Abstract
This systematic review assesses the effect of menopausal hormone therapy (MHT) on cardiovascular outcomes and risk factors in postmenopausal women with cardiovascular disease (CVD). The Medline, Embase and Cochrane databases were searched from inception to December 2022 for randomized controlled trials (RCTs) and observational studies using methodology from a previous Cochrane review. Quality assessment used the Cochrane risk of bias tool and Newcastle–Ottawa scale, respectively. From 5647 studies identified, 29 (23 RCTs and six observational studies) were included. Most studies were conducted in North America or Europe and investigated oral estrogens. Participants were older with varying frequency of cardiac risk factors and underlying CVD. No significant difference was observed between MHT users and controls regarding primary outcomes of non-fatal myocardial infarction, cardiovascular death or stroke. No difference in frequency of angina, heart failure and transient ischemic attacks was observed. Inconsistent effects of MHT on angiographic progression were seen and varied with glycemic status. Estradiol had a positive effect on flow-mediated dilatation. Limited studies identified differing effects of MHT on cardiac risk factors, varying with estrogen preparation. This study confirms no benefit of MHT for secondary CVD prevention, highlighting evidence limitations and the importance of shared decision-making when managing menopausal symptoms in women with CVD.
摘要
这项系统综述评估了绝经后激素治疗(MHT)对绝经后妇女心血管疾病(CVD)心血管结局和危险因素的影响。从开始到2022年12月, 使用先前Cochrane综述的方法搜索Medline、Embase和Cochrane数据库中的随机对照试验(RCT)和观察性研究。质量评估分别使用Cochrane偏倚风险工具和Newcastle–Ottawa量表。在已确定的5647项研究中, 纳入了29项(23项随机对照试验和6项观察性研究)。大多数研究在北美或欧洲进行, 并调查了口服雌激素。参与者年龄较大, 有不同频率的心脏危险因素和潜在的心血管疾病。MHT使用者和对照组在非致命性心肌梗死、心血管死亡或中风的主要转归方面没有观察到显著差异。心绞痛、心力衰竭和短暂性脑缺血发作的频率没有差异。MHT对血管造影进展的影响不一致, 且随血糖状况而变化。雌二醇对血流介导的扩张有积极作用。有限的研究确定了MHT对心脏危险因素的不同影响, 因雌激素制剂的不同而不同。这项研究证实了MHT对心血管疾病二级预防没有益处, 强调了证据的局限性和在处理心血管疾病女性更年期症状时共同决策的重要性。
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and a significant cause of morbidity in women [Citation1]. Adverse cardiometabolic changes associated with the menopause transition plus aging contribute to the increased risk of CVD in women with increasing incidence after age 60 years [Citation2]. Sex-specific differences in CVD risk factors, clinical presentation and risk perception contribute to CVD being ‘understudied, under-recognized, underdiagnosed and undertreated’ in women [Citation1]. Vasomotor symptoms affect up to 80% of women during the menopause transition with a median duration of 7.4 years [Citation3]. However, a significant proportion of postmenopausal women report vasomotor symptoms persisting past age 60 years [Citation4]. Multiple factors affect the duration of vasomotor symptoms, including ethnicity, obesity, smoking status, age at onset of symptoms, education level, perceived stress and depression [Citation3]. These factors also influence CVD risk in women and vasomotor symptoms are probably linked to CVD risk [Citation5]. Menopausal hormone therapy (MHT) is the most effective therapy for management of vasomotor symptoms and urogenital atrophy [Citation6], and guidelines agree that MHT initiated for management of vasomotor symptoms in healthy women before age 60 years or within 10 years of menopause is associated with a favorable risk–benefit profile [Citation7–10]. Age, time since menopause, MHT preparation and underlying cardiac risk influence the interaction between MHT and CVD [Citation11–13]. Variation exists regarding recommendations for the use of MHT in women with CVD risk factors or at high risk of CVD [Citation9,Citation10,Citation14]. A 2015 Cochrane review addressing the question of MHT and secondary CVD prevention concluded that there was no protective effect of MHT [Citation15]. Our aim was to conduct an updated systematic review to assess the effect of MHT on cardiovascular outcomes and CVD risk factors in postmenopausal women with established CVD.
Materials and methods
Search strategy
This systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement and registered in PROSPERO (CRD42022359604) [Citation16]. The Medline, Embase and Cochrane databases were searched from inception to December 2022 for randomized controlled trials (RCTs) and observational studies. The search was repeated in May 2023 to identify any further publications. MeSH search terms were adapted to each database and included the terms ‘post menopause’, ‘menopausal hormone therapy’ and ‘cardiovascular disease’ (Supplementary Table 1).
English language studies of postmenopausal women aged over 18 years with a diagnosis of established CVD that reported the effect of MHT were included. Studies including premenopausal or perimenopausal women or women without CVD were excluded. MHT included oral or transdermal estrogen with or without cyclic/continuous progestin; studies using only vaginal estrogen were excluded. Comparators included a placebo group or no therapy. Primary outcomes were non-fatal myocardial infarction, cardiovascular death or stroke. Secondary outcomes included angina, heart failure, transient ischemic attack (TIA), angiographic progression of CVD, and change in cardiovascular risk factors including lipid concentrations, blood pressure and glucose tolerance. The PICO (Patient, Intervention, Control, and Outcome) strategy is shown in Supplementary Table 2. Abstracts, case–control or cross-sectional studies, case reports, review articles and non-human studies were excluded.
Data extraction and quality assessment
Abstracts were independently reviewed by two reviewers (S.B. and R.G.) and any disagreements were resolved through discussion with a third reviewer (A.J.V.). A full text review of included studies and data extraction was conducted independently by two reviewers (S.B. and A.J.V.). Data collection included trial characteristics (author, year of publication, study design), participants (number, average age, baseline cardiovascular risk factors), intervention (type and dose of hormone therapy) and outcomes ( and Supplementary Table 3).
Table 1. Summary of included studies.
Assessment for risk of bias was conducted using the Cochrane risk of bias tool for RCTs as outlined in the Cochrane Handbook of Systematic Reviews of Interventions [Citation46]. The Newcastle–Ottawa scale was used for observational studies [Citation47].
Data synthesis
A random-effects model was used for synthesis of dichotomous data due to the anticipated level of heterogeneity in the included studies. Primary outcome results were expressed as risk ratios (RRs) with 95% confidence intervals (CIs). The heterogeneity of studies was tested using the I2 statistic, where I2 > 50% indicates substantial heterogeneity [Citation46]. ReviewManager software (RevMan version 5.4; The Cochrane Collaboration, 2020) was used for the meta-analysis. Due to the limited number of included studies in our analysis, we were unable to assess publication bias using a funnel plot [Citation46]. Studies that were not included in the analysis were presented narratively.
Results
Characteristics of selected studies
The search strategy yielded a total of 5647 studies published between 1972 and 2023, of which 29 studies were included in the systematic review ( and ). Of these 29 studies, 23 were RCTs and six were observational studies. Most studies were conducted in North America or Europe. Participants were older postmenopausal women, mean age >58 years for 28/29 studies (). All women had established CVD, with varying frequency of baseline cardiovascular risk factors (Supplementary Table 3). Most studies examined oral conjugated equine estrogens (CEE) combined with oral medroxyprogesterone acetate (MPA). Only six of the RCTs assessed transdermal estrogen ().
Figure 1. Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) flow diagram for new systematic reviews which included searches of databases, registers and other sources. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From Page et al. [16]. For more information, see http://www.prisma-statement.org/
![Figure 1. Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) flow diagram for new systematic reviews which included searches of databases, registers and other sources. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From Page et al. [16]. For more information, see http://www.prisma-statement.org/](/cms/asset/c4f21f40-6508-47ba-bf15-00b802585790/icmt_a_2273524_f0001_c.jpg)
Effect of MHT on cardiovascular events (primary outcomes)
Seven RCTs (n = 5359) assessing the impact of oral MHT on non-fatal myocardial infarction were included in the meta-analysis [Citation19,Citation21,Citation24,Citation28,Citation34,Citation38,Citation39] ( and ). Compared to placebo, MHT did not show any significant effect on non-fatal myocardial infarction (RR 0.97, 95% CI 0.80–1.18, p = 0.76, I2 = 0%) (). An RCT (n = 255) comparing transdermal estradiol (80 μg/day) versus no therapy reported no significant difference in overall CVD events (cardiac death, myocardial infarction or unstable angina) but was underpowered with a 40% withdrawal rate in the MHT group [Citation20].
Figure 2. Forest plots of primary outcomes: menopausal hormone therapy (MHT) versus controls on (a) non-fatal myocardial infarction, (b) cardiovascular disease (CVD) death and (c) stroke. CI, confidence interval; M-H, Mantel–Haenszel.
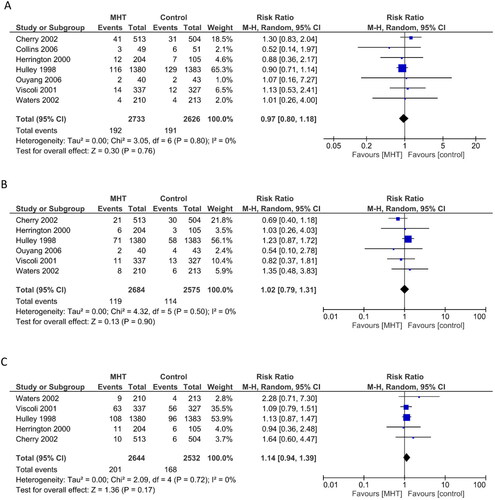
Six RCTs (n = 5259) reported the effect of MHT on CVD death and were included in the meta-analysis [Citation21,Citation24,Citation28,Citation34,Citation38,Citation39] (). No significant effect was observed compared to placebo (RR 1.02, 95% CI 0.79–1.31, p = 0.90, I2 = 0%) ().
Five RCTs (n = 5176) were included in the meta-analysis comparing the effect on stroke [Citation21,Citation24,Citation34,Citation38,Citation39] (). No significant effect of MHT was observed compared to placebo (RR 1.14, 95% CI 0.94–1.39, p = 0.17, I2 = 0%) (). Sub-analysis of the Heart and Estrogen/progestin Replacement Study (HERS) study reported no significant association between MHT and the risk of non-fatal or fatal stroke (relative hazard 1.18, 95% CI 0.83–1.66 and 1.61, 95% CI 0.73–3.55, respectively) [Citation37]. Increasing age, hypertension, atrial fibrillation, diabetes mellitus and current smoking were identified as independent risk factors for stroke [Citation37].
Six observational studies examined primary outcomes (). Sullivan et al. found a benefit of MHT on 5-year and 10-year survival after coronary artery bypass grafting; however, there were a number of methodological limitations with this study [Citation45]. The exposure group comprised only 92 women, compared to 1006 controls. The presence of diabetes and more severe CAD was associated with increased mortality, whereas estrogen use decreased the risk of death (RR 0.38, 95% CI 0.20–0.71). Frequency of revascularization (19% vs. 41%, p = 0.016) and major cardiac adverse events (19% vs. 43%; p = 0.010) was lower in MHT users compared to non-users in 129 women post coronary artery stenting, although follow-up duration was longer in MHT users (16 vs. 12 months, p = 0.037) [Citation42]. However, event-free survival at 3 years was higher in MHT users compared to non-users (67% vs. 46%, p = 0.02) [Citation42]. There was no significant difference between MHT users and non-users in adjusted 90-day mortality, stroke or composite CVD event rate and adjusted 1-year death rate in the largest study of 4029 women with acute coronary syndrome [Citation44]. No difference in the risk of recurrent myocardial infarction or CVD death in women post first myocardial infarction was observed between MHT users and non-users [Citation41]. A trend to reduced risk was noted with longer interval since commencing MHT; however, this may reflect survivor bias [Citation41]. No significant difference in CVD events at 1-year follow-up was observed between MHT users and non-users after coronary intervention [Citation43]. Fourteen-year follow-up of the ESPRIT RCT, where women post-myocardial infarction were randomized to 2 years of unopposed estradiol or placebo [Citation39], demonstrated no difference between groups regarding CVD death, stroke or ischemic heart disease overall and when stratified according to age 50–59 years and 60–69 years [Citation40].
Effect of MHT on other cardiovascular events or risk factors (secondary outcomes)
Angina, heart failure and TIA
Seven studies (six RCTs and one observational study) reported the effect of MHT on angina symptoms or hospitalization for unstable angina (). Clarke et al. reported that hospitalization due to unstable angina occurred predominantly within the first 2 years following randomization, with a non-significant trend for this to be higher in the MHT group [Citation20]. The five remaining RCTs reported no difference in angina between MHT or control groups [Citation21–24,Citation30]. One small retrospective cohort study reported a trend to less severe angina in estrogen users [Citation43].
Only two RCTs, HERS I and HERS II, reported hospitalization for heart failure (), both finding no significant difference between MHT and placebo groups [Citation22,Citation24].
Simon et al. analyzed prespecified secondary outcome data from HERS I regarding stroke and TIA [Citation37]. They found no effect of MHT on the risk of TIA. Other RCTs using oral estrogen, including HERS II and one using transdermal estrogen, reported no significant differences in the rate of TIA between MHT and control groups, although TIA was not a pre-specified outcome [Citation22,Citation24,Citation34,Citation39] ().
Angiographic progression of coronary artery disease
Seven studies (five RCTs and two observational studies) assessed the effect of MHT on angiographic progression of coronary artery disease (). Observational studies suggested a benefit of estrogen in preventing restenosis after coronary artery stenting [Citation42,Citation43]. However, this was not replicated in the RCTs. Three RCTs did not show any benefit of MHT on progression of coronary atherosclerotic lesions or the rate of repeat revascularization after percutaneous transluminal coronary angioplasty [Citation19,Citation24,Citation38]. One study found potential worsening of atherosclerotic disease in the MHT group; however, after adjusting for diabetes, this difference was no longer significant [Citation38]. Hodis et al. conducted a placebo-controlled trial assessing the effect of oral 17β-estradiol on the minimum lumen diameter and percent stenosis measured by angiogram [Citation26]. Whilst there was no difference between the intervention and control groups, the mean progression of stenosis was approximately twice as fast in participants with diabetes compared to those without. A sub-study of the WAVE trial showed increased progression of coronary atherosclerosis in diseased arteries in women with abnormal glucose tolerance compared to women with normal glucose tolerance, with significantly greater decreases in average (−0.047 vs. −0.009 mm/year, respectively; p = 0.001) and minimum (−0.06 mm vs. −0.01 mm/year, respectively; p = 0.005) lumen diameters [Citation27]. There was a reduction in minimum and average lumen diameters in non-diseased arteries in women receiving MHT with abnormal glucose tolerance, but not in those with normal glucose tolerance [Citation27].
Other CVD outcomes
Two RCTs assessed the effect of MHT on flow-mediated vasodilation, one using transdermal estradiol [Citation17] and the other using oral CEE [Citation25] (). Both studies observed significant increases in flow-mediated vasodilation in the MHT group compared to baseline but no differences between groups.
A HERS sub-study assessed progression of carotid atherosclerosis in 362 participants via ultrasound [Citation18]. Although intima-media thickness progressed in both groups compared with baseline, there was no significant difference in progression between groups.
Cardiovascular risk factors including lipid concentrations, blood pressure and glucose tolerance
Lipid concentrations were assessed in six RCTs as a primary outcome and four RCTs as a secondary outcome (). Most studies used oral estrogen and MPA. Three studies using transdermal estrogen found no significant differences in any lipid population between MHT users and controls [Citation20,Citation32,Citation35]. Of the studies using oral estrogen, a reduction in total cholesterol and low-density lipoprotein (LDL) and an increase in high-density lipoprotein (HDL) with variable or no effect on triglyceride concentrations were associated with MHT use [Citation19,Citation20,Citation25,Citation31,Citation36]. A potential confounding factor in these studies is statin therapy. Two small randomized crossover trials compared oral MHT to statin alone and the combination of MHT–statin [Citation25]. Statin or the MHT–statin combination was associated with lower triglyceride levels compared to MHT. Statin or the MHT–statin combination were superior to MHT alone, although the combination was not better than statin alone [Citation25,Citation36]. Of note, MHT alone did not achieve the lipid concentrations recommended for secondary CVD prevention.
A single study assessed the effect of either oral CEE or transdermal estrogen plus cyclical MPA compared to placebo on ambulatory blood pressure (ABP) [Citation35] (). Hypertension, well controlled on antihypertensive medication, was present in 26/60 participants at baseline. Compared to baseline, the group receiving CEE demonstrated a 4–5% increase in both day and night systolic ABP after 6 months of treatment (p < 0.05). At 12 months compared to baseline, night-time ABP decreased 9.6% in the CEE group and 22% in the placebo (p < 0.05). There was no significant change in ABP in the transdermal estrogen group. There were no significant treatment differences between groups. The study sample size was insufficient to assess change in mortality or cardiovascular events.
Two studies assessed the effect of MHT on glucose tolerance (). A sub-study of the HERS RCT investigated incident diabetes and fasting glucose levels [Citation29]. Fasting glucose levels were significantly higher in the placebo compared to the MHT group, in those with and without diabetes. Of the 2029 women who did not have diabetes at baseline, the cumulative incidence of diabetes was 6.2% in the MHT group and 9.5% in the placebo group. The observed 35% lower risk of diabetes (relative hazard 0.65, 95% CI 0.48–0.89; p = 0.006) with MHT persisted after adjustment for body mass index, weight change, dyslipidemia, hypertension and medications known to affect diabetes incidence. The second study assessed the effect of transdermal estrogen on insulin sensitivity and insulin secretion compared to the no treatment control group [Citation33]. Decreased insulin, increased C-peptide and improved insulin sensitivity were observed after 3 months of unopposed transdermal estrogen compared to controls. However, after the addition of MPA (14 days of treatment every 3 months) and at 12 months, there was no significant difference seen between the MHT group and controls. No significant difference was observed in fasting glucose, HBA1C or insulin secretion between groups at any timepoint.
Risk of bias
Most of the included studies had a low risk of bias. Some studies demonstrated concerns, mainly related to information regarding randomization and blinding. Three of the studies had a high risk of bias owing to missing outcome information or high numbers of loss to follow-up (Supplementary Figure 1 and Supplementary Table 4).
Discussion
Our systematic review and meta-analysis of eligible studies assessed the effect of MHT in women with pre-existing CVD on a range of CVD outcomes and risk factors. Most studies investigated the effect of oral estrogens and were conducted in older postmenopausal women aged >60 years. No significant differences were observed between MHT users and controls regarding the primary outcomes of non-fatal myocardial infarction, cardiovascular death and stroke. The frequency of angina, heart failure and TIA also did not differ between MHT and control groups. Mixed effects of MHT on angiographic progression were observed and varied with glycemic status. A positive effect of estradiol on flow-mediated dilatation was seen. Limited studies explored the effect of MHT on cardiac risk factors in women with pre-existing CVD. Although favorable effects on LDL and HDL levels were observed with oral estrogen and neutral effects with transdermal estrogen, statin therapy was superior to MHT. Transdermal but not oral estrogen was neutral with regard to blood pressure. MHT was associated with positive effects on glucose tolerance and diabetes incidence; however, diabetes may be associated with angiographic disease acceleration.
Our systematic search did not identify any new studies and thus our primary outcome findings are similar to a previous Cochrane review [Citation15]. Consistent with our study, a 2020 meta-analysis of 10 RCTs published since 2000 investigating MHT and CVD outcomes in postmenopausal women with and without CVD reported no significant association between MHT and cardiovascular death, myocardial infarction, coronary heart disease, angina and revascularization overall in those ‘with underlying disease’ [Citation48]. This meta-analysis also reported an increased risk of stroke associated with MHT in those ‘with underlying disease at baseline’ (nine studies; summary estimate 1.14, 95% CI 1.04–1.26) but not in those ‘without underlying disease’ [Citation48]. We reported a non-significant similar RR (1.14, 95% CI 0.94–1.39) which may reflect the fewer included studies. Our primary outcome findings are also consistent with an observational study investigating cardiovascular events in women who discontinued MHT following myocardial infarction, and did not find any significant difference between women who continued or discontinued therapy [Citation49]. Limitations of many of the included studies in this systematic review relate to the small sample sizes and low numbers of individual CVD events. Therefore, the lack of an observed effect of MHT on CVD outcomes may reflect low statistical power rather than physiological safety.
Although we and others observed no significant overall association between MHT use and CVD [Citation15,Citation48], post hoc analyses have reported transient increased CVD risk within the first year of follow-up, in both secondary and primary prevention studies [Citation24,Citation34,Citation50]. The HERS, the largest secondary prevention study, reported an increased risk of coronary heart disease events in only the first year of follow-up (adjusted relative hazard at 1 year 1.51, 95% CI 1.00–2.27) [Citation24]. Pro-arrhythmic, pro-thrombotic and pro-inflammatory effects of MHT were proposed as the potential underlying mechanisms and HERS II reported increased non-fatal ventricular arrythmias in the MHT group versus the placebo group (33 vs. 13; relative hazard 1.97) [Citation22]. A sub-study of HERS reported that in women not using a statin at baseline, MHT was significantly associated with increased risk of cardiac death and non-fatal myocardial infarction during the first year compared to placebo [Citation51]. Uncertainty persists secondary to methodological limitations, low event rates and confounders such as concurrent statin use, and is reflected in the borderline significance of the RRs.
Modifiers of CVD risk with MHT include age, time since menopause and MHT preparation. A 2019 meta-regression indicated that increasing age worsens the effect of MHT on stroke, TIA and systemic embolism [Citation12]. Kim et al. reported reduced risk of CVD death associated with early but not late initiation of MHT [Citation48]. Similar findings were reported by Nudy et al. [Citation12]; however, the reduced risk of cardiac events (cardiac mortality and non-fatal myocardial infarction) observed in younger MHT initiators aged <60 years old did not persist after exclusion of open-label studies. Interestingly, post-hoc analysis of the Raloxifene Use for the Heart (RUTH) placebo-controlled RCT, involving 10,101 women with established CVD (50%) or at high risk of CVD, reported a significant 41% lower incidence of coronary events with use of the selective estrogen receptor modulator, raloxifene, in women aged <60 years but no difference in women aged 60 years or over [Citation52]. Studies included in the current review predominately investigated oral estrogens, mainly 0.625 mg CEE, and oral MPA in combined MHT. A 2019 systematic review of postmenopausal women, predominately without prevalent CVD, reported mixed findings regarding estrogen dose and the risk of coronary heart disease events [Citation53]. Transdermal MHT may be associated with a beneficial or no effect on myocardial infarction risk [Citation53–55]. Vaginal estrogen was associated with reduced myocardial infarction and stroke risk in one national registry study [Citation53]. We were unable to assess the effect of transdermal therapy on stroke as included studies only involved oral estrogen therapy. Prior systematic reviews reported a dose-dependent increased risk of stroke with oral estrogen; however, no significant increased risk was observed with transdermal estradiol doses ≤50 μg [Citation53,Citation55]. Kim et al. also reported that combined MHT, late initiation and MHT duration ≥5 years increased the stroke risk [Citation48]. The type of progestogen may also modify CVD risk [Citation54]. Ischemic stroke risk was increased with norpregnane derivatives but not progesterone, pregnane or nortestosterone derivatives [Citation53]. MPA but not progesterone has been shown to attenuate the beneficial effects of estrogens [Citation54].
Our systematic review also addressed the effect of MHT on traditional cardiovascular risk factors as well as the effect of angiographic progression of CVD, specifically in women with known CVD. Oral estrogen is associated with beneficial changes in total cholesterol, LDL and HDL concentrations but adverse triglyceride changes [Citation56]. In contrast, transdermal estrogen is neutral or beneficial with regard to triglycerides, HDL and LDL [Citation56]. In the current analysis, although oral MHT had a favorable effect on lipid profile, most studies were of short duration (less than 12 months) and confounded by concurrent statin therapy. Additionally, we observed that MHT use did not achieve target lipid levels recommended for secondary CVD prevention. Our findings indicate that statin, rather than MHT, is the preferred therapy for hyperlipidemia in women with CVD.
Results differed in observational studies compared to the RCTs assessing angiographic outcomes. The observational data suggested a benefit of estrogen use in terms of prevention of restenosis after coronary intervention, whilst RCT data did not. This difference may reflect bias and confounders associated with observational studies including the effect and timing of prior MHT use. The Early versus late Intervention Trial with Estradiol (ELITE) RCT reported reduced carotid intima-media thickness progression in healthy women treated with MHT who were within 6 years of menopause compared with placebo or women 10 years or more postmenopause [Citation57].
Hypertension is the leading global CVD risk factor in women; conferring a higher risk of myocardial infarction and stroke compared with men [Citation1]. Data from the Women’s Health Initiative Observational study (n = 19,986; 98% without previous CVD) indicated lower risk of incident hypertension and lower systolic blood pressure with transdermal estrogen versus oral estrogen dose [Citation58]. Data from the French E3N cohort (n = 49,905) were consistent with these findings and also reported increased risk of incident hypertension with pregnane and norpregane derivatives but not progesterone or dydrogesterone [Citation59]. Our findings and evidence from these studies suggest transdermal estradiol (and micronized progesterone or dydrogesterone if required for combined MHT) as the preferred MHT [Citation11,Citation53,Citation54,Citation58,Citation59].
The Women’s Health Initiative study observed a 49% (combined MHT) and 61% (CEE alone) reduction in self-reported diabetes mellitus in postmenopausal women assigned to MHT versus placebo during intervention but not at follow-up [Citation60]. In the current systematic review, few studies specifically assessed the effect of MHT on glucose tolerance in women with CVD; however, there was a suggestion that MHT may reduce incident diabetes. Of note, studies investigating angiographic outcomes suggested worse progression of coronary atherosclerotic disease in the setting of diabetes. Taken together, this suggests that MHT should not be used with the expectation of reducing diabetes incidence or cardiovascular events after coronary intervention and the use of MHT should be reconsidered in women who have both CVD and diabetes.
A limitation of this study is that we only included English language studies. Participants were generally older and predominantly recruited from North America and Europe, which limits generalizability to younger postmenopausal women and other countries. Since these studies were conducted approximately 20 years ago, the increasing prevalence of cardiac risk factors, especially diabetes mellitus and obesity [Citation1], may limit the applicability of these null findings to the current population of postmenopausal women at potentially greater underlying cardiac risk. Most of the included studies involved oral CEE/MPA which is associated with a greater thromboembolic risk versus transdermal estrogen [Citation53,Citation54]. Furthermore, heterogeneity of the studies was observed, especially of those examining secondary outcomes.
Conclusion
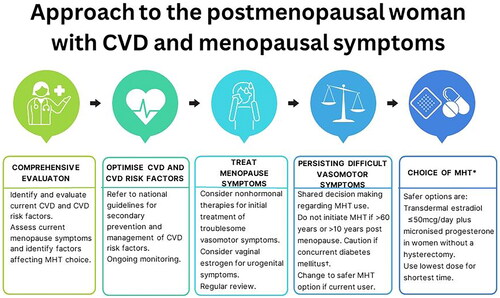
Our findings indicate that MHT does not provide secondary CVD prevention, although the risk of non-fatal MI, CVD death or stroke was not significantly increased with MHT. Depending on the preparation, MHT may have some beneficial effects on cardiovascular risk factors; however, these have not translated to improved CVD outcomes. Therefore, optimization of lipids, blood pressure and glycemic control as per CVD guidelines is essential [Citation61]. MHT is considered high risk in women with existing CVD or 10-year CVD risk ≥10% [Citation9,Citation13] and in the setting of uncontrolled cardiac risk factors including blood pressure ≥180/110 mmHg, total cholesterol >7.8 mmol/L and triglycerides > 4.5 mmol/L [Citation8,Citation9]. Non-hormonal therapy [Citation62] is indicated as first-line therapy for management of vasomotor symptoms in these women [Citation13,Citation53]. For women with established CVD and persisting vasomotor symptoms despite non-hormonal therapy, shared decision-making regarding the use of transdermal estradiol at doses of ≤50 μg (especially if previous hysterectomy) with concurrent statin use could be considered [Citation53]. Oral MHT should be avoided due to the effect on blood pressure and thromboembolism risk. Our findings also highlight the potential adverse effect of oral MHT where CVD and diabetes coexist with the potential for more rapid progression of coronary artery disease with MHT. In this setting, systemic MHT is best avoided. A suggested clinical approach is shown in . This systematic review also highlights research gaps related to the effect of transdermal MHT, use of different progestogens and MHT use in other patient populations.
Figure 3. Clinical approach to the management of menopausal symptoms in women with cardiovascular disease (CVD). †Diabetes mellitus may be associated with increased progression of atherosclerosis. *Oral menopausal hormone therapy (MHT) is associated with increased blood pressure and increased thrombotic risk.
Potential conflict of interest
A.J. Vincent is a current board member of the International Menopause Society and a member of the editorial board of Climacteric. The other authors have no competing interests to declare.
Source of funding
None.
Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download MS Word (33.5 KB)Supplemental Material
Download MS Word (13.5 KB)Supplemental Material
Download MS Word (17 KB)Supplemental Material
Download MS Word (248.9 KB)Acknowledgements
The authors thank Paula Todd, Liaison Librarian, Medicine, Nursing & Health Sciences, Pharmacy & Pharmaceutical Sciences, Monash University for their assistance with the systematic search.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Vogel B, Acevedo M, Appelman Y, et al. The lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. 19doi: 10.1016/S0140-6736(21)00684-X.
- Nappi RE, Chedraui P, Lambrinoudaki I, et al. Menopause: a cardiometabolic transition. Lancet Diabetes Endocrinol. 2022;10(6):442–456. doi: 10.1016/S2213-8587(22)00076-6.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063.
- Gartoulla P, Worsley R, Bell RJ, et al. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause. 2015;22(7):694–701. doi: 10.1097/GME.0000000000000383.
- Franco OH, Muka T, Colpani V, et al. Vasomotor symptoms in women and cardiovascular risk markers: systematic review and meta-analysis. Maturitas. 2015;81(3):353–361. doi: 10.1016/j.maturitas.2015.04.016.
- MacLennan AH, Broadbent JL, Lester S, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;2004Issue(4):CD002978. Art. No.: CD002978. doi: 10.1002/14651858.CD002978.pub2.
- Baber RJ, Panay N, Fenton A, IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–150. doi: 10.3109/13697137.2015.1129166.
- The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29(7):767–794.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236.
- National Institute for Health and Care Excellence (NICE). Menopause. 2015. NG23. [cited 2016 Aug 26]. https://www.nice.org.uk/Guidance/NG23
- Kaemmle LM, Stadler A, Janka H, et al. The impact of micronized progesterone on cardiovascular events – a systematic review. Climacteric. 2022;25(4):327–336. doi: 10.1080/13697137.2021.2022644.
- Nudy M, Chinchilli VM, Foy AJ. A systematic review and meta-regression analysis to examine the ‘timing hypothesis’ of hormone replacement therapy on mortality, coronary heart disease, and stroke. Int J Cardiol Heart Vasc. 2019;22:123–131. doi: 10.1016/j.ijcha.2019.01.001.
- Cho L, Kaunitz AM, Faubion SS, et al. Rethinking menopausal hormone therapy: for whom, what, when, and how long? Circulation. 2023;147(7):597–610. doi: 10.1161/CIRCULATIONAHA.122.061559.
- Gersh FL, O’Keefe JH, Lavie CJ. Postmenopausal hormone therapy for cardiovascular health: the evolving data. Heart. 2021;107(14):1115–1122. doi: 10.1136/heartjnl-2019-316323.
- Boardman HMP, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;2015(3):CD002229. doi: 10.1002/14651858.CD002229.pub4.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Blumel JE, Castelo-Branco C, Leal T, et al. Effects of transdermal estrogens on endothelial function in postmenopausal women with coronary disease. Climacteric. 2003;6(1):38–44. doi: 10.1080/cmt.6.1.38.44.
- Byington RP, Furberg CD, Herrington DM, et al. Effect of estrogen plus progestin on progression of carotid atherosclerosis in postmenopausal women with heart disease: HERS B-mode substudy. Arterioscler Thromb Vasc Biol. 2002;22(10):1692–1697. doi: 10.1161/01.atv.0000033514.79653.04.
- Cherry N, Gilmour K, Hannaford P, et al. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet. 2002;360(9350):2001–2008.
- Clarke SC, Kelleher J, Lloyd-Jones H, et al. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: the Papworth HRT atherosclerosis study. BJOG. 2002;109(9):1056–1062. doi: 10.1111/j.1471-0528.2002.01544.x.
- Collins P, Flather M, Lees B, et al. Randomized trial of effects of continuous combined HRT on markers of lipids and coagulation in women with acute coronary syndromes: WHISP pilot study. Eur Heart J. 2006;27(17):2046–2053. doi: 10.1093/eurheartj/ehl183.
- Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: heart and estrogen/progestin replacement study follow-up (HERS II). JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49.
- Hall G, Pripp U, Schenck-Gustafsson K, et al. Longterm effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas. 1998;28(3):235–242. doi: 10.1016/s0378-5122(97)00080-7.
- Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343(8):522–529. doi: 10.1056/NEJM200008243430801.
- Herrington DM, Werbel BL, Riley WA, et al. Individual and combined effects of estrogen/progestin therapy and lovastatin on lipids and flow-mediated vasodilation in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1999;33(7):2030–2037. doi: 10.1016/s0735-1097(99)00128-x.
- Hodis HN, Mack WJ, Azen SP, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349(6):535–545. doi: 10.1056/NEJMoa030830.
- Howard BV, Hsia J, Ouyang P, et al. Postmenopausal hormone therapy is associated with atherosclerosis progression in women with abnormal glucose tolerance. Circulation. 2004;110(2):201–206. doi: 10.1161/01.CIR.0000134955.93951.D5.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605.
- Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the heart and estrogen/progestin replacement study: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005.
- Khan MA, Hlatky MA, Liu MW, et al. Effect of postmenopausal hormone therapy on coronary heart disease events after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2003;91(8):989–991, A7. doi: 10.1016/s0002-9149(03)00121-8.
- Lamon-Fava S, Herrington DM, Reboussin DM, et al. Changes in remnant and high-density lipoproteins associated with hormone therapy and progression of coronary artery disease in postmenopausal women. Atherosclerosis. 2009;205(1):325–330. doi: 10.1016/j.atherosclerosis.2008.12.020.
- Os I, Hofstad AE, Brekke M, et al. The EWA (estrogen in women with atherosclerosis) study: a randomized study of the use of hormone replacement therapy in women with angiographically verified coronary artery disease. Characteristics of the study population. Effects ON lipids and lipoproteins. J Intern Med. 2000;247(4):433–441. doi: 10.1046/j.1365-2796.2000.00675.x.
- Os I, Os A, Abdelnoor M, et al. Insulin sensitivity in women with coronary heart disease during hormone replacement therapy. J Womens Health (Larchmt). 2005;14(2):137–145. doi: 10.1089/jwh.2005.14.137.
- Ouyang P, Tardif J-C, Herrington DM, et al. Randomized trial of hormone therapy in women after coronary bypass surgery. Evidence of differential effect of hormone therapy on angiographic progression of disease in saphenous vein grafts and native coronary arteries. Atherosclerosis. 2006;189(2):375–386. doi: 10.1016/j.atherosclerosis.2005.12.015.
- Pripp U, Hall G, Csemiczky G, et al. A randomized trial on effects of hormone therapy on ambulatory blood pressure and lipoprotein levels in women with coronary artery disease. J Hypertens. 1999;17(10):1379–1386. doi: 10.1097/00004872-199917100-00004.
- Sbarouni E, Kyriakides ZS, Kremastinos DTH. The effect of hormone replacement therapy alone and in combination with simvastatin on plasma lipids of hypercholesterolemic postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1998;32(5):1244–1250. doi: 10.1016/s0735-1097(98)00413-00416.
- Simon JA, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen-progestin Replacement Study (HERS). Circulation. 2001;103(5):638–642. doi: 10.1161/01.cir.103.5.638.
- Viscoli CM, Brass LM, Kernan WN, et al. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534.
- Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288(19):2432–2440. doi: 10.1001/jama.288.19.2432.
- Cherry N, McNamee R, Heagerty A, et al. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG. 2014;121(6):700–705; discussion 705. doi: 10.1111/1471-0528.12598.
- Heckbert SR, Kaplan RC, Weiss NS, et al. Risk of recurrent coronary events in relation to use and recent initiation of postmenopausal hormone therapy. Arch Intern Med. 2001;161(14):1709–1713. doi: 10.1001/archinte.161.14.1709.
- Khan MA, Liu MW, Singh D, et al. Long-term (three years) effect of estrogen replacement therapy on major adverse cardiac events in postmenopausal women after intracoronary stenting. Am J Cardiol. 2000;86(3):330–333. doi: 10.1016/s0002-9149(00)00926-7.
- O’Brien JE, Peterson ED, Keeler GP, et al. Relation between estrogen replacement therapy and restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 1996;28(5):1111–1118. doi: 10.1016/S0735-1097(96)00306-3.
- Parsons E, Newby LK, Bhapkar MV, et al. Postmenopausal hormone use in women with acute coronary syndromes. J Womens Health (Larchmt). 2004;13(8):863–871. doi: 10.1089/jwh.2004.13.863.
- Sullivan JM, El-Zeky F, Zwaag RV, et al. Effect on survival of estrogen replacement therapy after coronary artery bypass grafting. Am J Cardiol. 1997;79(7):847–850. doi: 10.1016/s0002-9149(97)00001-5.
- Higgins JT, Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. 2022. Available from: www.training.cochrane.org/handbook.
- Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000.
- Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10(1):20631. doi: 10.1038/s41598-020-77534-9.
- Bretler D-M, Hansen PR, Sørensen R, et al. Discontinuation of hormone replacement therapy after myocardial infarction and short term risk of adverse cardiovascular events: nationwide cohort study. BMJ. 2012;344(mar27 1):e1802–e1802. doi: 10.1136/bmj.e1802.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808.
- Herrington DM, Vittinghoff E, Lin F, et al. Statin therapy, cardiovascular events, and total mortality in the heart and estrogen/progestin replacement study (HERS). Circulation. 2002;105(25):2962–2967. doi: 10.1161/01.cir.0000019406.74017.b2.
- Collins P, Mosca L, Geiger MJ, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for the heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119(7):922–930. doi: 10.1161/CIRCULATIONAHA.108.817577.
- Oliver-Williams C, Glisic M, Shahzad S, et al. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: a systematic review. Hum Reprod Update. 2019;25(2):257–271. doi: 10.1093/humupd/dmy039.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15 Suppl 1:11–17. doi: 10.3109/13697137.2012.669624.
- Bezwada P, Shaikh A, Misra D. The effect of transdermal estrogen patch use on cardiovascular outcomes: a systematic review. J Womens Health (Larchmt). 2017;26(12):1319–1325. doi: 10.1089/jwh.2016.6151.
- Nie G, Yang X, Wang Y, et al. The effects of menopause hormone therapy on lipid profile in postmenopausal women: a systematic review and Meta-Analysis [systematic review. Front Pharmacol. 2022;13:850815. doi: 10.3389/fphar.2022.850815.
- Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. doi: 10.1056/NEJMoa1505241.
- Wild RA, Larson JC, Crandall CJ, et al. Hormone therapy formulation, dose, route of delivery, and risk of hypertension: findings from the women’s health initiative observational study (WHI-OS). Menopause. 2021;28(10):1108–1116. doi: 10.1097/GME.0000000000001828.
- Madika A-L, MacDonald CJ, Fournier A, et al. Menopausal hormone therapy and risk of incident hypertension: role of the route of estrogen administration and progestogens in the E3N cohort. Menopause. 2021;28(11):1204–1208. doi: 10.1097/GME.0000000000001839.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040.
- Thakkar A, Agarwala A, Michos ED. Secondary prevention of cardiovascular disease in women: Closing the gap. Eur Cardiol. 2021;16:e41. doi: 10.15420/ecr.2021.24.
- The 2023 nonhormone therapy position statement of the North American menopause society. Menopause. 2023;30(6):573–590.
