Abstract
Ischemic heart disease is the primary cause of cardiovascular disease (CVD) mortality in both men and women. Strategies targeting traditional modifiable risk factors are essential – including hypertension, smoking, dyslipidemia and diabetes mellitus – particularly for atherosclerosis, but additionally for stroke, heart failure and some arrhythmias. However, challenges related to education, screening and equitable access to effective preventative therapies persist, and are particularly problematic for women around the globe and those from lower socioeconomic groups. The association of female-specific risk factors (e.g. premature menopause, gestational hypertension, small for gestational age births) with CVD provides a potential window for targeted prevention strategies. However, further evidence for specific effective screening and interventions is urgently required. In addition to population-level factors involved in increasing the risk of suffering a CVD event, efforts are leveraging the enormous potential of blood-based ‘omics’, improved imaging biomarkers and increasingly complex bioinformatic analytic approaches to strive toward more personalized early disease detection and personalized preventative therapies. These novel tactics may be particularly relevant for women in whom traditional risk factors perform poorly. Here we discuss established and emerging approaches for improving risk assessment, early disease detection and effective preventative strategies to reduce the mammoth burden of CVD in women.
摘要
缺血性心脏病是男女性心血管疾病(CVD)死亡的主要原因。针对经典可变风险因素的管理策略是必不可少的, 包括高血压、吸烟、血脂异常和糖尿病, 尤其是动脉粥样硬化, 但也包括中风、心力衰竭和某些心律失常。然而, 与教育、筛查和公平获得有效预防疗法有关的挑战依然存在, 这对全球女社会经济地位较低女性群体来说尤其困难。女性特有的风险因素(如早绝经、妊娠期高血压、小胎龄分娩)与心血管疾病的关联, 为有针对性的预防策略提供了潜在的窗口。然而, 迫切需要进一步的证据来证明具体有效的筛查和干预措施。除了增加心血管疾病风险的人群因素外, 人们正在努力利用基于血液的“组学”的巨大潜力, 改进成像生物标志物和日益复杂的生物信息学分析方法, 努力实现更个性化的早期疾病筛查和个性化的预防治疗。这些新颖的策略可能特别适用于没有明显传统风险因素表现的女性。本研究讨论了现有的和新兴的方法, 以改善风险评估, 早期疾病筛查和有效的预防策略, 以减轻女性在心血管疾病方面的巨大负担。
Introduction
Ischemic heart disease (IHD) is the primary cause of cardiovascular disease (CVD)-related mortality in both men and women worldwide [Citation1,Citation2]. However, despite campaigns over many decades, awareness of the impact of CVD on women remains suboptimal, and there has been a poignant decline in ‘ideal cardiovascular health’ [Citation3,Citation4] – a composite of modifiable clinical health factors and health behaviors – and slowing of the overall reduction of the CVD burden for women over the last 10 years [Citation2,Citation5].
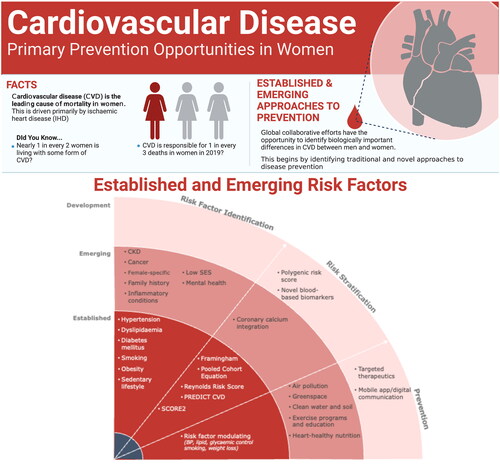
The key roles of hypertension, dyslipidemia, diabetes mellitus and cigarette smoking in driving CVD are well recognized at a population level and have been the target of primary prevention strategies for more than 50 years, resulting in a substantial reduction in population-adjusted mortality associated with coronary artery disease (CAD). However, access to screening and primary prevention programs has been less optimal for women [Citation6]. Ongoing improvements in education, screening and equitable access to effective preventative therapies are required (). In addition, individuals with no traditional risk factors develop life-threatening or fatal heart attacks, and the need for improved markers for risk assessment and ultimately detection of early disease to improve personalized prevention strategies is urgent.
Screening and treating traditional modifiable risk factors in women
Hypertension is the leading contributing risk factor for IHD globally [Citation7], with a particularly strong association in women [Citation8]. The linear relationship between blood pressure (BP) measures and CAD-related or stroke-related death extends into the ‘normal’ range [Citation9]. Consistent with this, data from the SPRINT trial showed that aggressive management with systolic BP targets below 120 mmHg provides greater protection against major cardiovascular events and death than more traditional goals [Citation10]. Women have additionally demonstrated worse non-IHD cardiovascular consequences of elevated BP compared to men, including left ventricular hypertrophy, heart failure with preserved ejection fraction and arterial stiffness [Citation11,Citation12].
There are a wide variety of available anti-hypertensive agents when considering pharmacotherapy, including many now accessible in generic forms. Little difference has been observed between the various BP-lowering drug classes if the target BP is achieved, despite theoretical biological benefits related to specific molecular pathways (e.g. angiotensin receptor blockade reducing cardiovascular nicotinamide adenine dinucleotide phosphate [NADPH] oxidase activity) [Citation13–15]. This knowledge has led to the opportunity for shared-decision approaches between a patient and her physician to achieve an agreed target with minimum side effects, pragmatically weighing individual absolute risk against patient tolerance. When escalation is required, using two or more agents at a lower dose has been observed to be more effective and better tolerated from a side effects perspective than a maximal dose of a single agent [Citation16,Citation17]. Weight loss, alcohol abstinence or reduction and exercise are all important lifestyle factors to be included in the overall patient management plan.
The causal role of hypercholesterolemia in atherosclerosis is well established [Citation18,Citation19]. In women specifically, significant increases in serum total and low-density lipoprotein (LDL) cholesterol have been noted within the first year of the final menstrual cycle and associated with carotid atherosclerosis [Citation20]. Lipid-modifying treatment using statins to inhibit HMG-CoA reductase reduces cardiovascular events and mortality in individuals with established CAD [Citation21,Citation22]. In primary prevention, evidence for starting statin treatment varies according to overall risk for the individual, with the absolute benefit of lowering LDL-C depending on the absolute CVD risk [Citation22]. There is a dearth of primary prevention evidence specifically in women. However, women eligible for statin treatment are less likely to be prescribed therapy or be at the target dose [Citation23]. Other agents such as ezetimibe may also be considered to achieve LDL lowering [Citation24], particularly in individuals who are intolerant of statins [Citation25]. More recently, monoclonal antibody and small interfering RNA strategies to inhibit PCSK9 have demonstrated powerful effects on serum LDL cholesterol [Citation26,Citation27], with a reduction in major adverse cardiovascular events (MACE) in those with established CAD on monoclonal antibody strategies [Citation26,Citation28]. The potential for only twice-yearly injections is attractive to both patients and health-care providers. Equitable access to such emerging therapies using newer technology will be key for global impact, with gender and socioeconomic status being important considerations.
Type 1 and type 2 diabetes are independent risk factors for atherosclerotic CVD, with the risk appearing to be more significant in females [Citation29]. Data from more than 850,000 participants across 64 studies found a 44% higher risk of CAD incidence in women with diabetes than in men [Citation30], and women with diabetes in the UK Biobank had a 29% higher risk of myocardial infarction compared with diabetic men [Citation31]. Prevention strategies include diet, reducing body mass index, increasing exercise and reducing sedentary behaviors. Whilst pharmacological control of blood sugar with traditional oral agents and insulin has been disappointing regarding the reduction of atherosclerotic and cardiovascular events [Citation32], new therapies targeting SGLT2 [Citation33–35] and GLP-1 [Citation36] have shown potential new promise. In the case of SGLT2 inhibitors, the most profound effect has been protection against heart failure events, with less efficacy for myocardial infarction and only achieving statistical significance in very large meta-analyses [Citation37]. However, SGLT2 inhibitors may have sex-based efficacy differences, with one meta-analysis showing a significant reduction of MACE risk in men but not in women [Citation38]. The latter findings may generally be related to poorer lipid level and BP control in women compared with men [Citation39].
Cigarette smoking is responsible for 50% of all avoidable deaths in smokers, with half of these deaths resulting from atherosclerotic CVD [Citation40]. Astonishingly, the CVD risk in young smokers (age <50 years) is five-fold higher than in non-smokers, with even higher risk in women versus men [Citation41]. Mechanisms include endothelial dysfunction with reduced nitric oxide bioavailability, increased platelet and macrophage activation, and a pro-inflammatory vascular environment that drives tissue remodeling [Citation42]. Age-adjusted smoking prevalence has decreased significantly over the past 30 years in both sexes, with significantly lower smoking rates in women compared to men. However, despite the widely acknowledged health impact of smoking, age-adjusted smoking prevalence has remained either unchanged or significantly increased for women in 66% of the countries of the world compared to 33.5% of countries for men [Citation43]. Even more concerningly, the rates of tobacco and e-cigarette smoking have been rising in young women, particularly those aged under 26 years [Citation2].
Preventative strategies need to include broader public health interventions to prevent smoking initiation, including public messaging, policy approaches – both packaging rules and higher taxation – and laws preventing smoking within indoor public locations. New Zealand has taken a particularly strict stance, legally banning the purchase of cigarettes or tobacco for ‘the next generation’, or more specifically anyone born after 1 January 2009. However, whilst smoking cessation is the most effective measure for reversing vascular injury and preventing associated events, the addictive nature makes success rates low even with nicotine replacement or anti-addiction pharmacotherapies such as bupropion [Citation44].
Whilst there is a strong association of obesity with CVD risk, diet and lifestyle interventions have been disappointing regarding their impact on improved cardiovascular outcomes [Citation45]. However, recently bariatric surgery has been found to reduce MACE incidence in several matched cohort studies [Citation46,Citation47], with statistically similar results between sexes remaining after adjustment [Citation46]. The complex pathogenesis, as well as physiological and psychologic impacts, of obesity have historically resulted in many proposed interventions, with both positive and grave cardiovascular consequences [Citation48,Citation49]. Evidence is emerging regarding the cardiovascular impact of some newer, specific pharmacological weight loss strategies (e.g. liraglutide [Citation50]) in the setting of diabetes and obesity. Further research is needed on the safety and impact of these therapies on MACE risk in non-diabetic, obese individuals. Sex-based differences in glycemic control with agents such as GLP-1 receptor agonists have been previously reported. Further research is ongoing regarding potential differential protective effects on MACE between sexes.
Risk scores and stratification
Data from large population studies examining cardiovascular risk factors and outcomes have led to the development of traditional risk algorithms. One of the first widely adopted scores was the Framingham Risk Score (FRS). The most recent major version from 2008 includes age, sex, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic BP, BP-lowering treatment, diabetes mellitus and current smoking as predictor variables for a CVD event (CAD, stroke, peripheral arterial disease or heart failure) [Citation51]. Whilst the FRS has been derived from a largely White American population, with recalibration the various iterations have demonstrated an ability to perform adequately in multiple ethnic groups [Citation52,Citation53]. The American College of Cardiology/American Heart Association-endorsed Pooled Cohort Equation (PCE) was the first model to include data from large populations of both White and Black Americans. The model includes the same predictor variables as the 2008 FRS, but includes only hard endpoints (fatal and non-fatal CAD and stroke) [Citation54]. The sex-specific Reynolds Risk Score was developed in a large prospective cohort of non-diabetic North Americans, and is distinct from the FRS and the PCE with the inclusion of high-sensitivity C-reactive protein (hs-CRP) [Citation55]. NZ PREDICT-1o highlights the importance of a risk score that is calibrated to the population it will be applied in, having been derived from more than 400,000 New Zealanders and performing substantially better in New Zealand than the PCE [Citation56]. In a similar vein, the European Society of Cardiology recommends using SCORE2, derived using 45 cohorts in 13 European countries. Importantly, SCORE2 considers the issues of age heavily weighting risk estimates in other algorithms, and provides age-specific and sex-specific 10-year absolute risk estimates [Citation57].
There are known and well-documented issues with applying CVD risk scores to new populations, including both over-estimation and under-estimation of risk. Despite these performance issues, expert consensus guidelines recommend incorporating 10-year CVD risk tools in the primary prevention setting [Citation9]. However, access barriers for women have been measured, with fewer undergoing CVD risk classification in the primary care setting compared to men [Citation6]. Five- and 10-year ‘absolute’ cardiovascular risk scores are heavily influenced by age, and thus do not provide maximum opportunity in younger populations for early risk identification and personalized prevention. Adjusting risk estimates for age and sex may allow individual patients to maximize their cardiovascular health across the whole of life, driving decisions around smoking cessation, exercise, BP reduction and metabolic optimization [Citation9]. Lastly, it is important to better understand the impact of sex-specific risk factors, such as a history of adverse pregnancy outcomes and early menopause, and how to incorporate these into risk assessments.
Female-specific and non-traditional risk markers
The recognized burden of CVD in women and the relatively poor performance of traditional risk algorithms have led researchers and clinicians to search for additional aids in non-traditional and sex-specific markers. Adverse pregnancy outcomes may provide some opportunity for early sex-specific risk identification and intervention. Gestational hypertension, gestational diabetes, preterm delivery and giving birth to a small for gestational age infant are prognostic in CVD [Citation58,Citation59]. Premature menopause and polycystic ovary syndrome are additional female-specific factors associated with CVD [Citation58]. Although the overall CVD risk is lower in young women compared with their male counterparts, it rises substantially after menopause [Citation60]. Several underlying mechanisms have been discussed and include changes in the lipid profile and body fat distribution [Citation61], with accelerated gains in fat mass and losses of lean mass during the menopause transition [Citation62]. Multi-disciplinary teams are encouraged to provide collaborative care for pregnant patients with CVD – including at a minimum obstetrics, primary care, cardiology and anesthesiology – to ensure risk is identified and effectively communicated, and personalized, comprehensive prenatal, delivery and postnatal strategies are implemented [Citation63]. Pregnancy represents a unique opportunity for risk assessment and initiation of risk factor management in women who are not otherwise begin followed medically. Whilst such a pathway is logical, there is a paucity of trial evidence demonstrating that the application of such risk assessment results in clinical benefit.
Systemic autoimmune inflammatory disease [Citation64], more common in women than in men, is also an important but under-recognized risk factor, in addition to cancer survivorship and exposure to mediastinal or breast radiation, or specific chemotherapies or immunotherapies [Citation65]. With regard to the latter, an increasing number of women survive breast cancer and are confronted with increased CVD associated with certain cancer therapies. Improved services for cancer survivors are providing opportunities for patient-specific optimization of cardiovascular health. A number of under-recognized risk factors for CAD are increasingly appreciated, including psychological, social, economic and cultural factors [Citation2]. Women, especially those from minority populations, are disproportionally affected by disparities in wealth, education and access to resources with impacts of cardiovascular health. Studies have found that lower socioeconomic status – including low income, low levels of education and living in disadvantaged areas – was strongly associated with CVD risk in women [Citation66,Citation67]. The strong association between mental health conditions and cardiovascular health and disease has been increasingly recognized [Citation68], ultimately with relevance for both women and men.
Sleep that is considered to be of sufficient duration and quality is associated with a lower risk of CVD in women [Citation69]. Poor quality has been independently associated with hypertension, arrhythmias, stroke, myocardial infarction and congestive heart failure [Citation70]. Women are more likely than men to report insufficient sleep duration. Self-reported sleep duration and insomnia have been associated with incident CVD in women. Among 86,329 women who reported on sleep in the Women’s Health Initiative Observational Study, higher insomnia scores or sleep duration of ≤5 h or ≥10 h had a higher risk of incident CVD [Citation71]. In the Sleep Heart Health Study, designed to look at the relationship between obstructive sleep apnea and CVD, women who had the shortest average apnea–hypopnea respiratory event duration during sleep – a measure of hypersensitivity and hyperarousability that is associated with insomnia – had a 32% increased hazard of all-cause mortality compared to women that had long-duration events [Citation72]. While the mechanisms by which sleep disturbances increase CVD risk remain incompletely characterized, the adverse effects of poor-quality sleep appear to affect women profoundly.
Elevated lipoprotein(a) (Lp(a)) is associated with high burden of atherosclerosis, and can occur independently of elevated LDL cholesterol [Citation73,Citation74]. Previously considered untreatable and a domain only for lipid specialists, Lp(a) is now emerging as a therapeutic target with the use of RNA technology [Citation75]. Future risk scoring systems should consider adding Lp(a) and other less traditional risk factors after the generation of appropriate evidence, which may particularly increase accurate prediction of the development of CAD in females.
Health literacy is an important, but frequently under-considered, factor in the screening and ongoing management of traditional cardiovascular risk factors. Access to education and culturally appropriate resources is an obvious driver of worse health literacy; a trend that is present in both higher and lower-income countries, but amplified in the latter. Significant improvements in the portion of women obtaining tertiary education qualifications have been made in many countries, and women have generally been reported as having higher average health literacy than men [Citation76,Citation77]. However, more than 1 in 10 adult women have a below basic health literacy level, limiting opportunities for CVD self-management [Citation77].
Community strategies for optimal cardiovascular health
Policy decisions have the potential for wide benefits on cardiovascular health of the community beyond education, through influencing the environment. This may include efforts to reduce air pollution, promote incidental exercise and activity, and improve food option quality.
Air pollution is recognized to be a key environmental CVD risk factor, with fine particulate matter <2.5 µm, nitrogen dioxide and ozone gas being some of the major contributing factors [Citation78]. Exposure to air pollution has been associated with increased risk of stroke and CAD, even at levels lower than currently allowable by public health policies [Citation79]. Extreme temperature events, reduction in green-space proximity in the form of civic foliage and public parks, and contamination of waterways and soils with toxins such as heavy metals are also important considerations requiring national and international policy focus [Citation80]. These factors tend to be particularly relevant to communities from low socioeconomic backgrounds.
Meta-analysis of studies investigating physical activity in the general population has shown a 14–20% lower risk of CAD in those who exercised 150–300 min per week at a moderate-intensity level [Citation81]. Importantly, the association of physical activity and reduced CAD risk was stronger in women than in men. However, further research is needed to better understand this observation, as physical activity has not necessarily been associated with enhanced CVD risk factor control in women compared to men [Citation82]. Globally, women may have reduced opportunities for moderate-to-vigorous physical activity due to cultural expectations surrounding gender norms, time reserved for domestic duties and religious limitations [Citation83].
Diet has a profound effect on health and affects all of the major CVD risk factors. Diets high in saturated and trans-fats create a more atherogenic lipid profile, and it is estimated that a 2% increase in calories from trans-fats results in a 23% increase in CVD incidence [Citation84]. Sex-based differences in cardiovascular risk factors, such as lipid profile and adipose distribution, have been well described and may be reflective of both biological and cultural diet differences [Citation85,Citation86]. Metabolically, lipoprotein lipase enzyme in women has demonstrated 1.99-fold increased activity compared to men [Citation87], highlighting important biological differences in nutrient utilization. Current recommendations to optimize cardiovascular health promote a diet inclusive of vegetables, fruits, whole grains, lean protein with minimal red meat intake and low levels of saturated and trans-fats [Citation80]. A recent meta-analysis estimated that higher adherence to a Mediterranean diet was associated with risk reductions for CVD incidence and CAD in women by 24% and 25%, respectively; diet adherence was not associated with significant differences in the incidence of stroke [Citation88].
A need for new solutions: beyond traditional risk factors
Until recently, risk algorithms derived from large population studies have been all that clinicians and patients had to predict the probability of CVD events and guide early preventative strategies. While these scores are helpful to determine the probability of a future CVD event, their role as an emerging tool with the ability to identify the emergence of the causal pathophysiology itself (e.g. atherosclerosis or early cardiomyopathy) is limited. As we have highlighted, it is not uncommon for patients to present with extensive atherosclerosis and life-threatening heart attacks with no standard modifiable cardiovascular risk factors (SMuRF) for CAD. This population comprises approximately 10–25% of initial ST elevation myocardial infarction (STEMI) presentations and experiences a surprisingly higher (>50% greater) 30-day mortality rate compared to those with at least one risk factor, a difference that is more pronounced in women [Citation89–91]. The ‘SMuRFless STEMI’ group highlights that while the existing risk scoring systems are beneficial on a population level, individuals can have critical, vulnerable, progressive coronary disease whilst masquerading as ‘low’ risk [Citation92]. Improved systems to detect causal pathophysiological changes are emerging. However, global thought leaders and policymakers need to consider the prospective evidence required for synergistic integration of these novel tools with traditional risk stratification, ultimately with translation to clinical guidelines [Citation93].
Computed tomography (CT) imaging techniques can identify CAD and are the most clinically advanced methods for early CAD disease detection. Given that we now have data showing that atherosclerosis is treatable and aggressive LDL-lowering strategies can achieve complete halting of progression and stabilization of phenotypes [Citation94–96], an imaging-guided, personalized therapeutic strategy now makes sense and is ideally initiated before an event. The strong association of coronary artery calcification (CAC) with atherosclerosis was first reported by Rumberger et al. in 1995 [Citation97], reflecting deposition of calcium phosphate hydroxyapatite crystals in the intimal extracellular matrix being a common and typical feature of atherosclerosis [Citation98]. Standardized measures of CAC utilizing non-contrast, ECG-gated CT acquisition are now reported in the Agatston score [Citation99]. The resulting CAC score (CACS), which is our only completely non-invasive marker of coronary atherosclerosis itself, is one of the most successful single markers of coronary events [Citation100,Citation101]. The main clinical role that has emerged in international guidelines and practice is based on studies demonstrating the ability for the CACS to reclassify patients from intermediate traditional risk groups into a high-risk or low-risk group [Citation100,Citation102,Citation103]. Here, findings are considered likely to have therapeutic influence. The unmet need regarding detection of subclinical disease in individuals without traditional risk factors has not been addressed to date, although there have been increasing calls for use of the CACS in this group. Early identification of plaque in this population would direct and personalize use of effective therapy, with statins shown to benefit patients with established CAD even with ‘low’ cholesterol [Citation22]. However, dedicated prospective studies are required in this group if this is to be considered in future.
Future opportunities: incorporating genomics and novel omic biomarkers of disease
Polygenic risk scores (PRSs) for CAD have been developed from large populations and clinical biobanks, and have expanded from a few single nucleotide polymorphisms to millions of variants. Novel analytic approaches have led to the creation of a ‘meta’ PRS consisting of 1.7 million genetic variants [Citation104]. This tool has demonstrated a nearly 4.2-fold increased risk of a CAD event in individuals in the top 20% of risk compared with those in the bottom 20%, and has been validated in American and Canadian cohorts of European descent [Citation105,Citation106]. A comparison of PRS performance between the sexes demonstrates similar performance in predicting CAD events and no statistical interaction between the PRS and sex has been observed [Citation104]. However, as expected, CAD risks are delayed in women compared to men across polygenic risk strata. Despite the promise of these large retrospective studies, there is a need to test the CAD PRS in prospective studies evaluating the feasibility, patient experience, impact on risk assessment and management, and health economic potential, as outlined in a recently published expert perspective [Citation107].
High-throughput, multi-omic platforms, paired with advanced coronary imaging and machine learning in large cohorts, provide us with the opportunity to hunt for the ‘holy grail’: a blood-based biomarker of coronary atherosclerosis activity and/or burden, which can be used to guide personalized approaches to CAD and myocardial infarction prevention [Citation108–110]. Considering gender in the derivation of multi-omic risk scores will increase the probability of successful translation and impact.
Summary and conclusion
CVD is a leading cause of morbidity and mortality globally, with IHD remaining a primary contributor in both men and women. Great gains have been made in identifying and managing both CVD and its risk factors, resulting in improved age-adjusted mortality. However, there is a concerning lack of direct focus and data on the impact of CVD in women and progress in reducing the CVD burden in women has slowed in recent years. The under-estimation of CVD risk remains an important issue and continued efforts are needed to raise awareness of CVD among health-care providers and women alike. Enhanced, ongoing collaborations between academic, clinical, policy and advocacy communities will allow us to identify and address evidence and implementation gaps and work toward reducing the burden of CVD in women, thereby improving overall cardiovascular health.
Potential conflict of interest
R. Mehran reports equity <1% in Applied Therapeutics, Elixir Medical and STEL, and <1% (spouse) in ControlRad; and non-financial support from the American Medical Association (Scientific Advisory Board), Society for Cardiovascular Angiography & Interventions (Women in Innovations Committee Member) and Faculty CRF. G. A. Figtree reports non-financial support from CAD Frontiers Pty Ltd, Prokardia Pty Ltd and Australian Cardiovascular Alliance; and patents (planned, pending or issued) for ‘Use of P2X7R antagonists in cardiovascular disease’ [PCT/AU2018/050905], ‘Methods for predicting coronary artery disease’ [AU202209266] and ‘Novel P2X7 Receptor Antagonists’ [PCT/AU2022/051400]. M. P. Gray, B. Vogel and J. A. Leopold report no relevant financial conflicts of interest.
Source of funding
Nil.
References
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010.
- Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. doi: 10.1016/S0140-6736(21)00684-X.
- Enserro DM, Vasan RS, Xanthakis V. Twenty-year trends in the American Heart Association cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham offspring study. J Am Heart Assoc. 2018;7(11):e008741. doi: 10.1161/jaha.118.008741.
- Corlin L, Short MI, Vasan RS, et al. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the Framingham offspring study. JAMA Cardiol. 2020;5(5):549–556. doi: 10.1001/jamacardio.2020.0109.
- Lopez AD, Adair T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int J Epidemiol. 2019;48(6):1815–1823. doi: 10.1093/ije/dyz143.
- Hyun KK, Redfern J, Patel A, et al. Gender inequalities in cardiovascular risk factor assessment and management in primary healthcare. Heart. 2017;103(7):492–498. doi: 10.1136/heartjnl-2016-310216.
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8.
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9.
- Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484.
- Wright JTJr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939.
- Gerdts E, Okin PM, de Simone G, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51(4):1109–1114. doi: 10.1161/hypertensionaha.107.107474.
- Wenger NK, Arnold A, Bairey Merz CN, et al. Hypertension across a Woman’s Life Cycle. J Am Coll Cardiol. 2018;71(16):1797–1813. doi: 10.1016/j.jacc.2018.02.033.
- Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336(7653):1121–1123. doi: 10.1136/bmj.39548.738368.BE.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338(may19 1):b1665–b1665. doi: 10.1136/bmj.b1665.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–921. doi: 10.1056/nejm199304013281303.
- Dickerson JE, Hingorani AD, Ashby MJ, et al. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet. 1999;353(9169):2008–2013. doi: 10.1016/s0140-6736(98)07614-4.
- Chow CK, Atkins ER, Hillis GS, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398(10305):1043–1052. doi: 10.1016/s0140-6736(21)01922-x.
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144.
- Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144–2153. doi: 10.1056/NEJMoa1604304.
- Matthews KA, El Khoudary SR, Brooks MM, et al. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48(1):70–76. doi: 10.1161/strokeaha.116.014743.
- Shepherd J. The West of Scotland Coronary Prevention Study: a trial of cholesterol reduction in Scottish men. Am J Cardiol. 1995;76(9):113C–117C. doi: 10.1016/s0002-9149(99)80480-9.
- Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5.
- Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005562. doi: 10.1161/circoutcomes.118.005562.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8.
- Serban MC, Banach M, Mikhailidis DP. Clinical implications of the IMPROVE-IT trial in the light of current and future lipid-lowering treatment options. Expert Opin Pharmacother. 2016;17(3):369–380. doi: 10.1517/14656566.2016.1118055.
- Sabatine MS, Giugliano RP, Pedersen TR. Evolocumab in patients with cardiovascular disease. N Engl J Med. 2017;377(8):787–788. doi: 10.1056/NEJMc1708587.
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174.
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C.
- Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–1551. doi: 10.1007/s00125-014-3260-6.
- de Jong M, Woodward M, Peters SAE. Diabetes, glycated hemoglobin, and the risk of myocardial infarction in women and men: a prospective cohort study of the UK biobank. Diabetes Care. 2020;43(9):2050–2059. doi: 10.2337/dc19-2363.
- Carbone S, Dixon DL, Buckley LF, et al. Glucose-lowering therapies for cardiovascular risk reduction in type 2 diabetes mellitus: state-of-the-art review. Mayo Clin Proc. 2018;93(11):1629–1647. doi: 10.1016/j.mayocp.2018.07.018.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925.
- Teo YH, Teo YN, Syn NL, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10(5):e019463. doi: 10.1161/JAHA.120.019463.
- Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96(1133):156–161. doi: 10.1136/postgradmedj-2019-137186.
- Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908. doi: 10.1161/JAHA.119.014908.
- Singh AK, Singh R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab Syndr. 2020;14(3):181–187. doi: 10.1016/j.dsx.2020.02.012.
- Wright AK, Kontopantelis E, Emsley R, et al. Cardiovascular risk and risk factor management in type 2 diabetes mellitus. Circulation. 2019;139(24):2742–2753. doi: 10.1161/circulationaha.118.039100.
- Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE.
- Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316(7137):1043–1047. doi: 10.1136/bmj.316.7137.1043.
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–515. doi: 10.1161/ATVBAHA.113.300156.
- GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi: 10.1016/s0140-6736(21)01169-7.
- Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142.
- Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849.
- Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation. 2021;143(15):1468–1480. doi: 10.1161/CIRCULATIONAHA.120.052386.
- Moussa O, Ardissino M, Heaton T, et al. Effect of bariatric surgery on long-term cardiovascular outcomes: a nationwide nested cohort study. Eur Heart J. 2020;41(28):2660–2667. doi: 10.1093/eurheartj/ehaa069.
- Gurtner HP. Pulmonale Hypertonie nach Appetizuglern [Pulmonary hypertension following appetite depressants]. Med Welt. 1972;23(29):1036–1041.
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/nejm199708283370901.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827.
- D’Agostino RBSr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/circulationaha.107.699579.
- D’Agostino RBSr, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. Jama. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180.
- Hurley LP, Dickinson LM, Estacio RO, et al. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3(2):181–187. doi: 10.1161/circoutcomes.108.831073.
- Goff DCJr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98.
- Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. Jama. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611.
- Pylypchuk R, Wells S, Kerr A, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391(10133):1897–1907. doi: 10.1016/s0140-6736(18)30664-0.
- SCORE Working Group and E. S. C. Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309.
- Garcia M, Mulvagh SL, Merz CNB, et al. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–1293. doi: 10.1161/CIRCRESAHA.116.307547.
- Ngo AD, Roberts CL, Figtree G. Association between interpregnancy interval and future risk of maternal cardiovascular disease-a population-based record linkage study. BJOG. 2016;123(8):1311–1318. doi: 10.1111/1471-0528.13729.
- El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):e506–e532. doi: 10.1161/cir.0000000000000912.
- Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women’s Health across the Nation. Arch Intern Med. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568.
- Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4(5):e124865. doi: 10.1172/jci.insight.124865.
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340.
- Durante A, Bronzato S. The increased cardiovascular risk in patients affected by autoimmune diseases: review of the various manifestations. J Clin Med Res. 2015;7(6):379–384. doi: 10.14740/jocmr2122w.
- Alexandre J, Cautela J, Ederhy S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology guidelines. J Am Heart Assoc. 2020;9(18):e018403. doi: 10.1161/JAHA.120.018403.
- Backholer K, Peters SAE, Bots SH, et al. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Commun Health. 2017;71(6):550–557. doi: 10.1136/jech-2016-207890.
- Jenkins KR, Ofstedal MB. The association between socioeconomic status and cardiovascular risk factors among middle-aged and older men and women. Women Health. 2014;54(1):15–34. doi: 10.1080/03630242.2013.858098.
- Rajan S, McKee M, Rangarajan S, et al. Association of symptoms of depression with cardiovascular disease and mortality in low-, middle-, and high-income countries. JAMA Psychiatry. 2020;77(10):1052–1063. doi: 10.1001/jamapsychiatry.2020.1351.
- Daugherty SL, Carter JR, Bourjeily G. Cardiovascular disease in women across the lifespan: the importance of sleep. J Womens Health (Larchmt). 2020;29(3):452–460. doi: 10.1089/jwh.2020.8331.
- St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444.
- Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the women’s health initiative. J Womens Health (Larchmt). 2013;22(6):477–486. doi: 10.1089/jwh.2012.3918 23651054.
- Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC.
- Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063.
- Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604.
- Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA therapeutics to lower blood levels of Lp(a). J Am Coll Cardiol. 2021;77(12):1576–1589. doi: 10.1016/j.jacc.2021.01.051.
- Clouston SAP, Manganello JA, Richards M. A life course approach to health literacy: the role of gender, educational attainment and lifetime cognitive capability. Age Ageing. 2017;46(3):493–499. doi: 10.1093/ageing/afw229.
- Kutner M, Greenberg E, Jin Y, et al. The health literacy of America’s adults: results from the 2003 National Assessment of Adult Literacy. Washington (DC): Education USDOE; 2006. p. 2006–2483.
- Al-Kindi SG, Brook RD, Biswal S, et al. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17(10):656–672. doi: 10.1038/s41569-020-0371-2.
- Wolf K, Hoffmann B, Andersen ZJ, et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. 2021;5(9):e620–e632. doi: 10.1016/S2542-5196(21)00195-9.
- Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121(2):162–180. doi: 10.1161/CIRCRESAHA.117.306458.
- Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710.
- 2018 Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee scientific report. Washington (DC): U.S. Department of Health and Human Services; 2018.
- Grace C, Begum R, Subhani S, et al. Prevention of type 2 diabetes in British Bangladeshis: qualitative study of community, religious, and professional perspectives. BMJ. 2008;337(v04 3):a1931–a1931. doi: 10.1136/bmj.a1931.
- Mozaffarian D, Katan MB, Ascherio A, et al. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–1613. doi: 10.1056/NEJMra054035.
- Wardle J, Haase AM, Steptoe A, et al. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27(2):107–116. doi: 10.1207/s15324796abm2702_5.
- Kiefer I, Rathmanner T, Kunze M. Eating and dieting differences in men and women. J Men’s Health Gender. 2005;2(2):194–201. doi: 10.1016/j.jmhg.2005.04.010.
- Pedersen SB, Jønler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;78(6):1354–1359. doi: 10.1210/jcem.78.6.8200937.
- Pant A, Gribbin S, McIntyre D, et al. Primary prevention of cardiovascular disease in women with a mediterranean diet: systematic review and meta-analysis. Heart. 2023;109(16):1208–1215. doi: 10.1136/heartjnl-2022-321930.
- Vernon ST, Coffey S, D’Souza M, et al. ST-Segment-Elevation Myocardial Infarction (STEMI) patients without standard modifiable cardiovascular risk factors-how common are they, and what are their outcomes? J Am Heart Assoc. 2019;8(21):e013296. doi: 10.1161/jaha.119.013296.
- Vernon ST, Coffey S, Bhindi R, et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur J Prev Cardiol. 2017;24(17):1824–1830. doi: 10.1177/2047487317720287.
- Figtree GA, Vernon ST, Hadziosmanovic N, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet. 2021;397(10279):1085–1094. doi: 10.1016/S0140-6736(21)00272-5.
- Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167(22):2437–2442. doi: 10.1001/archinte.167.22.2437.
- Figtree GA, Vernon ST. Coronary artery disease patients without standard modifiable risk factors (SMuRFs) – a forgotten group calling out for new discoveries. Cardiovasc Res. 2021;117(6):e76–e78. doi: 10.1093/cvr/cvab145.
- Gragnano F, Calabrò P. Role of dual lipid-lowering therapy in coronary atherosclerosis regression: Evidence from recent studies. Atherosclerosis. 2018;269:219–228. doi: 10.1016/j.atherosclerosis.2018.01.012.
- Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015;66(5):495–507. doi: 10.1016/j.jacc.2015.05.065.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. Jama. 2016;316(22):2373–2384. doi: 10.1001/jama.2016.16951.
- Rumberger JA, Schwartz RS, Simons DB, et al. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73(16):1169–1173. doi: 10.1016/0002-9149(94)90176-7.
- Otsuka F, Sakakura K, Yahagi K, et al. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34(4):724–736. doi: 10.1161/ATVBAHA.113.302642.
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t.
- Mohlenkamp S, Lehmann N, Moebus S, et al. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57(13):1455–1464. doi: 10.1016/j.jacc.2010.10.043.
- Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67(2):139–147. doi: 10.1016/j.jacc.2015.10.058.
- Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107(20):2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55.
- Kelkar AA, Schultz WM, Khosa F, et al. Long-term prognosis after coronary artery calcium scoring among low-intermediate risk women and men. Circ Cardiovasc Imaging. 2016;9(4):e003742. doi: 10.1161/CIRCIMAGING.115.003742.
- Inouye M, Abraham G, Nelson CP, et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72(16):1883–1893. doi: 10.1016/j.jacc.2018.07.079.
- Dikilitas O, He B, Bailey K, et al. Association of polygenic risk scores with premature coronary heart disease: a comparative study. J Am Coll Cardiol. 2019;73(9):1793. doi: 10.1016/s0735-1097(19)32399-x.
- Wünnemann F, Sin Lo K, Langford-Avelar A, et al. Validation of genome-wide polygenic risk scores for coronary artery disease in French Canadians. Circ Genom Precis Med. 2019;12(6):e002481. doi: 10.1161/circgen.119.002481.
- Nicholls SJ, Vernon S, Figtree GA. Taking the next steps to implement polygenic risk scoring for improved risk stratification and primary prevention of coronary artery disease. Eur J Prev Cardiol. 2022;29(4):580–587. doi: 10.1093/eurjpc/zwaa030.
- Vernon ST, Tang O, Kim T, et al. Metabolic signatures in coronary artery disease: results from the BioHEART-CT study. Cells. 2021;10(5):980. doi: 10.3390/cells10050980.
- Kott KA, Vernon S, Hansen T, et al. Single-cell immune profiling in coronary artery disease: the role of state-of-the-art immunophenotyping with mass cytometry in the diagnosis of atherosclerosis. J Am Heart Assoc. 2020;9(24):e017759. doi: 10.1161/JAHA.120.017759.
- Kott KA, Vernon ST, Hansen T, et al. Biobanking for discovery of novel cardiovascular biomarkers using imaging-quantified disease burden: protocol for the longitudinal, prospective, BioHEART-CT cohort study. BMJ Open. 2019;9(9):e028649. doi: 10.1136/bmjopen-2018-028649.