks and although not significant, the results from other parameters (i.e. fasting glucose and insulin) show the same trend toward an improved glucose tolerance. This is consistent with large randomized controlled trials in which a favorable effect of HT in delaying or reducing the onset of type 2 diabetes in women was observed [Citation45]. Nevertheless, this needs to be confirmed in larger cohorts of patients treated with E4.
Bone turnover, assessed by bone markers CTX-1 and osteocalcin, revealed that E4 dose-dependently reduces the level of CTX-1, a marker of the intensity of bone remodeling [Citation46]. The impact of E4 on osteocalcin after 12 weeks of treatment was not different from baseline but differed from placebo, which showed an increase in the level of osteocalcin. This is consistent with the role of estrogen in bone remodeling [Citation47] and supports the potential beneficial effect of E4 in preventing osteoporosis in menopausal women. Regarding the impact of SHBG on bone biomarkers and mineral density, a recent study that evaluated the effects of E2 in more than 600 postmenopausal women revealed a U-shaped effect suggesting that too high levels are detrimental to bone health while a slight increase may be beneficial [Citation48]. Importantly, contrary to E2, SHBG does not bind E4. The consequence of the absence of binding of E4 to human SHBG is that changes in the plasma levels of this protein will not influence the access of E4 to its target tissues contrary to E2 [Citation49]. This information, together with the reduction of CTX-1, may support the potential benefit of E4 at the dose of 15 mg to sustain bones’ health, but longer studies are required to confirm the benefit of E4 on bone mineral density as these effects may require 6–12 months to stabilize [Citation50].
Although these observations come from a small trial of limited duration, restraining the inclusion of a significant number of women from a broad range of age and BMI, the results are encouraging for the safety profile of E4, which may be explained by its distinct mode of action. E4 acts as an agonist on the nuclear estrogen receptor alpha (ERα) [Citation15,Citation51], and, in contrast to other estrogens, antagonizes the activity of the membrane ERα in several tissues including the breast [Citation13,Citation14]. Moreover, the selective action of E4 in tissues is distinctly different from those of selective estrogen receptor modulators [Citation14,Citation15]. E4 demonstrates numerous vascular protective actions that are independent of the membrane ERα and instead dependent on nuclear ERα. The distinct mode of action of E4 may thus explain its neutral effect on the liver [Citation12,Citation13]. Furthermore, E4 is metabolized in human hepatocytes to form glucuronide and sulfate conjugates [Citation52], making E4 an end-stage product of metabolism which does not generate long-lasting and cumulative metabolites that may lead to a prothrombotic state [Citation15,Citation53–55]. Collectively, the unique mode of action and metabolism of E4 may be responsible for the favorable effects observed on hemostasis and metabolic parameters.
In conclusion, treatment with E4 shows limited impact on hemostasis parameters, which may translate into a lower risk of VTE compared to other estrogens used in HT. Its effect on lipids and glucose metabolism further strengthens the potentially safe cardiovascular risk profile of E4, while the results observed on bone biomarkers are suggestive for a beneficial effect on bone turnover and remodeling. Taken together with the therapeutic benefit of E4 in relieving postmenopausal symptoms [Citation17], these data suggest the potential of an improved oral estrogen-only HT. Ongoing phase 3 trials will provide further insight into the safety profile of E4.
Potential conflict of interest
J.D. is CEO and founder of QUALIblood s.a. and reports personal fees and honorarium from Daiichi-Sankyo, Diagnostica Stago, DOASense, Gedeon Richter, Mithra Pharmaceuticals, Norgine, Portola, Roche and Roche Diagnostics. U.G. is a senior consultant at Mithra Pharmaceuticals. M.T. and M.J. are employees of Estetra SRL (an affiliate company of Mithra Pharmaceuticals). C.B. is an employee of QUALIblood s.a. R.A.L and W.H.U are members of the Scientific Advisory Board of Mithra Pharmaceuticals. J.-M.F. is co-founder of Mithra Pharmaceuticals, shareholder and member of the Board.
Supplemental Material
Download PDF (577.4 KB)Acknowledgements
The authors wish to thank the investigators in the participating centers for conducting the E4Relief trial [Citation17]. The authors also thank the members of the Donesta Scientific Advisory Boards for their valuable advice. Medical writing support was provided by Jan Egberts, Nazanin Hakimzadeh and Mireille Gerrits at Terminal 4 Communications, Hilversum, The Netherlands. Maud Hennion (Pharmalex Belgium) provided statistical support.
Additional information
Funding
References
- Lobo RA, Pickar JH, Stevenson JC, et al. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis. 2016;254:282–290.
- Manson JE, Kaunitz AM. Menopause management–getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83–95.
- Blondon M, van Hylckama Vlieg A, Wiggins KL, et al. Differential associations of oral estradiol and conjugated equine estrogen with hemostatic biomarkers. J Thromb Haemost. 2014;12(6):879–886.
- Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
- Harrington LB, Blondon M, Cushman M, et al. The cross-sectional association between vasomotor symptoms and hemostatic parameter levels in postmenopausal women. Menopause. 2017;24(4):360–370.
- Miller VT. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273(3):199.
- Lobo RA, Bush T, Carr BR, et al. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertil Steril. 2001;76(1):13–24.
- Casanova G, Radavelli S, Lhullier F, et al. Effects of nonoral estradiol-micronized progesterone or low-dose oral estradiol-drospirenone therapy on metabolic variables and markers of endothelial function in early postmenopause. Fertil Steril. 2009;92(2):605–612.
- Terauchi M, Honjo H, Mizunuma H, et al. Effects of oral estradiol and levonorgestrel on cardiovascular risk markers in postmenopausal women. Arch Gynecol Obstet. 2012;285(6):1647–1656.
- Abot A, Fontaine C, Buscato M, et al. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor alpha modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;6(10):1328–1346.
- Arnal JF, Lenfant F, Metivier R, et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97(3):1045–1087.
- Foidart JM, Gaspard U, Pequeux C, et al. Unique vascular benefits of estetrol, a native fetal estrogen with specific actions in tissues (NEST). In: Brinton RD, Genazzani AR, Simoncini T, et al. editors. Sex steroids’ effects on brain, heart and vessels. ISGE Series. Cham: Springer International Publishing; 2019. p. 169–195.
- Gerard C, Arnal JF, Jost M, et al. Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause. Expert Rev Clin Pharmacol. 2022;15(2):121–137.
- Coelingh Bennink HJ, Verhoeven C, Zimmerman Y, et al. Clinical effects of the fetal estrogen estetrol in a multiple-rising-dose study in postmenopausal women. Maturitas. 2016;91:93–100.
- Gaspard U, Taziaux M, Mawet M, et al. A multicenter, randomized study to select the minimum effective dose of estetrol (E4) in postmenopausal women (E4Relief): part 1. Vasomotor symptoms and overall safety. Menopause. 2020;27(8):848–857.
- Utian WH, Gaspard U, Jost M, et al. Estetrol, the next generation of hormone therapy: Results of a phase 2b dose-finding study in postmenopausal women (E4 relief); NAMS, San Diego, CA, USA, October 5, 2018.
- Douxfils J, Morimont L, Delvigne AS, et al. Validation and standardization of the ETP-based activated protein C resistance test for the clinical investigation of steroid contraceptives in women: an unmet clinical and regulatory need. Clin Chem Lab Med. 2020;58(2):294–305.
- Favresse J, Lippi G, Roy PM, et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548–577.
- Eilertsen AL, Dahm AEA, Hoibraaten E, et al. Relationship between sex hormone binding globulin and blood coagulation in women on postmenopausal hormone treatment. Blood Coagul Fibrinolysis. 2019;30(1):17–23.
- Pastori D, Violi F. Blood hormones and venous thromboembolic events: lack of association or lack of standardization? Thromb Haemost. 2018; Nov118(11):1847–1849.
- Roetker NS, MacLehose RF, Hoogeveen RC, et al. Prospective study of endogenous hormones and incidence of venous thromboembolism: the atherosclerosis risk in communities study. Thromb Haemost. 2018;118(11):1940–1950.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573–1580.
- Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med. 2006;166(7):772–780.
- Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227–1231.
- Kluft C, Zimmerman Y, Mawet M, et al. Reduced hemostatic effects with drospirenone-based oral contraceptives containing estetrol vs. ethinyl estradiol. Contraception. 2017;95(2):140–147.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102(6):396–402.
- Morimont L, Dogne JM, Douxfils J. Letter to the editors-in-Chief in response to the article of Abou-Ismail, et al. entitled Estrogen and thrombosis: a bench to bedside review. Thrombosis Res. 2020;192:40–51. Erratum: Thrombosis Res. 2020;193:221–223.
- Post MS, Christella M, Thomassen LG, et al. Effect of oral and transdermal estrogen replacement therapy on hemostatic variables associated with venous thrombosis: a randomized, placebo-controlled study in postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23(6):1116–1121.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20(6):463–475.
- Coelingh Bennink HJT, Verhoeven C, Zimmerman Y, et al. Pharmacodynamic effects of the fetal estrogen estetrol in postmenopausal women: results from a multiple-rising-dose study. Menopause. 2017;24(6):677–685.
- Coelingh Bennink HJT, Zimmerman Y, Verhoeven C, et al. A dose-escalating study with the fetal Estrogen estetrol in healthy men. J Clin Endocrinol Metab. 2018;103(9):3239–3249.
- Ropponen A, Aittomaki K, Vihma V, et al. Effects of oral and transdermal estradiol administration on levels of sex hormone-binding globulin in postmenopausal women with and without a history of intrahepatic cholestasis of pregnancy. J Clin Endocrinol Metab. 2005;90(6):3431–3434.
- Vehkavaara S, Hakala-Ala-Pietila T, Virkamaki A, et al. Differential effects of oral and transdermal estrogen replacement therapy on endothelial function in postmenopausal women. Circulation. 2000;102(22):2687–2693.
- Nachtigall LE, Raju U, Banerjee S, et al. Serum estradiol-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, estradiol, and estrone levels. Menopause. 2000;7(4):243–250.
- Serin IS, Ozçelik B, Başbuğ M, et al. Long-term effects of continuous oral and transdermal estrogen replacement therapy on sex hormone binding globulin and free testosterone levels. Eur J Obstet Gynecol Reprod Biol. 2001;99(2):222–225.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14(6):985–994.
- van Vliet HA, Frolich M, Christella M, et al. Association between sex hormone-binding globulin levels and activated protein C resistance in explaining the risk of thrombosis in users of oral contraceptives containing different progestogens. Hum Reprod. 2005;20(2):563–568.
- Morimont L, Haguet H, Dogne JM, et al. Combined oral contraceptives and venous thromboembolism: review and perspective to mitigate the risk. Front Endocrinol. 2021;12:769187.
- Arlt W. Androgen therapy in women. Eur J Endocrinol. 2006;154(1):1–11.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause. JAMA Intern Med. 2017;177(10):1471–1479.
- Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98(1):83–90.
- Goodman MP. Are all estrogens created equal? A review of oral vs. transdermal therapy. J Womens Health. 2012;21(2):161–169.
- Mauvais-Jarvis F, Manson JE, Stevenson JC, et al. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188.
- Chubb SA. Measurement of C-terminal telopeptide of type I collagen (CTX) in serum. Clin Biochem. 2012;45(12):928–935.
- Li J, Zhang H, Yang C, et al. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34(4):367–379.
- Zhu Z, Zhao J, Fang Y, et al. Association between serum estradiol level, sex hormone binding globulin level, and bone mineral density in middle-aged postmenopausal women. J Orthop Surg Res. 2021;16(1):648.
- Hammond GL, Hogeveen KN, Visser M, et al. Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells. Climacteric. 2008;11(Suppl 1):41–46.
- Reid IR, Eastell R, Fogelman I, et al. A comparison of the effects of raloxifene and conjugated equine Estrogen on bone and lipids in healthy postmenopausal women. Arch Intern Med. 2004;164(8):871–879.
- Guivarc’h E, Buscato M, Guihot AL, et al. Predominant role of nuclear versus membrane estrogen receptor alpha in arterial protection: implications for estrogen receptor alpha modulation in cardiovascular prevention/safety. JAHA. 2018;7(13):e008950.
- Visser M, Foidart J-M, Coelingh Bennink HJT. In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism. Climacteric. 2008;11(Suppl 1):64–68.
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11(Suppl 1):47–58.
- Bagot CN, Marsh MS, Whitehead M, et al. The effect of estrone on thrombin generation may explain the different thrombotic risk between oral and transdermal hormone replacement therapy. J Thromb Haemost. 2010;8(8):1736–1744.
- Douxfils J, Morimont L, Bouvy C. Oral contraceptives and venous thromboembolism: focus on testing that may enable prediction and assessment of the risk. Semin Thromb Hemost. 2020;46(8):872–886.
