Abstract
Objective
This study aimed to evaluate the self-reported satisfaction of Spanish postmenopausal women currently treated for vulvovaginal atrophy (VVA) symptoms.
Methods
The CRETA (CRoss sectional European sTudy on Adherence) is a multicenter cross-sectional study conducted in 29 public and private hospitals in Spain, which enrolled postmenopausal women receiving treatment with ospemifene, local hormone therapy (HT) or vaginal moisturizers for VVA. After the prior informed consent of the patients, sociodemographic and treatment perception data were collected using a structured questionnaire.
Results
Among 752 women who completed the survey, the satisfaction score was significantly higher for the group treated with ospemifene (mean 8.3 ± 1.4) compared with the local HT group (7.2 ± 1.7) and the vaginal moisturizer group (6.5 ± 2.1) according to a 10-point Likert scale (p < 0.0001). Compared to vaginal moisturizers and local HT, participants treated with ospemifene reported the highest adherence (96.7% vs. 70.2% and 78.6%, respectively) and the lowest number of missed doses in the last month (0.6 ± 1.3 standard deviation [SD] vs. 3.5 ± 4.3 SD and 2.0 ± 2.8 SD, respectively) (p < 0.0001). Ospemifene was significantly perceived as easy to use (83.9% vs. 44.9% and 58.6%, respectively; p < 0.0001), efficacious in reducing the time to relieve symptoms (17.1% vs. 7.0% and 6.7%, p = 0.0005 and p = 0.0006, respectively) and convenient for sexual life (53.1% vs. 25.6% and 42.3%, p < 0.0001 and p = 0.0234, respectively).
Conclusions
Among postmenopausal women with VVA, treatment with ospemifene has the most positive perceptions and the highest overall satisfaction level and could be an optimal therapeutic approach, maximizing patient adherence.
摘要
目的: 本研究旨在评估目前正在进行外阴阴道萎缩(VVA)症状治疗的西班牙绝经后妇女的自我报告满意度。
方法: CRETA(欧洲关于依从性的横断面研究)是一项在西班牙29家公立和私立医院进行的多中心横断面研究, 招募接受奥培米芬、局部激素治疗(HT)或VVA阴道保湿剂治疗的绝经后妇女。在患者知情同意后, 使用结构化问卷收集社会人口学和治疗感知数据。
结果: 在完成调查的752名妇女中, 根据10级李克特量表评分, 奥培米芬治疗组的满意度得分(平均8.3 ± 1.4)显著高于局部HT组(7.2 ± 1.7)和阴道保湿剂组(6.5 ± 2.1)(P < 0.0001)。与阴道保湿剂和局部HT相比, 接受奥培米芬治疗的受试者在最后一个月报告的依从性最高(分别为96.7% VS 70.2%和78.6%), 漏服药的次数最少(分别为0.6 ± 1.3SD VS 3.5 ± 4.3SD和2.0 ± 2.8SD)(p < 0.0001)。奥培米芬被认为更易于应用(分别为83.9% VS 44.9%和58.6%;p<0.0001), 缩短症状缓解所需时间(分别为17.1% VS 7.0%和6.7%, p = 0.0005和p = 0.0006), 并且性生活方便(分别为53.1%VS 25.6%和42.3%, p<0.00001和p = 0.0234)
结论: VVA的绝经后妇女对奥培米芬治疗的感受最积极、总体满意度最高, 奥培米芬可能是最佳的治疗方法, 最大限度地提高患者的依从性。
Introduction
Vulvovaginal atrophy (VVA) is a chronic and progressive condition characterized by histological-functional and environmental alterations in the genitals associated with aging and hypoestrogenism. VVA is considered part of the genitourinary syndrome of menopause, a terminology to emphasize the various genital, sexual and urinary symptoms associated with a decrease in estrogen and other sex steroids due to menopause [Citation1]. Low circulating estrogen levels that occur following menopause result in histological and physiological changes in genital tissues. The histological changes lead to decreased flexibility and elasticity of the vaginal vault and blood flow. Besides, an elevated vaginal pH [Citation2] related to vaginal microbiota changes [Citation3] leads to a decrease in the epithelial barrier and lubrication function [Citation4]. Epidemiological data have shown that about 50% of postmenopausal women experience VVA symptoms as a natural physiological consequence of estrogen loss [Citation5]. In European countries, the prevalence of postmenopausal VVA is around 80%, and 65% of women experience VVA within 1 year since menopause [Citation6]. The most common reported symptoms of VVA are vaginal dryness and pain during intercourse (dyspareunia attributed to VVA), followed by itching, dysuria and burning [Citation7] and, less commonly, vaginal tenderness and vaginal bleeding [Citation8,Citation9].
VVA tends to worsen throughout the years after menopause, requiring long-term therapy to achieve good results and avoid the recurrence of symptoms when treatment is stopped. First-line therapy approved for less severe symptoms includes non-hormonal vaginal moisturizers with supplemental vulvar and vaginal lubricants for sexual activity [Citation10,Citation11]. In addition, hormonal medications such as local estrogen therapy, intravaginal dehydroepiandrosterone and oral ospemifene may be effective in the management of moderate–severe VVA symptoms and for those whose symptoms are not resolved with non-hormonal therapies [Citation11]. Ospemifene is an oral selective estrogen receptor modulator with proven efficacy for improving the vaginal maturation index, vaginal pH, dyspareunia and vaginal dryness [Citation12–14].
However, despite available treatment options, the condition’s high prevalence and the impact on women’s lives, VVA is under-reported and underdiagnosed, and therefore undertreated or treated late [Citation15,Citation16], with approximately 7% of women affected reporting use of prescribed therapies [Citation10]. Indeed, average adherence rates to long-term pharmacological treatments for chronic illnesses in developed countries are only about 50%. The World Health Organization (WHO) considers the poor adherence to treatment of chronic diseases as a global problem of striking magnitude with an impact even higher in developing countries [Citation17]. The WHO has defined adherence as ‘the extent to which a person’s behavior – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider’ [Citation18]. The process of adherence is determined by a set of complex and interrelated factors grouped into five domains (patient-centered, therapy-related, social and economic, healthcare system and disease) [Citation19,Citation20]. The consequences of poor medication adherence include compromised treatment efficacy, worsened clinical outcomes [Citation21], a substantial negative effect on patients’ quality of life, and increased healthcare costs and financial burden for society [Citation22]. Patient satisfaction with medication is a major determinant of medication adherence, influencing treatment-related behaviors, such as the possibility of continuing to use their medication, taking their medication correctly and adhering to medication regimens [Citation23]. Therefore, the present study aims to describe and evaluate women’s satisfaction with current treatment for VVA and to identify the patient-related factors affecting medication adherence in Spain.
Methods
Study design
This multicenter, cross-sectional, descriptive, observational study was conducted in 29 gynecology offices of private and public hospitals across different Spanish regions from 9 June 2020 to 11 September 2021 (NCT04607707). Ethical approval for this study was given in all of the involved centers by the Ethics Committee for Research with medicinal products of the León and Bierzo Health Areas.
Objectives
The main objective of the CRETA (CRoss sectional European sTudy on Adherence) was to analyze the satisfaction of postmenopausal women with their current treatment for VVA symptoms. Secondary outcomes included evaluation of medication adherence, healthcare professional’s opinion and women’s quality of life. This article focuses on the main objective and the secondary outcomes related to medication adherence as reported by patients.
Sample size calculation
The study aimed to collect anonymized data from 900 women in 60 different private and public centers. This objective assumed that 50% of those approached would give informed consent for their data to be collected and analyzed, allowing a recruitment rate of 50% to be estimated with an accuracy of 3% (95% asymptotic two-sided confidence interval).
Study participants
Eligible participants were women with natural menopause (defined as the presence of amenorrhea for at least 12 months) with a clinical diagnosis of mild, moderate or severe VVA and currently receiving treatment with ospemifene, local hormone therapy (HT) or vaginal moisturizers according to the datasheet for at least 3 months. In this way, all of the women included are already aware of their situation. They are all informed women and aware of their condition. What is represented in this study are real-life data and standard clinical practice in Spain, recruiting women already diagnosed and treated (with any of the three study design options) at least 3 months before being included in the study. Women were also required to have given their written informed consent to participate in the study. Exclusion criteria were lack of treatment for VVA, current concomitant treatment with more than one drug for VVA (except lubricants) or phytotherapy, treatment discontinuation for VVA due to an adverse effect associated with the use of ospemifene, local HT or vaginal moisturizers and pregnancy, breast-feeding or use of concurrent treatment with any systemic HT inducing amenorrhea.
Gynecologists at each participation site were asked to recruit the first five consecutive patients for each treatment group who attended a routine visit and met all the selection criteria. However, not all centers could recruit the expected number of patients, mainly because the recruiting process was interfered with due to the increased demand for care caused by the COVID-19 pandemic. Thus, in order to reach the expected sample size, gynecologists from the other participating centers were asked to recruit additional women meeting the eligibility criteria.
Data collection
After taking written informed consent from the patients, data were collected in a single routine visit using a structured questionnaire that included 48 questions about patients’ sociodemographic data, the relationship between women and their gynecologist, perceptions about the VVA condition, and women’s satisfaction and medication adherence; lastly, patients were requested to complete a validated health-related quality of life Cervantes scale [Citation24]. The questionnaire was administered on paper to women in the gynecology consulting rooms, and the participants were asked to complete an anonymous written survey. Then, completed surveys were returned in a sealed envelope to a gynecologist to ensure confidentiality. The questionnaires were electronically scanned and the data were automatically captured and uploaded to an electronic health record, specifically prepared for the purpose of this study.
Gynecologists also completed an online questionnaire which included 41 items regarding their professional experience treating VVA, data about the treatments prescribed to their patients, the preferences, the pros and cons, the perceived adherence and the efficacy of each treatment considered in this study, and their perception on what their patients thought about the condition and the treatments.
Statistical analysis
Qualitative outcomes analysis was performed using the chi-square test and Fisher’s exact test in non-normally distributed data. Comparisons of quantitative outcomes between different groups were performed by the Student t-test, while the Mantel–Haenszel test was used to compare ordinal variables. The statistical significance was considered as p < 0.05. Statistical analyses were performed by the SAS software package, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Population characteristics
A sample size of 900 patients was initially calculated and, finally, 831 were recruited for the study. Of them, 79 were excluded due to lack of current treatment (n = 20), concomitant treatment with more than one medication (n = 53) and unknown current treatment (n = 6). The remaining 752 women met all of the selection criteria and completed the questionnaire. Among them, 40.2% (n = 302) were treated with vaginal moisturizers, 31.8% (n = 239) with local HT and 28.1% (n = 211) with ospemifene.
In the total number of centers, 74% were private centers and 26% public centers. This reflects the reality of clinical practice because most of the VVA treatments are not financed by the national health system, and many women choose private healthcare for this condition.
The baseline demographics and clinical characteristics of the population are presented in . Most patients (71.3%) were aged 51–65 years, were sexually active (81.2%), had children (mostly one or two) (68.5%) and had not undergone any cesarean section (81.7%). Menopause duration was between 1 and 10 years for most women (67.9%).
Table 1. Baseline demographics and clinical characteristics of the population.
Relationship between patients and gynecologists and patient’s knowledge on VVA
More than half of the patients (57.9%) referred that the conversation about VVA with their gynecologists was brought up by themselves. A vast majority of the patients (around 98%) were satisfied with the information received from their gynecologists about the disease and treatment initiation. Additionally, 88.9% of women participated in shared decision-making regarding the treatment, and 93.7% considered that they were appropriately informed about the need to maintain a long-term treatment. The features most frequently recognized by the overall population as typical symptoms of VVA were vaginal dryness (89.6% of respondents), followed by dyspareunia (67.8%), itching (42.7%), burning (42.3%) and decreased sexual desire (39.2%). However, women treated with ospemifene seemed more familiar with some of the symptoms, since higher proportions of women recognized dyspareunia (81.5% vs. 28.3% for moisturizers, p < 0.001; and 67.8% for local HT, p = 0.0011), itching (51.2% vs. 33.1% for moisturizers, p < 0.0001) and burning (52.6% vs. 32.1% for moisturizers, p < 0.001) as typical VVA symptoms.
Subjective VVA symptoms and impact on daily life
Women with VVA receiving ospemifene complained of more severe symptoms when initiating the present treatment than those treated with vaginal moisturizers (45.0% vs. 20.4%, p < 0.0001) or local HT (45.0% vs. 38.6%, p = 0.0178). The most bothersome symptom was vaginal dryness, reported by 91.4% of women. Other common bothersome symptoms were dyspareunia (70.7%), burning (45.8%) and itching (44.4%), although no statistically significant differences were found between treatment groups. Patients rated the relevance of their sexual life on a 10-point Likert scale (0 = ‘none’ to 10 = ‘a lot’). The highest score was reported by women treated with ospemifene (7.3 ± 2.1) and was statistically significant compared to vaginal moisturizers (6.6 ± 2.6, p = 0.0035) and local HT (6.5 ± 2.6, p = 0.0009).
Participants’ experiences regarding VVA treatments
In 64.2% of the patients, the current treatment for VVA was their first treatment, with the highest percentage in the group treated with vaginal moisturizers (75.1%), followed by the local HT group (66.0%) and, finally, the ospemifene group (46.7%) (p < 0.05). Indeed, in the ospemifene group, 41.1% of women had used more than two prior treatments before switching to the current medication. This is totally understandable, since its labeling reflected an indication for second-line or third-line treatment, after the use of other previously prescribed treatments.
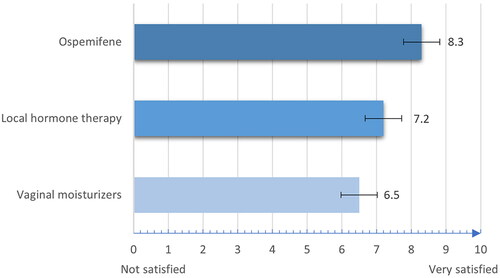
Regarding satisfaction with VVA treatment, participants were asked to rate how satisfactory these therapies were on a 10-point Likert scale (where 0 indicated ‘not at all satisfactory’ and 10 indicated ‘extremely satisfactory’). Although the mean ± standard deviation (SD) score for the overall sample was 7.2 ± 1.9, the satisfaction score was significantly higher for the ospemifene group with a mean value of 8.3 ± 1.4 in comparison to 7.2 ± 1.7 for the local HT group and 6.5 ± 2.1 for the vaginal moisturizer group (p < 0.0001) ().
Figure 1. Satisfaction ratings of the different vulvovaginal atrophy therapy formulations. All differences were statistically significant (p < 0.0001). Bars represent the standard deviation from the mean.
Most participants (84.7%) stated that they would be willing to follow their current treatment long term, the highest percentage being for the ospemifene group (90.9%, n = 190, p = 0.003 vs. moisturizers and p = 0.1373 vs. local HT), followed by the local HT group (86.2%, n = 200, p = 0.0396 vs. moisturizers) and the vaginal moisturizer group (79.3%, n = 237). Women using ospemifene had the highest adherence to treatment, with 96.7% communicating that they followed the treatment according to their doctor’s instructions in contrast to 70.2% for moisturizers and 78.6% for local HT (p < 0.001). In this line, the lowest number of missed doses in the last month was reported for the ospemifene group, with a mean value of 0.6 ± 1.3, followed by the local HT (2.0 ± 2.8) and vaginal moisturizer (3.5 ± 4.3) groups (p < 0.0001) (). Interestingly, treatment compliance was considered highly relevant by 84.1% of women within the ospemifene group in contrast to 74.9% and 82.4% within the moisturizer and local HT groups, respectively (p < 0.0001). Indeed, a higher proportion of women receiving ospemifene (47.4%) believed that poor adherence had negative consequences on the efficacy of the treatment than those treated with moisturizers (28.8%, p < 0.0001) and local HT (35.2%, p = 0.0012).
Table 2. Responses provided by surveyed women on medication adherence.
Regarding preferences about the administration route, 54.4% of all participants showed a predilection for an oral pill. Among women with comorbidities, in the case that add-on medication for VVA symptoms was required, the vaginal route was preferred by women users of vaginal moisturizers (54.3%; n = 159) and local HT (62.9%; n = 149), whereas the oral route was preferred by women for treatment with ospemifene (93.3%; n = 195) (p < 0.0001).
The mean time to VVA symptom improvement with current treatment was 1.8 ± 1.8 months. Vaginal dryness was the VVA symptom experiencing the highest relief in all treatment groups and was reported by 93.7% of women. As reported by patients, ospemifene significantly improved the following symptoms in comparison to the other treatments: dyspareunia (79.3% of respondents vs. 69.3% for local HT, p = 0.0168; and 61.6% for vaginal moisturizers, p < 0.0001) and decreased sexual desire (26.9% of respondents vs. 18.2% for local HT, p = 0.0297; and 14.0% for vaginal moisturizers, p = 0.0005).
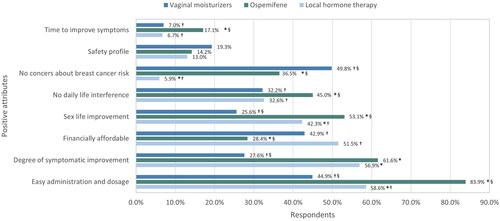
Participants also expressed their views on the advantages associated with VVA treatment (). Ospemifene showed significant differences in comparison to other treatments for the following positive attributes: route of administration easy to use (83.9% of respondents, p < 0.0001), positive impact on sexual life (53.1%, p < 0.05), lack of interference on woman’s life (45.0%, p < 0.05) and efficacious in reducing the time to improve VVA symptoms (17.1%, p < 0.05). The significant advantages described for local HT and vaginal moisturizers compared to ospemifene were being financially affordable (51.5% and 42.9% vs. 28.4%, p < 0.001 and p = 0.0011, respectively) and, only for vaginal moisturizers, its lack of concern about breast cancer (49.8% vs. 5.9% for local HT, p < 0.0001; and vs. 36.5% for ospemifene, p < 0.0029). Among the disadvantages perceived by surveyed women about the different formulations for VVA treatment, participants using ospemifene were mostly worried because of the medication price (92.2%; p < 0.0001) and safety profile (54.9%, p < 0.0006). However, only 20.2% of participants were willing to abandon the ospemifene treatment due to any of these disadvantages, compared to 35.1% (p = 0.0006) with local HT and 37.2% (p < 0.0001) with vaginal moisturizers. Breast cancer risk was the most common concern among the women treated with local HT (67.3%, p < 0.0001), while in the vaginal moisturizer group this aspect was the least important for participants.
Discussion
Summary of main findings
The CRETA evaluated woman’s satisfaction and adherence with current therapeutic options among 752 Spanish postmenopausal women. The most common treatment was vaginal moisturizers, followed by local HT and ospemifene. Interestingly, women in the ospemifene group complained of more severe symptoms and a greater impact on their sexual life than those treated with vaginal moisturizers or local HT. In general terms, all of the treatment options were reported as effective in relieving VVA symptoms, although statistically significant differences were found in favor of ospemifene to improve some symptoms, such as dyspareunia and decreased sexual desire, measured by the sexuality domain of the validated health-related quality of life Cervantes scale [Citation24]. Based on the study’s findings, patients treated with ospemifene reported significantly greater adherence and lower mean number of missed doses in the last month compared to the other treatment groups. The main finding of the current study is the high satisfaction expressed by symptomatic participants with VVA treated with ospemifene, which was significantly greater than that reported with moisturizers or local HT. In addition, most participants stated that they would be willing to follow their current treatment long term and highlighted the route of administration easy to use, positive impact on sexual life, lack of interference on woman’s life and shorter time to improve VVA symptoms as positive attributes associated with ospemifene treatment compared to the other therapies. On the other hand, the high medication price and safety profile were the main disadvantages perceived by women using ospemifene.
It must be noted that the good results attributed to ospemifene in terms of satisfaction, adherence and better improvement of some symptoms, such as dyspareunia and decreased sexual desire, were obtained in a cohort of patients with more unfavorable features compared to the moisturizer and local HT cohorts. Women treated with ospemifene also reported a better understanding of the condition, suggesting a higher cultural background. Indeed, the ospemifene cohort reported more severe symptoms and included a higher proportion of women who had received other medications for AVV before ospemifene, suggesting that previous treatments were not efficient or satisfactory enough. Recently, it has been suggested that despite ospemifene improving both moderate and severe AVV symptoms, the earlier treatment may be associated with a greater benefit when symptoms are moderate rather than severe [Citation25].
Comparison with existing literature
In our cohort of women, vaginal dryness was rated as the most prevalent and the most bothersome AVV symptom. Several studies have analyzed the prevalence and impact of VVA symptoms experienced by women during menopause [Citation9,Citation26–33]. In line with our findings, the most common symptom among these studies was vaginal dryness, with the European Vulvovaginal Epidemiological Survey (EVES) study reporting an estimated prevalence of up to 90%. Similarly, the European Real Women’s Views of Treatment Options for Menopausal Vaginal Changes (REVIVE) survey showed that the most common VVA-associated symptoms in European postmenopausal women were vaginal dryness (70.0%) followed by vaginal irritation (32.7%) [Citation34]. In the VIVA (Vaginal Health: Insights, Views, and Attitudes) survey, lower proportions of postmenopausal women were concerned about vaginal dryness (36%) and dyspareunia (24%), although those percentages increased (83% and 42%, respectively) when only the women specifically reporting vaginal discomfort were considered [Citation35].
In our study, the highest adherence to treatment was reported in women receiving the daily dose of ospemifene. A recent 12-month study including 86,946 patients also described a higher adherence for the ospemifene group compared to local HT (40% vs. 21%) [Citation36]. Although previous head-to-head studies comparing the satisfaction associated with the different VVA treatments are not available, a clear relationship between medical treatment satisfaction and patient compliance has been proposed [Citation37,Citation38]; with the consequent potential impact on perceived treatment effectiveness [Citation39]. In our head-to-head study, the treatment satisfaction was highest with ospemifene, reaching a mean score higher than 8 points out of 10. Moreover, most participants stated that they would be willing to follow their current treatment long term. Patient preference has also been directly linked to the method of administration [Citation36]. In this context, considering all treatment groups, more than half of the participants in our survey stated a clear preference for an oral route of administration for the treatment of VVA problems. The oral route also was the preferred route of administration among the group of women with comorbidities who had been treated with oral ospemifene for their VVA symptoms in the case that add-on medication for VVA symptoms was required. Similar to these findings, Nappi et al. found that 54.0% of participants preferred an oral pill, whereas only 46.0% preferred or somewhat preferred a vaginally administered product for the treatment of VVA problems [Citation34]. Our results showed that ospemifene treatment was perceived as a route of administration easy to use, efficacious in reducing the time to relieve VVA symptoms and positively impacting sexual life without interfering with a woman’s life. Findings from this study underscore the importance of a patient-friendly formulation for the treatment of VVA suggested by previous studies since product attributes influence women’s adherence to treatment and should assist healthcare professionals in optimizing treatment [Citation16].
Limitations and strengths
Among the limitations of our study, we highlight the possible selection bias. Women who completed the survey were currently in treatment for VVA symptoms and had a high level of knowledge about VVA and treatment acceptability, which might be higher than that found in untreated patients. Other limitations of this study are the consequence of the characteristics and nature of surveys. Our results came from a self-reported questionnaire, which suffers from a certain recall bias effect and the limitations of reporting subjective symptoms from an objective condition. Despite these limitations, the cohort size of our study is large enough to support the reliability of our findings. Furthermore, the majority of currently available questionnaires that assess VVA of menopausal women focus principally on VVA symptoms, whereas our questionnaire was specifically designed using women’s own words as the basis of the survey to assess also satisfaction about VVA medications and adherence to treatment.
Potential clinical value
The present findings highlight the perceived satisfaction and adherence to different treatments in postmenopausal women with VVA and provide new insights about women’s perceptions regarding the advantages and disadvantages of different formulations for VVA treatment. Based on the study’s findings, ospemifene clinical characteristics give the opportunity for long-term therapies for VVA and increase the adherence and persistence to treatment, representing an innovative evolution that can help reduce the burden of symptoms and the consequences of postmenopausal VVA. Additionally, our findings can contribute to the development of new methods to improve patient adherence throughout VVA treatment. Improvement of treatment satisfaction and adherence will not only enhance the quality of life experienced by postmenopausal women but will also help to follow and restore their sexual and emotional well-being.
Conclusions
The CRETA has examined the self-reported satisfaction of Spanish postmenopausal women currently treated for VVA symptoms. The main finding of our study is the high satisfaction and adherence reported by participants with VVA treated with ospemifene besides a lower number of missed doses compared to vaginal moisturizers and local HT. Respondents preferred oral formulations for VVA treatment, and ospemifene was perceived as easy to use, effective in reducing the time to relieve VVA symptoms (especially dyspareunia and decreased sexual desire) and convenient for sexual life. Our findings suggest that ospemifene can help reduce the burden of VVA symptoms and could be an optimal therapeutic approach used in the treatment of postmenopausal VVA while maximizing patient adherence and reducing the missing medication doses.
Summary sentences for table of contents
According to Spanish postmenopausal women’s perceptions, treatment with ospemifene for vulvovaginal atrophy (VVA) symptoms demonstrates significantly greater satisfaction and adherence when compared with other VVA treatments such as vaginal moisturizers and local hormone therapy. Participants also highlight several positive attributes associated with ospemifene treatment, such as easy management, efficacy, rapid action and convenience for sexual life.
Potential conflict of interest
Shionogi S.L.U. sponsored the study and provided support for the medical writing assistance by Kalispera medical writing S.L. R.S.-B. has received consulting fees and speaker fees from Shionogi. S.P. has received consulting fees and speaker fees outside the submitted work from Shionogi; grants from Arkochim, Bayer Healthcare, Novo Nordisk, Lacer, Sandoz and Theramex; grants and personal fees from Exeltis, Gedeon Richter, Procare Health and Serelys; and personal fees from Alma Laser, Pfizer and Procaps. M.J.C. has received consulting fees and speaker fees from Shionogi. M.V.d.D.P.d.Z. has received speaker fees from Shionogi. A.J.G.C. and J.J.Q.M. are employees of Shionogi S.L.U. For the remaining authors, no conflicts of interest were declared.
Source of funding
The study received financial support from Shionogi S.L.U.
Acknowledgements
The authors thank Kalispera medical writing S.L. for providing medical writing support.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13697137.2024.2327254)
References
- Portman DJ, Gass MLS. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the international society for the study of women’s sexual health and the North American menopause society. Menopause. 2014;21(10):1063–1068.
- Qi W, Li H, Wang C, et al. The effect of pathophysiological changes in the vaginal milieu on the signs and symptoms of genitourinary syndrome of menopause (GSM). Menopause. 2020;28(1):102–108.
- Shardell M, Gravitt PE, Burke AE, et al. Association of vaginal microbiota with signs and symptoms of the genitourinary syndrome of menopause across reproductive stages. J Gerontol A Biol Sci Med Sci. 2021;76(9):1542–1550.
- Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997;314(4):228–231.
- Palacios S, Nappi RE, Bruyniks N, et al. The European Vulvovaginal Epidemiological Survey (EVES): prevalence, symptoms and impact of vulvovaginal atrophy of menopause. Climacteric. 2018;21(3):286–291.
- Nappi RE, Seracchioli R, Salvatore S, et al. Impact of vulvovaginal atrophy of menopause: prevalence and symptoms in Italian women according to the EVES study. Gynecol Endocrinol. 2019;35(5):453–459.
- Palma F, Xholli A, Cagnacci A. The most bothersome symptom of vaginal atrophy: evidence from the observational AGATA study. Maturitas. 2018;108:18–23.
- Kingsberg SA, Wysocki S, Magnus L, et al. Vulvar and vaginal atrophy in postmenopausal women: findings from the revive (REal women’s views of treatment options for menopausal vaginal changes) survey. J Sex Med. 2013;10(7):1790–1799.
- Cagnacci A, Carbone MM, Palma F. Prevalence and association between objective signs and subjective symptoms of vaginal atrophy: the AGATA study. Menopause. 2016;23(10):1139–1145.
- Faubion SS, Kingsberg SA, Clark AL, et al. The 2020 genitourinary syndrome of menopause position statement of the North American menopause society. Menopause. 2020;27(9):976–992.
- Hirschberg AL, Bitzer J, Cano A, et al. Topical estrogens and non-hormonal preparations for postmenopausal vulvovaginal atrophy: an EMAS clinical guide. Maturitas. 2021;148:55–61.
- Alvisi S, Baldassarre M, Martelli V, et al. Effects of ospemifene on vaginal epithelium of post-menopausal women. Gynecol Endocrinol. 2017;33(12):946–950.
- Alvisi S, Baldassarre M, Gava G, et al. Structure of epithelial and stromal compartments of vulvar and vaginal tissue from women with Vulvo-Vaginal atrophy taking ospemifene. J Sex Med. 2018;15(12):1776–1784.
- Goldstein SW, Winter AG, Goldstein I. Improvements to the vulva, vestibule, urethral meatus, and vagina in women treated with ospemifene for moderate to severe dyspareunia: a prospective vulvoscopic pilot study. Sex Med. 2018;6(2):154–161.
- Krychman M, Graham S, Bernick B, et al. The women’s empower survey: women’s knowledge and awareness of treatment options for vulvar and vaginal atrophy remains inadequate. J Sex Med. 2017;14(3):425–433.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s empower survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413–424.
- Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35(3):207–207.
- World Health Organization. Adherence to long-term therapies: evidence for action. World Health Organization. 2003. https://apps.who.int/iris/handle/10665/42682
- Gast A, Mathes T. Medication adherence influencing factors – an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):1–17.
- Jin J, Sklar GE, Sen OV, et al. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286.
- Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15.
- Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530.
- Alam MM, Sikdar P, Kumar A, et al. Assessing adherence and patient satisfaction with medication: validation of TSQM in emerging markets. Int J Pharm Healthc Mark. 2018;12(4):409–432.
- Palacios S, Ferrer-Barriendos J, Parrilla JJ, et al. [Health-related quality of life in the spanish women through and beyond menopause. Development and validation of the cervantes scale]. Med Clin. 2004;122(6):205–211.
- Palacios S, Panay N, Sánchez-Borrego R, et al. Earlier treatment of vulvovaginal atrophy in post-menopausal women may improve treatment outcomes. J Gynecol Women’s Heal. 2019;16(1):555928.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5(1):437–447.
- Simon JA, Kokot-Kierepa M, Goldstein J, et al. Vaginal health in the United States: results from the vaginal health: insights, views & attitudes survey. Menopause. 2013;20(10):1043–1048.
- Nappi RE, Kingsberg S, Maamari R, et al. The closer (CLarifying vaginal atrophy’s impact on SEx and relationships) survey: implications of vaginal discomfort in postmenopausal women and in male partners. J Sex Med. 2013;10(9):2232–2241.
- Nappi RE, Particco M, Biglia N, et al. Attitudes and perceptions towards vulvar and vaginal atrophy in Italian post-menopausal women: evidence from the European REVIVE survey. Maturitas. 2016;91:74–80.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying vaginal atrophy’s impact on sex and relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21(2):137–142.
- Caruso S, Cianci S, Amore FF, et al. Quality of life and sexual function of naturally postmenopausal women on an ultralow-concentration estriol vaginal gel. Menopause. 2016;23(1):47–54.
- Palma F, Volpe A, Villa P, et al. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Pastore LM, Carter RA, Hulka BS, et al. Self-reported urogenital symptoms in postmenopausal women: women’s health initiative. Maturitas. 2004;49(4):292–303.
- Nappi RE, Palacios S, Panay N, et al. Vulvar and vaginal atrophy in four european countries: evidence from the european revive survey. Climacteric. 2016;19(2):188–197.
- Nappi RE, Kokot-Kierepa M. Vaginal health: insights, views and attitudes (VIVA) – results from an international survey. Climacteric. 2012;15(1):36–44.
- Faught BM, Soulban G, Yeaw J, et al. Ospemifene versus local estrogen: adherence and costs in postmenopausal dyspareunia. J Comp Eff Res. 2019;8(13):1111–1123.
- Naidu A. Factors affecting patient satisfaction and healthcare quality. Int J Health Care Qual Assur. 2009;22(4):366–381.
- Barbosa CD, Balp MM, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48.
- Volpicelli Leonard K, Robertson C, Bhowmick A, et al. Perceived treatment satisfaction and effectiveness facilitators among patients with chronic health conditions: a Self-Reported survey. Interact J Med Res. 2020;9(1):e13029.