Abstract
Menopause is a cardiometabolic transition with many women experiencing weight gain and redistribution of body fat. Hormonal changes may affect also several dimensions of well-being, including sexual function, with a high rate of female sexual dysfunction (FSD), which displays a multifactorial etiology. The most important biological factors range from chronic low-grade inflammation, associated with hypertrophic adipocytes that may translate into endothelial dysfunction and compromised blood flow through the genitourinary system, to insulin resistance and other neuroendocrine mechanisms targeting the sexual response. Psychosocial factors include poor body image, mood disorders, low self-esteem and life satisfaction, as well as partner’s health and quality of relationship, and social stigma. Even unhealthy lifestyle, chronic conditions and putative weight-promoting medications may play a role. The aim of the present narrative review is to update and summarize the state of the art on the link between obesity and FSD in postmenopausal women, pointing to the paucity of high-quality studies and the need for further research with validated end points to assess both biomarkers of obesity and FSD. In addition, we provide general information on the diagnosis and treatment of FSD at menopause with a focus on dietary interventions, physical activity, anti-obesity drugs and bariatric surgery.
摘要
更年期是心脏代谢的转变, 许多女性经历体重增加和身体脂肪的重新分布。激素变化也可能影响健康的几个方面, 包括性功能, 女性性功能障碍(FSD)的发生率很高, 这表现为多因素病因。最重要的生物学因素从与肥大脂肪细胞相关慢性低度炎症, 可能转化为内皮功能障碍和通过泌尿生殖系统的血流受损、胰岛素抵抗和其他针对性反应的神经内分泌机制。心理社会因素包括身体形象不佳、情绪障碍、自尊和生活满意度低, 以及伴侣的健康和关系质量, 以及社会耻辱。甚至不健康的生活方式、慢性疾病和可能的增重药物也可能起作用。本叙述性综述的目的是更新和总结绝经后女性肥胖和FSD之间联系的最新进展, 指出缺乏高质量的研究, 需要进一步研究验证终点, 以评估肥胖和FSD的生物标志物。此外, 我们还提供了更年期FSD诊断和治疗的一般信息, 重点是饮食干预、体力活动、减肥药物和减肥手术。
Introduction
In recent decades, the worldwide prevalence of obesity has increased dramatically and female gender is associated with about twice the risk of having severe obesity [Citation1]. The World Health Organization (WHO) defines overweight as body mass index (BMI) ≥ 25 kg/m2, obesity as BMI ≥ 30 kg/m2 and severe obesity as BMI ≥ 40 kg/m2 [Citation2]. In 2016, according to the WHO, approximately 1.9 billion adults were overweight and 650 million were obese [Citation2]. From 1990 to 2017, the global deaths and disability-adjusted life years attributable to an increased BMI have more than doubled for both females and males, depending on the socio-demographic index [Citation3]. Obesity is a multifaceted chronic condition associated with significant medical complications in women, aside from contributing to a reduction in life expectancy [Citation4]. Indeed, benefits of weight loss are highly relevant for general health and quality of life [Citation5], as well as reproductive [Citation6] and sexual [Citation7] health. A 5% reduction in body weight is clinically meaningful [Citation8], even though a 5–10% goal has been recommended by the 2013 guidelines for managing overweight and obesity [Citation9].
However, the classification of obesity by BMI alone does not provide adequate information on health status and the potential risk of future adverse clinical outcomes. In fact, although obesity is often associated with various comorbidities, a subgroup of obese patients does not seem to have an evident cardiometabolic risk and the concept of metabolically healthy obesity (MHO) has been derived from these clinical observations [Citation10]. To date, the definition of MHO is still a work in progress and stratification of obese subjects, based on metabolic health status, seems crucial to optimize prevention and treatment strategies [Citation11]. A systematic review outlined that the prevalence of MHO is highly variable, ranging between 6% and 75%, mainly because of the different worldwide diagnostic criteria [Citation12]. A recent effort to summarize the multitude of available definitions of MHO pointed to normal levels of blood glucose, high-density lipoproteins (HDL-C) and high-sensitivity C-reactive protein (CRP) and the lack of hypertension, hypertriglyceridemia and insulin resistance based on the homoeostatic model assessment of insulin resistance (HOMA-IR) in obese individuals [Citation13]. The cardiometabolic risk in obese patients is directly related to the number and severity of metabolic abnormalities. However, it is still unclear whether MHO should be considered a benign condition or simply a transitional stage toward metabolically unhealthy obesity. Overall, the current evidence appears to show that although MHO is associated with a substantially lower cardiometabolic risk than metabolically unhealthy obesity, there is no protection against this risk [Citation14]. Interestingly, MHO appears to be more prevalent in women than in men and decreases with age [Citation15]. However, a lower prevalence of MHO has been found in postmenopausal women compared to premenopausal women [Citation16] and a transition from MHO to metabolically unhealthy obesity has been identified in about 30% of postmenopausal women, suggesting the importance of the changes in the sex hormone environment [Citation16]. Indeed, abdominal adiposity measured through the waist circumference (WC), a widely used representative visceral obesity index alone or as a ratio with hip circumference [Citation17], could be a risk factor for vasomotor symptoms early in the menopausal transition and seems to be protective at later stages [Citation18].
Aside from being a cardiometabolic transition [Citation19], menopause represents a crucial phase in a woman’s life, influencing several dimensions of well-being including sexual function and relationship [Citation20]. The WHO defines sexual health as a fundamental component of overall health and well-being of individuals, couples and families [Citation21]. Weight gain, changes in body composition and body image are major issues in women at midlife [Citation22] and may be comorbid with the manifestation of sexual dysfunction, which is also very relevant at this life stage [Citation23]. Despite the high prevalence of weight-related conditions and the importance of addressing cardiometabolic health in women with several postmenopausal comorbidities [Citation24], including female sexual dysfunction (FSD) [Citation25], few studies have investigated the association between obesity and sexual health at menopause. Moreover, the possible inter-relationship between different clinical phenotypes of obesity and specific FSD diagnoses remains unclear in the menopausal population.
The aim of the present narrative review is to update and summarize the state of the art on the link between obesity and FSD in postmenopausal women. A better understanding of this relationship could provide valuable insights into how to comprehensively manage sexual symptoms and distress associated with obesity and its potential menopausal comorbidities. First, we briefly describe the two variables of interest: obesity and FSD after menopause. Then, we review main findings on etiological factors, according to the biopsychosocial model, as well as on the diagnosis and treatment strategies of FSD in postmenopausal women with obesity. Finally, we provide some final remarks to encourage the implementation of research and practice in this area of women’s midlife health.
Obesity in postmenopausal women
The menopausal transition is associated with an increase and redistribution of body fat, resulting in a change from the gynoid to the android pattern [Citation17]. A longitudinal follow-up of a cohort of the Study of Women’s Health Across the Nation (SWAN), including 1246 women with a mean age of 47.1 years, showed that fat mass and BMI start to increase 5–6 years before menopause onset [Citation26]. These two variables continue to increase up to 4 years or more after menopause [Citation26], with the development of central adiposity [Citation27].
White adipose tissue in humans is dependent on gender and age [Citation28]. From puberty, normal estrogen levels result in hyperplastic adipose tissue growth and women accumulate fat mainly in the subcutaneous district, particularly in the hips and thighs [Citation29]. Such a gynoid pattern of fat distribution has been correlated with a reduced risk of developing metabolic diseases [Citation29]. Conversely, men tend to store fat in visceral deposits, the so-called visceral adipose tissue (VAT) [Citation30]. However, in menopausal women, reduced estrogen levels lead to metabolically unhealthy hypertrophic VAT expansion, which has greater pro-inflammatory activity than subcutaneous deposits and is associated with an increased risk of cardiometabolic diseases [Citation19]. Of note, distinct inflammatory signatures, depending on their location, characterize abdominal and femoral adipocytes in postmenopausal women [Citation31].
Basic science has demonstrated that the declining estrogen levels play a pivotal role in changes in body composition. In the mice model, ovariectomy induces central abdominal fat accumulation, which improves after 17β-estradiol administration [Citation32]. In humans, surgical menopause accelerates weight gain in a greater proportion than does the natural menopause and contributes to fat redistribution [Citation33]. Furthermore, a decrease in estrogen levels increases the hypothalamic release of gonadotropin-releasing hormone (GnRH), which in turn stimulates the anterior pituitary gland to produce luteinizing hormone (LH) and follicle stimulating hormone (FSH) [Citation34]. FSH may play an important role in regulating fat metabolism in postmenopausal women. In particular, the binding of FSH to the Gi protein α-subunit (Gαi), an inhibitory coupling protein of the FSH receptor (FSHR) expressed in VAT, promotes lipid biosynthesis [Citation35]. On the other hand, estrogen decline influences homeostatic (caloric intake and energy expenditure) and hedonic feeding behavior directly modulating neural circuits, which are also sensitive to mood and sleep changes accompanying the menopause [Citation36,Citation37]. The contribution of FSH elevation and hormonal decline to total energy homeostasis, eating disorders, fat gain and distribution needs further investigation that may lead to an improved management of the metabolic health of postmenopausal women. In the Women’s Health Initiative (WHI) Observational Study, anthropometric measures positively correlated with androgens and their metabolites [Citation38] and abdominal obesity, hyperinsulinemia and insulin resistance appeared in relationship with ‘relative functional hyperandrogenism’, due to the reduction of sex hormone binding globulin (SHBG) concentration and the consequent increase of free testosterone in women with a previous history of polycystic ovary syndrome [Citation39]. Interestingly, adipose tissue itself may represent an ‘intracrine’ source of androgen synthesis because it has a complex set of enzymes that activate and inactivate androgens, thus regulating the local androgenic environment [Citation40]. However, gender may play a role in the context of androgen metabolism, fat presence and distribution because obesity-related secondary hypogonadism emerges only in males [Citation41].
Menopause and female sexual dysfunction
Sexual function declines progressively across the menopausal transition, with approximately 40–50% of all women reporting at least one sexual symptom, which is not always associated with distress [Citation42]. There is significant comorbidity among sexual symptoms in postmenopausal women [Citation43] and their multidimensional etiology [Citation44] requires appropriate diagnosis [Citation45] and treatment [Citation46]. The two most well-studied clinical conditions compromising sexuality at menopause are vulvovaginal atrophy/genitourinary syndrome of menopause (GSM) and hypoactive sexual desire disorder (HSDD), resulting from the significant drop in circulating estrogen levels and the progressive decline of androgens with age [Citation47]. Other aspects, such as mood (anxiety and depression) [Citation44] and personality [Citation48], as well the quality of partner relationships and the level of intimacy [Citation49], play an important role in modulating sexual satisfaction.
FSD identifies a heterogeneous group of disorders that involves sexual desire, arousal, orgasm or sexual pain, which are common at menopause and often remain underdiagnosed and undertreated in clinical practice [Citation23]. The most used psychometric screening tool to assess domains of sexual function is the Female Sexual Function Index (FSFI) [Citation50]. In formulating an appropriate diagnosis of FSD, sexual distress manifesting as concern, frustration, hopelessness or distressing behaviors plays a key role [Citation42] and should be assessed by the revised version of the Female Sexual Distress Scale [Citation51]. From a diagnostic standpoint, controversy revolves around the classification of dysfunctional sexual desire and arousal because the fifth version of the Diagnostic and Statistical Manual of Mental Disorders conceptualized desire and arousal disorders as part of a single clinical entity, namely ‘Female Sexual Interest and Arousal Disorder (FSIAD)’ [Citation52]. By contrast, in line with the Fourth International Committee on Sexual Medicine [Citation53], the International Society for the Study of Women’s Sexual Health (ISSWSH) advocated to maintain HSDD and female sexual arousal disorder as distinct conditions, which may reflect different etiological mechanisms requiring specific treatments [Citation54].
Female sexual dysfunction in postmenopausal women with obesity
There is a strong research evidence (from animal to human) to support the association between obesity and male sexuality, namely erectile dysfunction [Citation55]. Given sex-specific patterns in adipose tissue accumulation, obesity might affect sexual function in different ways across the sexes [Citation28]. Obesity may have an impact on symptom clustering in midlife women [Citation56], but its role in the occurrence of specific FSD has been less investigated [Citation57]. A recent systematic review and meta-analysis focusing on the global prevalence of sexual dysfunction in women with variable age distributions indicated that 26.9% (95% confidence interval 13.5–46.5) were overweight and 49.7% (95% confidence interval 35.8–63.5) were obese [Citation58]. Unfortunately, in a very large sample of women (aged 18 years or older) self-reporting sexual problems (any, desire, arousal and orgasm) and associated distress, reliable BMI data could not be included as a covariate of FSD, which was more common in women aged 45–64 years (14.8%) than in younger (10.8%) or older (8.9%) women [Citation59].
The relationship between obesity and female sexual function has been investigated mainly in clinical samples of postmenopausal women and the variation in outcomes may result from the diversity of culture and healthcare systems [Citation22,Citation57]. The Collaborative Group for Research of the Climacteric in Latin America (REDLINC) has conducted extensive research in Latin America indicating that obesity affects many dimensions of menopausal quality of life, including sexual health [Citation60]. A cross-sectional study used the FSFI to compare sexuality in 221 Brazilian obese and normal-weight postmenopausal women with a mean age of 54.3 ± 5 years [Citation61]. The authors found significantly lower desire and arousal scores in the obese group than in the normal BMI group (p = 0.043 and p = 0.028, respectively) [Citation61]. In an early study, Kirchengast et al. reported that body weight and BMI were significantly related to the degree of decreased sexual interest in a sample of 171 postmenopausal women [Citation62]. A higher percentage of sexual symptoms has been reported in Spanish obese postmenopausal women with abdominal obesity [Citation63], whereas in a longitudinal cohort of midlife Chinese women followed up for 10 years mild bothered sexual functioning was significantly associated with general obesity, but not with central obesity [Citation64]. In an Italian sample of women (44% postmenopausal) with and without FSD (FSFI total score < 23), free from diseases known to affect sexual function, the FSFI strongly correlated with BMI (r = −0.72, p = 0.0001), but not with the waist-to-hip ratio (r = −0.09, p = 0.48), in women with FSD [Citation65]. Interestingly, in a recent cross-sectional US study performed by analyzing medical records of 6688 women seeking consultation for menopause-related or sexual health-related concerns and being overweight or obese was associated with sexual inactivity and greater odds of having FSD among sexually active women [Citation66]. However, multivariable analysis showed that other factors with an impact on body weight and sexual function, including age, level of education, reproductive stage, medication use and mood disturbances, contributed to FSD in women with overweight and obesity [Citation66], confirming the importance of the biopsychosocial approach in sexual medicine [Citation23,Citation54].
Etiology
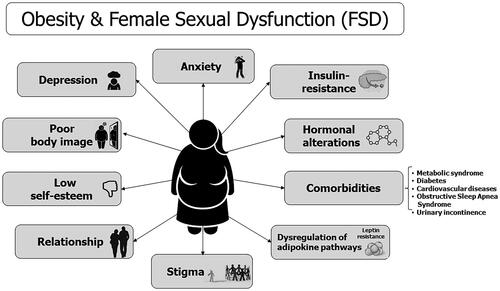
The etiology of FSD in postmenopausal women with obesity is complex and multifactorial, and the most important factors potentially implicated in the clinical manifestation of distressing sexual symptoms are shown in .
Figure 1. Biopsychosocial etiologies of female sexual dysfunction in postmenopausal women with obesity.
Inflammatory and endocrine factors
Adipose tissue is not only a simple inactive site to store energy, but also behaves as an active endocrine organ, responsible for the secretion of adipokines, cytokines and chemokines [Citation67]. The main effects of some of these are presented in .
Table 1. Expression and role of the main adipokines, cytokines and chemokines which may be relevant to the etiology of female sexual dysfunction (FSD) in postmenopausal women with obesity.
Under physiological conditions, the molecules secreted by adipocytes contribute to the maintenance of body homeostasis and can modulate the activity of the immune system [Citation68]. In individuals with obesity, a dysregulation of the adipokine and cytokine pathways causes the onset of chronic low-grade inflammation starting from a localized inflammation of the adipose tissue to a systematic state. Interestingly, inflammatory biomarkers of adiposity have been linked to vasomotor symptoms early in the menopausal transition, concomitant with the cardiometabolic shift in some women [Citation68]. Hypertrophic adipocytes produce non-esterified fatty acids, which induce local macrophages to secrete high levels of tumor necrosis factor-α (TNFα), which, in turn, stimulates adipocytes to produce more non-esterified fatty acids, pro-inflammatory cytokines such as interleukin-1β (IL-1β) and IL-6, acute phase proteins, and chemokines that attract more monocytes/macrophages into the adipose tissue [Citation69]. Such an inflammatory state may translate into endothelial dysfunction and compromised blood flow through the genitourinary system [Citation70]. Similar to men, the regulation of blood flow to the sexual organs in women is under the control of the nitric oxide (NO)/cGMP pathway [Citation71]. An inverse correlation has been found between BMI and clitoral blood flow, assessed by Doppler ultrasound in 71 women with FSD (mean age 44.8 ± 13.1 years) [Citation71]. Interestingly, women with obesity and/or with the metabolic syndrome (MS) had significantly higher vascular resistance indices, suggesting that the presence of these comorbidities may influence the arterial blood flow to the clitoris [Citation72]. Therefore, the reduced availability of NO and the consequent impairment of blood distribution to the genitourinary system may impair genital arousal contributing to FSD in obese women. In addition, some recent studies are addressing the relationship between innate immune system-induced inflammation, especially the Toll-like receptors and FSD, because of their role in initiating vascular and tissue impairments related to sexual dysfunction [Citation73].
Among the various dysregulated adipokines produced by hypertrophic adipocytes, leptin, a 16-kDa adipokine secreted mainly from white adipose tissue, is the most studied due to its reproductive implications in both women and men, while fewer data are available for other adipokines [Citation74]. Serum leptin levels are positively associated with body fat percentage and adipocyte size [Citation75], and the hypothalamus–pituitary–gonadal axis (HPG) becomes resistant to leptin signaling [Citation76]. Leptin is defined as a ‘satiety hormone’ because it inhibits appetite by acting centrally and its potential modulation of sexual function is indirect through important effects on hypothalamic GnRH secretion mediated by other peptides, including kisspeptin [Citation76]. Animal studies have shown that a high-fat diet and central leptin resistance decrease kisspeptin expression in both the rostral periventricular region of the third ventricle and the arcuate nucleus [Citation77], which is fundamental in the inhibition of GnRH-secreting neurons related to obesity. Kisspeptin is also important in other aspects of reproductive behavior from rodents to humans, including sexuality [Citation78], and its pharmacological use is under investigation in women with HSDD [Citation79].
Hypertrophic adipocytes also promote insulin resistance. VAT enhances the delivery of free fatty acids to the liver, leading to reduced hepatic insulin clearance. This further increases circulating insulin levels, leading to hyperinsulinemia. Increased production of adipokines and inflammatory mediators significantly affects insulin signaling in insulin-responsive tissues, promoting systemic insulin resistance and hyperinsulinemia [Citation80]. In patients with obesity, insulin resistance-induced hyperinsulinemia reduces the secretion of SHBG by the liver, altering the balance of steroid hormones in the body, with higher concentrations of bioavailable testosterone in postmenopausal women [Citation81]. Of note, endogenous estrogen and androgen levels do not explain the relationship between SHBG and the HOMA-IR, which is partially independent of BMI in postmenopausal women [Citation82]. Whether this may be relevant to domains of sexual response in individuals with obesity is underexplored. Women with supra-physiological testosterone levels tend to increase both abdominal visceral and subcutaneous adipose tissue [Citation83] with an interference in sexual function, particularly when they have obesity [Citation7].
VAT can lead to a higher rate of estrogen-dependent cancers, such as endometrial and breast cancer [Citation84], which are distressing conditions in women with obesity [Citation17]. This is the result of an increased aromatase activity and thus increased conversion of androgens to estrogens, which are primarily produced by the adipose tissue in postmenopausal women [Citation84]. An early study demonstrated that circulating estrone and 17β-estradiol increased by more than 40% in obese (BMI > 30 kg/m2) compared to normal-weight (BMI < 27 kg/m2) postmenopausal women [Citation85]. In multivariate analyses, obesity was an independent factor associated with higher vaginal estrogenization in a large sample of US postmenopausal women (age range 57–85 years) [Citation86]. However, bariatric surgery did not change the prevalence of vaginal dryness in a longitudinal analysis of data from 69 women (age range 35–72 years) [Citation87], pointing to other contributors rather than hormonal factors in the amelioration of sexual function postoperatively [Citation88].
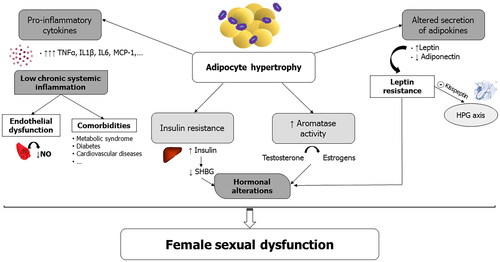
summarizes the main inflammatory and endocrine factors likely involved in FSD associated with obesity in general.
Figure 2. Main inflammatory and endocrine factors likely involved in female sexual dysfunction (FSD) associated with obesity. HPG, hypothalamic–pituitary–gonadal; IL6/1β, interleukin-6/1β; MCP-1, monocyte attractant protein-1; NO, nitric oxide; SHBG, sex hormone binding globulin; TNFα, tumor necrosis factor-α.
Psychosocial factors
Psychological distress, poor body image, low self-esteem and general well-being often characterize postmenopausal women with obesity with a possible interference in sexual function [Citation22,Citation89]. An increased risk of developing depression is strongly associated with obesity [Citation90], but also with the menopausal transition [Citation91]. In this life stage, weight gain and increased BMI have been related to anxiety, depression and low life satisfaction [Citation92]. A review of eight studies assessing health-related quality of life among women older than 55 years described that postmenopausal women with BMI > 30 kg/m2 had a poor health-related quality of life, particularly regarding physical function, energy, vitality and health perception [Citation93]. A possible link between mood, FSD and obesity is the use of psychopharmacological therapies because some of them induce sexual side effects [Citation94] and cause an increase in body weight [Citation95]. In a prospective observational large cohort study in the USA, women with overweight or obesity at baseline were more likely to be taking antidepressants, and taking at least one putative weight-promoting medication was associated with a greater significant increase in BMI and WC over the course of 3 years compared to women not on these medications [Citation96].
General and sexual health of the partner also plays a major role in FSD at midlife [Citation97], especially in the presence of obesity and unhealthy lifestyle due to their negative impact on cardiovascular and metabolic function [Citation98]. Another important issue to consider is the stigma and prejudice experienced by obese individuals [Citation99]. This also plays an important role in the context of romantic relationships because individuals presenting with overweight or obesity, particularly women, are at a disadvantage compared to those of normal weight [Citation100]. Weight-based stigma has been shown to correlate with body, relationship and sexual dissatisfaction, and with disordered eating behaviors [Citation100].
Therefore, during female midlife the complex inter-relationships between excess body weight, physical well-being and mental health have many consequences and ramifications on sexual function that should be further investigated.
Weight-related comorbidities
In addition to being a possible independent risk factor for FSD, obesity exerts its impact on sexuality because of related cardiometabolic comorbidities [Citation101,Citation102] and other chronic conditions [Citation4]. The association between FSD and the MS in postmenopausal women, irrespective of the presence of overweight and obesity, is well studied but results are mixed [Citation25]. In a postmenopausal sample of older overweight women from the Rancho Bernardo Study, the MS was associated with decreased sexual activity, desire and satisfaction in all women and with many dysfunctional sexual domains in those who were sexually active; coronary artery disease was more prevalent in women with low sexual activity [Citation103]. In a cross-sectional study conducted with a sample of 291 postmenopausal Brazilian women aged 40–65 years, the prevalence of HSDD was significantly higher among those with the MS (BMI 30.2 ± 5.8 kg/m2) than among those without (BMI 26.5 ± 4.4 kg/m2) [Citation104]. Women with hypertension and increased triglycerides (p = 0.03) also had a higher prevalence of HSDD than did those without these conditions [Citation104]. The role of triglycerides seemed crucial in the risk of presenting FSD in an Italian cross-sectional clinical sample of postmenopausal women, irrespective of the presence of the MS [Citation105]. Other Brazilian data confirmed a high prevalence of the MS and FSD in postmenopausal women, but did not show an association between both conditions [Citation106,Citation107]. FSD was rather significantly associated with married status, time since menopause (6–10 years), occurrence of climacteric symptoms and a history of sexual abuse [Citation106] and with partner’s sexual dysfunction [Citation107]. Even in a Turkish sample of women of middle and low-socioeconomic class, the rate of FSD increased postmenopause, but the MS status did not make a difference for FSD [Citation108].
In contrast to males, who frequently report erectile dysfunction and other sexual symptoms as a long-term complication of diabetes mellitus, controversies on a similar pattern in women still exist [Citation25,Citation102,Citation109]. The potential hypogonadism associated with a history of diabetes mellitus [Citation110] is counteracted in women by a more common history of polycystic ovary syndrome associated with hyperinsulinemia and hyperandrogenism that may persist in menopause [Citation39], complicating the possible investigation of a link between androgen milieu, cardiometabolic risk and FSD. Even the possibility that FSD may be considered a marker of increased cardiovascular risk is still under investigation and only adequate methodologies may help us to solve the issue [Citation111]. Indeed, it is quite difficult to link vascular and structural impairments of female genital tissues observed in animal models of atherosclerosis, hypertension, diabetes and obesity to sexual experiences of patients with chronic diseases [Citation112]. A higher rate of reporting menopausal symptoms was evident in a sample of postmenopausal Finnish women with various comorbidities, but a lower sexual function was associated only with diabetes [Citation113]. A recent meta-analysis including 25 studies showed that the overall prevalence of FSD in 3892 women aged 18–72 years with type 2 diabetes mellitus was 68.6% (95% confidence interval 61.6–75.3%), with a statistically significant increase over time [Citation114].
Sleep disorders and obstructive sleep apnea have been significantly associated with FSD in premenopausal women with obesity, especially when nocturnal hypoxia was documented [Citation115]. During the menopause, sleep disorders are very frequent [Citation116] and a positive association between obstructive sleep apnea and FSD has been reported [Citation117]. After adjusting for potential confounders, poor sleep quality was associated with reduced sexual function, suggesting the importance of individual well-being for a healthy sexuality [Citation117].
Pelvic floor dysfunction, including several types of urinary incontinence and lower urinary tract symptoms, is another common problem in women with obesity [Citation118] particularly after the menopause that may interfere with sexual function [Citation119]. Reasons range from mechanical to inflammatory and metabolic effects, all potentially contributing to an impairment of the neurovascular and neuromuscular substrates of the healthy sexual response [Citation119]. Unfavorable obesity-related microbiomes in several areas of the female body, including the vagina [Citation120], may exert adverse sexual effect but this area of research is still underexplored. In a very large cross-sectional US sample of postmenopausal women (age range 50–79 years), an elevated prevalence of urogenital symptoms (severe discharge, irritation, itching, dysuria) was associated with obesity and diabetes [Citation121].
Other obesity comorbidities may influence women’s sexuality with aging, as indicated by the evidence that people in good physical health are more likely to be sexually active with a partner [Citation122]. Interestingly enough, sexually active life expectancy is shorter for women, but women lose less years of sexually active life as a result of poor health than men [Citation123], reinforcing the idea that intimacy and other intrapersonal and interpersonal components are relevant to sexual satisfaction in women [Citation20,Citation49].
Diagnosis
Counseling is the cornerstone of adequate management of FSD in the menopause consultation because it helps to uncover sexual symptoms and to inform women about the multitude of treatment options [Citation124]. A patient-centered communication offering a general explanation about the possible changes in sexual function may predispose women to talk about their own experience [Citation54]. Very recently, Kingsberg et al. proposed the POSIT model for counseling, in which women receive ‘Permission’ to raise sexual issues and the health-care professional may ‘Offer’ appropriate information providing ‘Specific’ suggestions according to the level of expertise, and referring the patient to intensive ‘Therapy’ if needed [Citation125]. The main goal is to address the presence of GSM and/or HSDD because they may co-occur and reinforce each other, but, most importantly, they can display different etiologies and require tailored treatments [Citation46,Citation47,Citation125].
Screeners, psychometric validated questionnaires (FSFI, Female Sexual Distress Scale – revised, etc.) and semi-structured interviews may be used in routine practice of menopause to explore each domain of the sexual response (desire, arousal, lubrication, orgasm, satisfaction and pain) and to assess the level of distress associated with the presence of sexual complaints [Citation45–47,Citation54]. Objective signs associated with GSM symptoms should be collected in order to establish an appropriate treatment plan [Citation46,Citation125], taking into account the concomitant presence of HSDD [Citation23].
A comprehensive medical history is mandatory to evaluate evidence regarding the impact of obesity and related conditions in the manifestation of FSD with the ultimate goal of improving both sexual and health outcomes [Citation47]. Collecting standard anthropometric information (weight, height, WC) and blood pressure along with routine blood testing to assess cardiometabolic health should be a standard of menopausal care [Citation19]. In particular, WC is a cardiovascular disease risk marker that is independent of BMI [Citation126]. Newer anthropometric indexes, such as a body shape index, visceral adiposity index and lipid accumulation product, are not routinely used, even though they may be more accurate to evaluate cardiometabolic risk [Citation17,Citation127]. Other laboratory tests (thyroid, prolactin, cortisol, androgens) may play a role in selected cases to rule out secondary causes of FSD, but are not indicated in the assessment of menopausal sexual function [Citation23,Citation46]. In particular, androgen insufficiency is a clinical diagnosis and androgen levels are not included in diagnostic algorithms [128].
Objective non-invasive approaches such as 3D high-frequency vaginal ultrasound, to measure the anterior and posterior walls of the vagina [Citation129], and color Doppler ultrasound, to evaluate the clitoral pulsatility index, reflecting vascular resistance, are presently performed only for research purposes [Citation130].
A full psychosocial history is equally important to identify FSD contributors associated with mental health [Citation131] that may also be useful to set up effective counseling for weight management in postmenopausal women with obesity. In this regard, information on diet, eating behavior, previous weight-reduction strategies, physical activity and other lifestyle factors, taking into account cultural norms, environmental factors and socioeconomic barriers, are also fundamental [Citation132].
Treatment
The management of obesity may be challenging in postmenopausal women [Citation133] as it is in the management of FSD [Citation47,Citation125]. Recent insights into the pathophysiology of obesity have led to the discovery of several promising drug targets and novel therapeutic strategies [Citation134]; meanwhile, the development of pharmacological agents for FSD has been quite slow, with only a few therapies available mainly for premenopausal women [Citation135]. We could argue that the burden of obesity is enormous from several standpoints [Citation4], whereas FSD is not a life-threatening condition with a multidimensional etiology, which is difficult to target from a pathophysiological standpoint [Citation136]. However, there is a neuroendocrine link between sexual function and weight management deserving further investigation [Citation137]. Two drugs are available in the USA and Canada for the treatment of secondary acquired HSDD: flibanserin and bremelanotide [Citation136]. Of note, the use of flibanserin, an oral neuroactive serotoninergic agent, was associated with statistically significant weight loss relative to placebo in a post-hoc analysis of clinical trials in which premenopausal and postmenopausal study patients were not selected for being overweight/obese and were not expecting a weight reduction [Citation138]. On the other hand, bremelanotide, an injectable agonist of the central melanocortin system, which is a promising therapeutic target for treating various metabolic disorders including obesity, was efficacious and safe in almost every category of BMI, when subgroup analyses were performed in premenopausal women [Citation139]. Interestingly enough, in two phase 1 randomized controlled trials, twice-a-day exposure to bremelanotide, at a dose different from that used in HSDD, was effective in reducing caloric intake and weight loss in women with obesity (age range 18–55 years) [Citation140].
Nowadays, many hormonal possibilities are available to treat menopausal symptomatology, including sexual symptoms, or to target specific conditions like GSM and HSDD [Citation125,Citation141], and some novel pharmacotherapies are in development [Citation142]. An international survey has shown that postmenopausal women are very much concerned with weight gain and its control, even more than with vaginal health and GSM complaints [Citation143]. When women with obesity are symptomatic, menopause hormone therapy can be safely used [Citation144] with a preference for transdermal estradiol to improve both symptoms and cardiometabolic risks [Citation22,Citation145,Citation146]. There are no signals in the literature that local estrogen therapy cannot be safely used for the treatment of GSM also in women with obesity [Citation147]. Even ospemifene, an oral selective estrogen receptor modulator targeting GSM symptoms, has been proven safe in a 5-year post-authorization safety study [Citation148]. A recent systematic review and meta-analysis including 43 studies and 8480 postmenopausal women showed that testosterone administration is associated with a significant increase in the number of satisfying sexual events and sexual desire [Citation149]. Based on these data, the Global Position Statement on the use of testosterone therapy for women recommended a dose within the physiological premenopausal range to treat HSDD in postmenopausal women [Citation150]. Clinical and biochemical monitoring should be planned to avoid any supra-physiologic dosage of testosterone and treatment should be stopped if it is not effective after 6 months [Citation150]. A clinical practice guideline provided standards for safely prescribing testosterone to women with HSDD, pointing to systemic transdermal testosterone as the safest route and underlying the importance to address also modifiable factors or comorbidities such as relationship or mental health problems [Citation128]. Randomized controlled trials of testosterone therapy have excluded women at high cardiometabolic disease risk [Citation149], and therefore its prescription in women with obesity cannot be considered completely safe. However, non-oral testosterone therapies (percutaneous and injectable) did not cause significant adverse effects on lipid profiles, glucose metabolism and blood pressure [Citation150]. Other potential positive health benefits of testosterone replacement in postmenopausal women remain to be confirmed in future investigations [Citation151].
What obesity and FSD have in common is the importance to address the topic in a sensible manner, helping the patient to overcome barriers and fears of not being able to improve [Citation99,Citation137]. In addition, lifestyle and behavioral medicine may be effective in both conditions and targeted to specific profiles of menopausal women [Citation152]. No single therapy works for all women and individualization of treatment is always the key for adherence and satisfaction. In the following, we will revise briefly the main therapeutic strategies for obesity that have shown a positive effect on FSD with a focus on postmenopausal women.
Dietary interventions
The role of diet in sexual health is an area of study that has been gaining attention in recent decades, but there is a paucity of information in postmenopausal women with obesity to demonstrate an effect on FSD [Citation153]. A meta-analysis of population-based research pointed to some evidence that a healthy diet was related to a lower risk of FSD, with similar findings in clinical and non-clinical samples [Citation154].
A variety of diets have been developed to counteract overweight and obesity. The most studied diet for improving many chronic gynecologic diseases, including FSD, is the Mediterranean diet [Citation155], which is a pillar of cardiometabolic and reproductive improvement in the context of diabetes type 2 in both sexes [Citation156]. Previous solid data have shown that a Mediterranean-type diet improved markers of inflammation, endothelial function and insulin resistance [Citation157], an effect that could be associated with an improvement in sexual function observed in women (mean age 42.3 ± 4.5 years; mean BMI 28.8 ± 2.8 mg/m2) with the MS over 2 years [Citation158]. A recent position statement of the European Menopause and Andropause Society (EMAS) focused on short-term and long-term health effects of the Mediterranean diet on menopause [Citation159]. The authors suggest that long-term high adherence to the Mediterranean diet may protect the cardiovascular system, maintain or improve bone mineral density, help prevent cognitive decline, and reduce the risk of breast cancer and all-cause mortality in perimenopausal and postmenopausal women. Moreover, short-term adherence may improve vasomotor symptoms, mood, symptoms of depression and cardiovascular risk factors [Citation159]. Even if there is no mention of the effects on FSD, the reduction of chronic low-grade inflammation may be helpful to ameliorate FSD in postmenopausal women with obesity by lowering the general risk of non-communicable diseases [Citation160].
Among the different options developed to address overweight and obesity, ketogenic diets have gained increasing interest for the treatment of obesity and its overall comorbidities in women across the entire reproductive life span, including menopause [Citation161]. This dietary intervention mimics fasting through a marked reduction in carbohydrates (<30 g/day, approximately corresponding to ∼13% of daily energy intake), which prompts energy intake to resort to the β-oxidation of fatty acids, hence synthesizing acetyl-CoA [Citation162]. In low glucose availability, the escape of acetyl-CoA from the tricarboxylic acid cycle contributes to the formation of ketone bodies, which exert an anorexigenic effect [Citation162]. There is a consensus on the role of a very-low-calorie ketogenic diet in the management of metabolic diseases because it is effective for weight loss, reduction of VAT and improvement of metabolic parameters and markers of inflammation [Citation160]. Moreover, there is a weak recommendation with a medium level of evidence to suggest a weight-loss program with a very-low-calorie ketogenic diet in males with obesity and hypogonadism in order to increase plasma androgen levels and to improve sexual function [Citation163]. The only data linking very-low-calorie ketogenic diets and sexuality in women with obesity were collected in a very small sample (age range 18–65 years, BMI ≥ 30 kg/m2) over 4 months [Citation164]. There was a statistically significant improvement in the mean total FSFI score and in arousal and lubrication, with a significant improvement of orgasm only during the maximal ketosis phase of the diet [Citation164].
Physical activity
Participation in physical activity has been associated with a lower risk of FSD [Citation154]. Several mechanisms can be involved, from an increase in the production of endorphins [Citation165] or in the activity of the sympathetic nervous system [Citation166] to a decrease of cardiometabolic risk factors influencing the manifestation of FSD [Citation167]. Also, a positive effect of continued participation in physical activity on sexuality may be mediated by self-efficacy, body attractiveness and maintenance of healthy BMI levels in women (70% overweight or with obesity) at midlife [Citation168]. On the other hand, a recent multivariate analysis conducted with a sample of both premenopausal and postmenopausal women indicated that physical activity was associated with better sexual function and clitoral vascularization and lower sexual distress and reduced odds, but strenuous exercise might be harmful to sexuality [Citation169].
Physical activity may have a role in alleviating some menopausal symptoms [Citation170,Citation171], but results on sexual symptoms are mixed [Citation172]. A longitudinal study of the effects of free testosterone and other psychosocial variables on sexual function across the menopause stated that exercise was clearly associated with sexual satisfaction [Citation173]. In a cross-sectional analysis of data on sexual functioning, having social support and being physically active were associated with enhanced sexual engagement and enjoyment in US midlife women (age range 41–68 years) [Citation174]. On the other hand, in a cross-sectional study involving 2201 Korean women (age range 44–56 years) obesity and physical activity were the main modifiable factors associated with symptom severity, but physical activity did not seem to influence scores of the sexual domain of the MENQOL (The Menopause-Specific Quality of Life) questionnaire [Citation175]. Moreover, in a recent study conducted in a small sample of menopausal women (age range 50–64 years), moderate vigorous physical activity, measured via accelerometer, and adiposity were not significantly associated with sexual well-being and several aspects of the MENQOL [Citation176]. However, it was evident that physical activity may influence sexual well-being and the MENQOL indirectly by reducing adiposity, depressive symptoms and chronic conditions [Citation176].
Studies conducted in postmenopausal women with obesity specifically designed to assess the effect of physical activity on FSD are lacking. Based on data obtained with a multidisciplinary approach including a hypocaloric diet combined with physical exercise in 44 premenopausal women with obesity and FSD, Aversa et al. found a significant improvement in many domains of sexual function assessed with the FSFI and a reduction in body weight and BMI [Citation177]. The evaluation of reactive hyperemia and some metabolic and anthropometric parameters allowed speculation that FSD reduction could be related to an improvement in endothelial function and insulin resistance [Citation177].
Anti-obesity drugs
The field of obesity pharmacotherapies is rapidly growing, with a significant potential impact on non-communicable diseases and health-related quality of life [Citation178]. Six medications (orlistat, phentermine/topiramate, naltrexone/bupropion, liraglutide, semaglutide, tirzepatide), acting on discrete etiologic components of non-syndromic obesity, are presently US Food and Drug Administration (FDA) approved and other drugs are currently being investigated [Citation178]. To our knowledge, no studies have investigated the relationship between anti-obesity therapies and female sexual function. Only benefits of liraglutide, a long-acting glucagon-like peptide 1 (GLP-1) receptor agonist, on reproductive and sexual function, and on metabolic parameters, in obesity-associated functional hypogonadism in males are available [Citation179,Citation180]. The FDA recommended monitoring adverse events related to mental illness/depression and stopping treatment in the case of symptom development [Citation178].
In the last few years, a plethora of dietary compounds, plants and bioactive phytochemicals have been reported as promising sources for developing new treatment strategies for obesity and associated complications [Citation181], but no specific data were collected about FSD.
Finally, it is relevant to mention metformin, the first-line therapy for type 2 diabetes, because it is a drug widely used in the reproductive setting that has been shown weight-loss benefits in large cohort studies [Citation182]. While a positive effect of metformin on sexual function has been clearly shown in diabetic men and in those with cardiometabolic comorbidities [Citation183], only scant positive data are available namely in premenopausal women with prediabetes and diabetes, with the strength of a positive effect on domains of sexual function depending on the degree of insulin resistance [Citation184].
Bariatric surgery
Positive health outcomes following bariatric surgery are numerous and those on sexuality are probably indirect, deriving from a significant improvement of many biopsychosocial variables [Citation185]. Favorable sexual changes following weight loss may include an increase in the frequency of different sexual positions during intercourse and a significant improvement in the impact of urinary incontinence on quality of life [Citation186,Citation187]. A recent systematic review and meta-analysis including 16 observational studies and 953 women with a mean age of 39.4 ± 4.2 years showed that bariatric surgery significantly improves the total FSFI score and all sexual domains with the exception of pain. However, a sub-analysis by age demonstrated that the reduction in FSDs was only significant for women aged <40 years, while patients aged >40 years did not show a significant improvement in the total FSFI score and most subdomains excepting desire (p = 0.0034) [Citation188]. These findings may be likely explained by the proportion of menopausal, married and sexually active women in the studies included in the analysis [Citation188].
Final remarks
Menopause is a turning point in many aspects of women’s health, including changes in body weight and fat distribution, and in sexual functioning. Menopausal women with obesity display some bio-psycho-social risk factors to develop FSD and many chronic comorbidities are involved in the manifestation of sexual symptoms and associated distress. However, available data point to the need for further research for an in-depth understanding of the possible link between weight excess and an impairment of the sexual response. In fact, we do not have even a clue about the amount of weight loss that may translate into clinically meaningful benefits in women with FSD.
Investigation should focus on the role of abdominal obesity and, most importantly, of metabolic health as key determinants of FSD risk. Indeed, metabolic categories in menopausal women may drive such risk more than obesity alone. Stratification of subjects according to inflammatory biomarkers of adiposity along with other clinical and metabolic variables will help to clarify the issue. Also, neuroendocrine signals related to reproductive history and mental health should be taken into account to provide further insights into the impairment of some sexual domains associated with obesity in menopausal women. For this purpose, it is mandatory to identify objective measures of sexual functioning in clinical practice, being aware that they do not show a straight correlation with subjective sexual experiences. In addition, even though sexual health may represent a good surrogate marker of general health status, psychosocial variables play a very important role in sexual satisfaction in menopausal women and this may be valid also in individuals with obesity.
All this notwithstanding, the standard of practice to ensure sexual health in menopausal women with obesity is still under definition. Counseling should be a priority following several steps. First, uncovering the presence of sexual concerns. Then, helping to identify the etiology of possible FSD according to a bio-psycho-social approach. Next, promoting healthy behaviors, including self-care, eating well, exercising regularly, avoiding smoking, excessive alcohol and other risky substances, and paying attention to sleep quality and mental well-being. After that, managing obesity and its comorbidities, along with establishing appropriate multidimensional treatments for FSD diagnoses at menopause. Finally, aiming to achieve ‘femgevity’, a new term in line with the WHO definition of female ‘healthy aging’ as ‘the process of developing and maintaining functional ability that enables well-being in older age’ [Citation189,p.28]. Indeed, we believe that the amelioration of sexual well-being by reducing weight excess and associated health risks in midlife women is an essential component of healthy longevity and may contribute to gender equality and empowerment of the female population.
Potential conflict of interest
R. E. Nappi had past financial relationships (lecturer, member of advisory boards and/or consultant) with Boehringer Ingelheim, Ely Lilly, Endoceutics, Palatin Technologies, Pfizer Inc., Procter & Gamble Co., TEVA Women’s Health Inc. and Zambon SpA; and at present has ongoing relationships with Abbott, Astellas, Bayer HealthCare AG, Besins Helathcare, Exeltis, Fidia, Gedeon Richter, HRA Pharma, Merck & Co., Novo Nordisk, Organon & Co., Shionogi Limited, Theramex and Viatris. None of these are relevant to the present work. All the other authors have nothing to disclose.
Acknowledgements
The authors would like to thank Hellas Cena (Professor of Dietetics and Clinical Nutrition, University of Pavia, Italy) and Alberto Beretta (MD, PhD, president and chief scientific officer of SoLongevity, Milan, Italy) for a very inspiring discussion on the determinants of female healthy aging that gave origin to the new term ‘femgevity’. R. E. Nappi and L. Cucinella, as members of the European Campus of City-Universities (EC2U) Alliance, received funding at the University of Pavia, Italy, for the activities of Work Package 4 ‘Life-long wellbeing and healthy aging’.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Leeners B, Geary N, Tobler PN, et al. Ovarian hormones and obesity. Hum Reprod Update. 2017;23(3):300–321. doi: 10.1093/humupd/dmw045.
- WHO defining obesity and overweight. [cited 2023 Jul 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Dai H, Alsalhe TA, Chalghaf N, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the global burden of disease study. PLoS Med. 2020;17(7):e1003198. doi: 10.1371/journal.pmed.1003198.
- Ahmad NN, Butsch WS, Aidarous S. Clinical management of obesity in women: addressing a lifecycle of risk. Obstet Gynecol Clin North Am. 2016;43(2):201–230. doi: 10.1016/j.ogc.2016.01.007.
- Gill L, Mackey S. Obstetrician-gynecologists’ strategies for patient initiation and maintenance of antiobesity treatment with glucagon-like peptide-1 receptor agonists. J Womens Health (Larchmt). 2021;30(7):1016–1027. doi: 10.1089/jwh.2020.8683.
- Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–e2709. doi: 10.1210/clinem/dgaa285.
- Rowland DL, McNabney SM, Mann AR. Sexual function, obesity, and weight loss in men and women. Sex Med Rev. 2017;5(3):323–338. doi: 10.1016/j.sxmr.2017.03.006.
- Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring). 2015;23(12):2319–2320. doi: 10.1002/oby.21358.
- Jensen MD, Ryan DH, Apovian CM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25_suppl_2):S102–S138. [Database] doi: 10.1161/01.cir.0000437739.71477.ee.
- Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129(10):3978–3989. doi: 10.1172/JCI129186.
- Tsatsoulis A, Paschou SA. Metabolically healthy obesity: criteria, epidemiology, controversies, and consequences. Curr Obes Rep. 2020;9(2):109–120. doi: 10.1007/s13679-020-00375-0.
- Rey-López JP, de Rezende LF, Pastor-Valero M, et al. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15(10):781–790. doi: 10.1111/obr.12198.
- Tanriover C, Copur S, Gaipov A, et al. Metabolically healthy obesity: misleading phrase or healthy phenotype? Eur J Intern Med. 2023;111:5–20. doi: 10.1016/j.ejim.2023.02.025.
- Stefan N, Schulze MB. Metabolic health and cardiometabolic risk clusters: implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. 2023;11(6):426–440. doi: 10.1016/S2213-8587(23)00086-4.
- van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14(1):9. doi: 10.1186/1472-6823-14-9.
- Kabat GC, Wu WY, Bea JW, et al. Metabolic phenotypes of obesity: frequency, correlates and change over time in a cohort of postmenopausal women. Int J Obes (Lond). 2017;41(1):170–177. doi: 10.1038/ijo.2016.179.
- Opoku AA, Abushama M, Konje JC. Obesity and menopause. Best Pract Res Clin Obstet Gynaecol. 2023;88:102348. doi: 10.1016/j.bpobgyn.2023.102348.
- Gold EB, Crawford SL, Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women’s Health Across the Nation (SWAN). Menopause. 2017;24(1):9–26. doi: 10.1097/GME.0000000000000723.
- Nappi RE, Chedraui P, Lambrinoudaki I, et al. Menopause: a cardiometabolic transition. Lancet Diabetes Endocrinol. 2022;10(6):442–456. doi: 10.1016/S2213-8587(22)00076-6.
- Thomas HN, Thurston RC. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: a narrative review. Maturitas. 2016;87:49–60. doi: 10.1016/j.maturitas.2016.02.009.
- WHO defining sexual health. [cited 2023 Jul 29]. https://www.who.int/health-topics/sexual-health#tab=tab_1.
- Davis SR, Castelo-Branco C, Chedraui P, et al. Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–429. doi: 10.3109/13697137.2012.707385.
- Simon JA, Davis SR, Althof SE, et al. Sexual well-being after menopause: an international menopause society white paper. Climacteric. 2018;21(5):415–427. doi: 10.1080/13697137.2018.1482647.
- Pérez-López FR, Chedraui P, Gilbert JJ, et al. Cardiovascular risk in menopausal women and prevalent related co-morbid conditions: facing the post-Women’s Health Initiative era. Fertil Steril. 2009;92(4):1171–1186. doi: 10.1016/j.fertnstert.2009.06.032.
- Cipriani S, Simon JA. Sexual dysfunction as a harbinger of cardiovascular disease in postmenopausal women: how far are we? J Sex Med. 2022;19(9):1321–1332. doi: 10.1016/j.jsxm.2022.06.007.
- Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4(5):e124865. doi: 10.1172/jci.insight.124865.
- Greendale GA, Han W, Finkelstein JS, et al. Changes in regional fat distribution and anthropometric measures Across the menopause transition. J Clin Endocrinol Metab. 2021;106(9):2520–2534. doi: 10.1210/clinem/dgab389.
- Pan R, Chen Y. Fat biology and metabolic balance: on the significance of sex. Mol Cell Endocrinol. 2021;533:111336. doi: 10.1016/j.mce.2021.111336.
- Steiner BM, Berry DC. The regulation of adipose tissue health by estrogens. Front Endocrinol (Lausanne). 2022;13:889923. doi: 10.3389/fendo.2022.889923.
- Valencak TG, Osterrieder A, Schulz TJ. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017;12:806–813. doi: 10.1016/j.redox.2017.04.012.
- Lempesis IG, Hoebers N, Essers Y, et al. Distinct inflammatory signatures of upper and lower body adipose tissue and adipocytes in women with normal weight or obesity. Front Endocrinol (Lausanne). 2023;14:1205799. doi: 10.3389/fendo.2023.1205799.
- Stubbins RE, Najjar K, Holcomb VB, et al. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14(1):58–66. doi: 10.1111/j.1463-1326.2011.01488.x.
- Gibson CJ, Thurston RC, El Khoudary SR, et al. Body mass index following natural menopause and hysterectomy with and without bilateral oophorectomy. Int J Obes (Lond). 2013;37(6):809–813. doi: 10.1038/ijo.2012.164.
- Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause. Nat Rev Dis Primers. 2015;1(1):15004. doi: 10.1038/nrdp.2015.4.
- Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca(2+)/CREB pathway. Aging Cell. 2015;14(3):409–420. doi: 10.1111/acel.12331.
- De Jesus AN, Henry BA. The role of oestrogen in determining sexual dimorphism in energy balance. J Physiol. 2023;601(3):435–449. doi: 10.1113/JP279501.
- Rivera HM, Stincic TL. Estradiol and the control of feeding behavior. Steroids. 2018;133:44–52. doi: 10.1016/j.steroids.2017.11.011.
- Oh H, Wild RA, Manson JE, et al. Obesity, height, and serum androgen metabolism among postmenopausal women in the women’s health initiative observational study. Cancer Epidemiol Biomarkers Prev. 2021;30(11):2018–2029. doi: 10.1158/1055-9965.EPI-21-0604.
- Millán-de-Meer M, Luque-Ramírez M, Nattero-Chávez L, et al. PCOS during the menopausal transition and after menopause: a systematic review and meta-analysis. Hum Reprod Update. 2023;29(6):741–772. doi: 10.1093/humupd/dmad015.
- Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113(10):1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x.
- Fernandez CJ, Chacko EC, Pappachan JM. Male obesity-related secondary hypogonadism – pathophysiology, clinical implications and management. Eur Endocrinol. 2019;15(2):83–90. doi: 10.17925/EE.2019.15.2.83.
- Nappi RE, Cucinella L, Martella S, et al. Female sexual dysfunction (FSD): prevalence and impact on quality of life (QoL). Maturitas. 2016;94:87–91. doi: 10.1016/j.maturitas.2016.09.013.
- Nappi RE, Verde JB, Polatti F, et al. Self-reported sexual symptoms in women attending menopause clinics. Gynecol Obstet Invest. 2002;53(3):181–187. doi: 10.1159/000058371.
- Nappi RE, Albani F, Santamaria V, et al. Hormonal and psycho-relational aspects of sexual function during menopausal transition and at early menopause. Maturitas. 2010;67(1):78–83. doi: 10.1016/j.maturitas.2010.05.008.
- Nappi RE. New attitudes to sexuality in the menopause: clinical evaluation and diagnosis. Climacteric. 2007;10 Suppl 2:105–108. doi: 10.1080/13697130701599876.
- Cucinella L, Martini E, Tiranini L, et al. Menopause and female sexual dysfunctions. Minerva Obstet Gynecol. 2022;74(3):234–248. doi: 10.23736/S2724-606X.22.05001-1.
- Nappi RE, Cucinella L. Sexuality, pelvic floor/vaginal health and contraception at menopause. Best Pract Res Clin Obstet Gynaecol. 2022;81:85–97. doi: 10.1016/j.bpobgyn.2021.11.006.
- Barbagallo F, Cucinella L, Tiranini L, et al. Relationship between personality traits and sexual function in symptomatic postmenopausal women. Maturitas. 2022;166:50–57. doi: 10.1016/j.maturitas.2022.08.010.
- Thomas HN, Hess R, Thurston RC. Correlates of sexual activity and satisfaction in midlife and older women. Ann Fam Med. 2015;13(4):336–342. doi: 10.1370/afm.1820.
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208.
- Derogatis L, Clayton A, Lewis-D’Agostino D, et al. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5(2):357–364. doi: 10.1111/j.1743-6109.2007.00672.x.
- American Psychiatric Association. 2013). Diagnostic and statistical manual of mental disorders (5th ed.). doi: 10.1176/appi.books.9780890425596.
- McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement From the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13(2):135–143. doi: 10.1016/j.jsxm.2015.12.019.
- Parish SJ, Hahn SR, Goldstein SW, et al. The international society for the study of women’s sexual health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc. 2019;94(5):842–856. doi: 10.1016/j.mayocp.2019.01.009.
- Moon KH, Park SY, Kim YW. Obesity and erectile dysfunction: from bench to clinical implication. World J Mens Health. 2019;37(2):138–147. doi: 10.5534/wjmh.180026.
- Harlow SD, Karvonen-Gutierrez C, Elliott MR, et al. It is not just menopause: symptom clustering in the Study of Women’s Health Across the Nation. Womens Midlife Health. 2017;3(1):2. doi: 10.1186/s40695-017-0021-y.
- Shah MB. Obesity and sexuality in women. Obstet Gynecol Clin North Am. 2009;36(2):347–360, ix. ix. doi: 10.1016/j.ogc.2009.04.004.
- Salari N, Hasheminezhad R, Sedighi T, et al. The global prevalence of sexual dysfunction in obese and overweight women: a systematic review and meta-analysis. BMC Womens Health. 2023;23(1):375. doi: 10.1186/s12905-023-02544-4.
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970–978. doi: 10.1097/AOG.0b013e3181898cdb.
- Tserotas K, Blümel JE. Menopause research in Latin America. Climacteric. 2019;22(1):17–21. doi: 10.1080/13697137.2018.1540565.
- Silva GMDD, Lima SMRR, Reis BFD, et al. Evaluation of obesity influence in the sexual function of postmenopausal women: a cross-sectional study. Rev Bras Ginecol Obstet. 2019;41(11):660–667. doi: 10.1055/s-0039-1700795.
- Kirchengast S, Hartmann B, Gruber D, et al. Decreased sexual interest and its relationship to body build in postmenopausal women. Maturitas. 1996;23(1):63–71. doi: 10.1016/0378-5122(95)00954-x.
- Llaneza P, Iñarrea J, Gonzalez C, et al. Differences in health related quality of life in a sample of spanish menopausal women with and without obesity. Maturitas. 2007;58(4):387–394. doi: 10.1016/j.maturitas.2007.09.013.
- Tang R, Fan Y, Luo M, et al. General and central obesity are associated With increased severity of the VMS and sexual symptoms of menopause among Chinese women: a longitudinal study. Front Endocrinol (Lausanne). 2022;13:814872. doi: 10.3389/fendo.2022.814872.
- Esposito K, Ciotola M, Giugliano F, et al. Association of body weight with sexual function in women. Int J Impot Res. 2007;19(4):353–357. doi: 10.1038/sj.ijir.3901548.
- Faubion SS, Fairbanks F, Kuhle CL, et al. Association between body mass index and female sexual dysfunction: a cross-sectional study from the data registry on experiences of aging, menopause, and sexuality. J Sex Med. 2020;17(10):1971–1980. doi: 10.1016/j.jsxm.2020.07.004.
- Rodríguez A, Ezquerro S, Méndez-Giménez L, et al. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab. 2015;309(8):E691–714. doi: 10.1152/ajpendo.00297.2015.
- Thurston RC, Chang Y, Mancuso P, et al. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women’s Health Across the Nation. Fertil Steril. 2013;100(3):793–800. doi: 10.1016/j.fertnstert.2013.05.005.
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13.
- Maiorino MI, Bellastella G, Giugliano D, et al. From inflammation to sexual dysfunctions: a journey through diabetes, obesity, and metabolic syndrome. J Endocrinol Invest. 2018;41(11):1249–1258. doi: 10.1007/s40618-018-0872-6.
- Nappi R, Salonia A, Traish AM, et al. Clinical biologic pathophysiologies of women’s sexual dysfunction. J Sex Med. 2005;2(1):4–25. doi: 10.1111/j.1743-6109.2005.20102.x.
- Maseroli E, Fanni E, Cipriani S, et al. Cardiometabolic risk and female sexuality: focus on clitoral vascular resistance. J Sex Med. 2016;13(11):1651–1661. doi: 10.1016/j.jsxm.2016.09.009.
- Calmasini FB, Klee N, Webb RC, et al. Impact of immune system activation and vascular impairment on male and female sexual dysfunction. Sex Med Rev. 2019;7(4):604–613. doi: 10.1016/j.sxmr.2019.05.005.
- Tsatsanis C, Dermitzaki E, Avgoustinaki P, et al. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones (Athens). 2015;14(4):549–562. doi: 10.14310/horm.2002.1649.
- Schrauwen P, van Marken Lichtenbelt WD, Westerterp KR, et al. Effect of diet composition on leptin concentration in lean subjects. Metabolism. 1997;46(4):420–424. doi: 10.1016/s0026-0495(97)90059-7.
- Smith JT, Acohido BV, Clifton DK, et al. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18(4):298–303. doi: 10.1111/j.1365-2826.2006.01417.x.
- Childs GV, Odle AK, MacNicol MC, et al. The importance of leptin to reproduction. Endocrinology. 2021;162(2):bqaa204. doi: 10.1210/endocr/bqaa204.
- Mills EG, Yang L, Abbara A, et al. Current perspectives on kisspeptins role in behaviour. Front Endocrinol (Lausanne). 2022;13:928143. doi: 10.3389/fendo.2022.928143.
- Thurston L, Hunjan T, Ertl N, et al. Effects of kisspeptin administration in women with hypoactive sexual desire disorder: a randomized clinical trial. JAMA Netw Open. 2022;5(10):e2236131. doi: 10.1001/jamanetworkopen.2022.36131.
- Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17(1):47–66. doi: 10.1038/s41574-020-00431-8.
- Moccia P, Belda-Montesinos R, Monllor-Tormos A, et al. Body weight and fat mass across the menopausal transition: hormonal modulators. Gynecol Endocrinol. 2022;38(2):99–104. doi: 10.1080/09513590.2021.2004395.
- Davis SR, Robinson PJ, Moufarege A, et al. The contribution of SHBG to the variation in HOMA-IR is not dependent on endogenous oestrogen or androgen levels in postmenopausal women. Clin Endocrinol (Oxf). 2012;77(4):541–547. doi: 10.1111/j.1365-2265.2011.04301.x.
- Janssen I, Powell LH, Jasielec MS, et al. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity (Silver Spring). 2015;23(2):488–494. doi: 10.1002/oby.20974.
- Schmandt RE, Iglesias DA, Co NN, et al. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042.
- Cauley JA, Gutai JP, Kuller LH, et al. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129(6):1120–1131. doi: 10.1093/oxfordjournals.aje.a115234.
- Lindau ST, Dude A, Gavrilova N, et al. Prevalence and correlates of vaginal estrogenization in postmenopausal women in the United States. Menopause. 2017;24(5):536–545. doi: 10.1097/GME.0000000000000787.
- Goughnour SL, Thurston RC, Althouse AD, et al. Assessment of hot flushes and vaginal dryness among obese women undergoing bariatric surgery. Climacteric. 2016;19(1):71–76. doi: 10.3109/13697137.2015.1094782.
- Bates JN, Pastuszak AW, Khera M. Effect of body weight on sexual function in men and women. Curr Sex Health Rep. 2019;11(1):52–59. doi: 10.1007/s11930-019-00192-0.
- Esfahani SB, Pal S. Obesity, mental health, and sexual dysfunction: a critical review. Health Psychol Open. 2018;5(2):2055102918786867. doi: 10.1177/2055102918786867.
- de Wit L, Luppino F, van Straten A, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230–235. doi: 10.1016/j.psychres.2009.04.015.
- Maki PM, Kornstein SG, Joffe H, et al. Board of trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25(10):1069–1085. doi: 10.1097/GME.0000000000001174.
- Llaneza P, García-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120–130. doi: 10.1016/j.maturitas.2011.11.017.
- Jones GL, Sutton A. Quality of life in obese postmenopausal women. Menopause Int. 2008;14(1):26–32. doi: 10.1258/mi.2007.007034.
- Fooladi E, Bell RJ, Davis SR. Management strategies in SSRI-associated sexual dysfunction in women at midlife. Climacteric. 2012;15(4):306–316. doi: 10.3109/13697137.2012.658461.
- Gill H, Gill B, El-Halabi S, et al. Antidepressant medications and weight change: a narrative review. Obesity (Silver Spring). 2020;28(11):2064–2072. doi: 10.1002/oby.22969.
- Stanford FC, Cena H, Biino G, et al. The association between weight-promoting medication use and weight gain in postmenopausal women: findings from the Women’s Health Initiative. Menopause. 2020;27(10):1117–1125. doi: 10.1097/GME.0000000000001589.
- Jannini EA, Nappi RE. Couplepause: a new paradigm in treating sexual dysfunction during menopause and andropause. Sex Med Rev. 2018;6(3):384–395. doi: 10.1016/j.sxmr.2017.11.002.
- Mollaioli D, Ciocca G, Limoncin E, et al. Lifestyles and sexuality in men and women: the gender perspective in sexual medicine. Reprod Biol Endocrinol. 2020;18(1):10. doi: 10.1186/s12958-019-0557-9.
- Westbury S, Oyebode O, van Rens T, et al. Obesity stigma: causes, consequences, and potential solutions. Curr Obes Rep. 2023;12(1):10–23. doi: 10.1007/s13679-023-00495-3.
- Côté M, Bégin C. Review of the experience of weight-based stigmatization in romantic relationships. Curr Obes Rep. 2020;9(3):280–287. doi: 10.1007/s13679-020-00383-0.
- Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594.
- Miner M, Esposito K, Guay A, et al. Cardiometabolic risk and female sexual health: the Princeton III summary. J Sex Med. 2012;9(3):641–651; quiz 652. doi: 10.1111/j.1743-6109.2012.02649.x.
- Trompeter SE, Bettencourt R, Barrett-Connor E. Metabolic syndrome and sexual function in postmenopausal women. Am J Med. 2016;129(12):1270–1277.e1. doi: 10.1016/j.amjmed.2016.03.039.
- Dutra da Silva GM, Rolim Rosa Lima SM, Reis BF, et al. Prevalence of hypoactive sexual desire disorder among sexually active postmenopausal women with metabolic syndrome at a public hospital clinic in Brazil: a cross-sectional study. Sex Med. 2020;8(3):545–553. doi: 10.1016/j.esxm.2020.05.008.
- Martelli V, Valisella S, Moscatiello S, et al. Prevalence of sexual dysfunction among postmenopausal women with and without metabolic syndrome. J Sex Med. 2012;9(2):434–441. doi: 10.1111/j.1743-6109.2011.02517.x.
- Dombek K, Capistrano EJ, Costa AC, et al. Metabolic syndrome and sexual function in postmenopausal women. Arch Endocrinol Metab. 2016;60(6):545–553. doi: 10.1590/2359-3997000000194.
- Politano CA, Valadares AL, Pinto-Neto A, et al. The metabolic syndrome and sexual function in climacteric women: a cross-sectional study. J Sex Med. 2015;12(2):455–462. doi: 10.1111/jsm.12749.
- Yoldemir T, Garibova N, Atasayan K. The association between sexual dysfunction and metabolic syndrome among Turkish postmenopausal women. Climacteric. 2019;22(5):472–477. doi: 10.1080/13697137.2019.1580256.
- Maseroli E, Scavello I, Vignozzi L. Cardiometabolic risk and female sexuality-part I. Risk factors and potential pathophysiological underpinnings for female vasculogenic sexual dysfunction syndromes. Sex Med Rev. 2018;6(4):508–524. doi: 10.1016/j.sxmr.2018.02.009.
- Thong EP, Codner E, Laven JSE, et al. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020;8(2):134–149. doi: 10.1016/S2213-8587(19)30345-6.
- Maseroli E, Scavello I, Vignozzi L. Cardiometabolic risk and female sexuality-part II. Understanding (and overcoming) gender differences: the key role of an adequate methodological approach. Sex Med Rev. 2018;6(4):525–534. doi: 10.1016/j.sxmr.2018.03.004.
- Angulo J, Hannan JL. Cardiometabolic diseases and female sexual dysfunction: animal studies. J Sex Med. 2022;19(3):408–420. doi: 10.1016/j.jsxm.2021.12.009.
- Katainen RE, Engblom JR, Siirtola TJ, et al. Climacteric symptoms in middle-aged women with chronic somatic diseases. Maturitas. 2016;86:17–24. doi: 10.1016/j.maturitas.2016.01.005.
- Rahmanian E, Salari N, Mohammadi M, et al. Evaluation of sexual dysfunction and female sexual dysfunction indicators in women with type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11(1):73. doi: 10.1186/s13098-019-0469-z.
- Fanfulla F, Camera A, Fulgoni P, et al. Sexual dysfunction in obese women: does obstructive sleep apnea play a role? Sleep Med. 2013;14(3):252–256. doi: 10.1016/j.sleep.2012.11.023.
- Proserpio P, Marra S, Campana C, et al. Insomnia and menopause: a narrative review on mechanisms and treatments. Climacteric. 2020;23(6):539–549. doi: 10.1080/13697137.2020.1799973.
- Kling JM, Manson JE, Naughton MJ, et al. Association of sleep disturbance and sexual function in postmenopausal women. Menopause. 2017;24(6):604–612. doi: 10.1097/GME.0000000000000824.
- Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev. 2018;19(12):1735–1745. doi: 10.1111/obr.12756.
- Chilaka C, Toozs-Hobson P, Chilaka V. Pelvic floor dysfunction and obesity. Best Pract Res Clin Obstet Gynaecol. 2023;90:102389. doi: 10.1016/j.bpobgyn.2023.102389.
- Garg A, Ellis LB, Love RL, et al. Vaginal microbiome in obesity and its impact on reproduction. Best Pract Res Clin Obstet Gynaecol. 2023;90:102365. doi: 10.1016/j.bpobgyn.2023.102365.
- Pastore LM, Carter RA, Hulka BS, et al. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303. doi: 10.1016/j.maturitas.2004.06.019.
- Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762–774. doi: 10.1056/NEJMoa067423.
- Lindau ST, Gavrilova N. Sex, health, and years of sexually active life gained due to good health: evidence from two US population based cross sectional surveys of ageing. BMJ. 2010;340(mar09 2):c810–c810. doi: 10.1136/bmj.c810.
- Al-Azzawi F, Bitzer J, Brandenburg U, et al. Therapeutic options for postmenopausal female sexual dysfunction. Climacteric. 2010;13(2):103–120. doi: 10.3109/13697130903437615.
- Kingsberg SA, Adler B, Metropoulos J, et al. The yin and yang of GSM and low sexual desire. Climacteric. 2023;26(4):323–328. doi: 10.1080/13697137.2023.2194529.
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973.
- Valenzuela PL, Carrera-Bastos P, Castillo-García A, et al. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. 2023;20(7):475–494. doi: 10.1038/s41569-023-00847-5.
- Parish SJ, Simon JA, Davis SR, et al. International society for the study of women’s sexual health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18(5):849–867. doi: 10.1016/j.jsxm.2020.10.009.
- Balica AC, Cooper AM, McKevitt MK, et al. Dyspareunia related to GSM: association of total vaginal thickness via transabdominal ultrasound. J Sex Med. 2019;16(12):2038–2042. doi: 10.1016/j.jsxm.2019.08.019.
- Nappi RE, Ferdeghini F, Sampaolo P, et al. Clitoral circulation in postmenopausal women with sexual dysfunction: a pilot randomized study with hormone therapy. Maturitas. 2006;55(3):288–295. doi: 10.1016/j.maturitas.2006.04.014.
- Kemp JVA, Kumar V, Saleem A, et al. Examining associations between women’s mental health and obesity. Psychiatr Clin North Am. 2023;46(3):539–549. doi: 10.1016/j.psc.2023.04.009.
- Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79–132. doi: 10.1210/er.2017-00253.
- Kapoor E, Collazo-Clavell ML, Faubion SS. Weight gain in women at midlife: a concise review of the pathophysiology and strategies for management. Mayo Clin Proc. 2017;92(10):1552–1558. doi: 10.1016/j.mayocp.2017.08.004.
- Angelidi AM, Belanger MJ, Kokkinos A, et al. Novel noninvasive approaches to the treatment of obesity: from pharmacotherapy to gene therapy. Endocr Rev. 2022;43(3):507–557. doi: 10.1210/endrev/bnab034.
- Nappi RE, Tiranini L, Martini E, et al. Medical treatment of female sexual dysfunction. Urol Clin North Am. 2022;49(2):299–307. doi: 10.1016/j.ucl.2022.02.001.
- Nappi RE, Gardella B. What are the challenges in prescribing pharmacotherapy for female sexual dysfunctions? Expert Opin Pharmacother. 2019;20(7):777–779. doi: 10.1080/14656566.2019.1582644.
- Simon JA, Kingsberg SA, Goldstein I, et al. Weight loss in women taking flibanserin for Hypoactive Sexual Desire Disorder (HSDD): insights Into potential mechanisms. Sex Med Rev. 2019;7(4):575–586. doi: 10.1016/j.sxmr.2019.04.003.
- Kornstein SG, Simon JA, Apfel SC, et al. Effect of flibanserin treatment on body weight in premenopausal and postmenopausal women with hypoactive sexual desire disorder: a post hoc analysis. J Womens Health (Larchmt). 2017;26(11):1161–1168. doi: 10.1089/jwh.2016.6230.
- Simon JA, Kingsberg SA, Portman D, et al. Prespecified and integrated subgroup analyses from the RECONNECT phase 3 studies of bremelanotide. J Womens Health (Larchmt). 2022;31(3):391–400. doi: 10.1089/jwh.2021.0225.
- Spana C, Jordan R, Fischkoff S. Effect of bremelanotide on body weight of obese women: data from two phase 1 randomized controlled trials. Diabetes Obes Metab. 2022;24(6):1084–1093. doi: 10.1111/dom.14672.
- Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmacother. 2015;16(6):875–887. doi: 10.1517/14656566.2015.1020791.
- Nappi RE, Tiranini L, Cucinella L, et al. Pharmacotherapy for female sexual dysfunctions (FSDs): what is on the market and where is this field heading? Expert Opin Pharmacother. 2023;24(1):135–143. doi: 10.1080/14656566.2022.2066997.
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) – results from an international survey. Climacteric. 2012;15(1):36–44. doi: 10.3109/13697137.2011.647840.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with medical conditions. Best Pract Res Clin Endocrinol Metab. 2021;35(6):101578. doi: 10.1016/j.beem.2021.101578.
- Lobo RA, Gompel A. Management of menopause: a view towards prevention. Lancet Diabetes Endocrinol. 2022;10(6):457–470. doi: 10.1016/S2213-8587(21)00269-2.
- Davis SR, Baber RJ. Treating menopause – MHT and beyond. Nat Rev Endocrinol. 2022;18(8):490–502. doi: 10.1038/s41574-022-00685-4.
- Nappi RE, Tiranini L, Martini E, et al. Different local estrogen therapies for a tailored approach to GSM. Climacteric. 2023;26(4):361–366. doi: 10.1080/13697137.2023.2218998.
- Palacios S, Nappi RE, Cancelo MJ, et al. Expert opinion on the treatment of vulvovaginal atrophy with ospemifene based on new evidence. Climacteric. 2023;26(4):388–391. doi: 10.1080/13697137.2023.2190881.
- Islam RM, Bell RJ, Green S, et al. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019;7(10):754–766. doi: 10.1016/S2213-8587(19)30189-5.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. Climacteric. 2019;22(5):429–434. doi: 10.1080/13697137.2019.1637079.
- Nappi RE. Testosterone for women: green light for sex, amber light for health? Lancet Diabetes Endocrinol. 2019;7(10):738–739. doi: 10.1016/S2213-8587(19)30251-7.
- Jannini EA. SM = SM: the interface of systems medicine and sexual medicine for facing non-communicable diseases in a gender-dependent manner. Sex Med Rev. 2017;5(3):349–364. doi: 10.1016/j.sxmr.2017.04.002.
- Towe M, La J, El-Khatib F, et al. Diet and female sexual health. Sex Med Rev. 2020;8(2):256–264. doi: 10.1016/j.sxmr.2019.08.004.
- Allen MS, Walter EE. Health-related lifestyle factors and sexual dysfunction: a meta-analysis of population-based research. J Sex Med. 2018;15(4):458–475. doi: 10.1016/j.jsxm.2018.02.008.
- Kudesia R, Alexander M, Gulati M, et al. Dietary approaches to women’s sexual and reproductive health. Am J Lifestyle Med. 2021;15(4):414–424. doi: 10.1177/15598276211007113.
- Longo M, Scappaticcio L, Caputo M, et al. Mediterranean diet in type 2 diabetes: an updated overview of pharmacological activities of cardiometabolic and reproductive outcomes. Curr Opin Pharmacol. 2021;60:27–33. doi: 10.1016/j.coph.2021.06.005.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440.
- Esposito K, Ciotola M, Giugliano F, et al. Mediterranean diet improves sexual function in women with the metabolic syndrome. Int J Impot Res. 2007;19(5):486–491. doi: 10.1038/sj.ijir.3901555.
- Cano A, Marshall S, Zolfaroli I, et al. The Mediterranean diet and menopausal health: an EMAS position statement. Maturitas. 2020;139:90–97. doi: 10.1016/j.maturitas.2020.07.001.
- Sánchez-Sánchez ML, García-Vigara A, Hidalgo-Mora JJ, et al. Mediterranean diet and health: a systematic review of epidemiological studies and intervention trials. Maturitas. 2020;136:25–37. doi: 10.1016/j.maturitas.2020.03.008.
- Camajani E, Feraco A, Verde L, et al. Ketogenic diet as a possible non-pharmacological therapy in main endocrine diseases of the female reproductive system: a practical guide for nutritionists. Curr Obes Rep. 2023;12(3):231–249. doi: 10.1007/s13679-023-00516-1.
- Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18(3):193–216.
- Caprio M, Infante M, Moriconi E, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Invest. 2019;42(11):1365–1386. doi: 10.1007/s40618-019-01061-2.
- Castro AI, Gomez-Arbelaez D, Crujeiras AB, et al. Effect of a very low-calorie ketogenic diet on food and alcohol cravings, physical and sexual activity, sleep disturbances, and quality of life in obese patients. Nutrients. 2018;10(10):1348. doi: 10.3390/nu10101348.
- Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, et al. Exercise and the stress system. Hormones (Athens). 2005;4(2):73–89.
- Lorenz TA, Meston CM. Acute exercise improves physical sexual arousal in women taking antidepressants. Ann Behav Med. 2012;43(3):352–361. doi: 10.1007/s12160-011-9338-1.
- Helmrich SP, Ragland DR, Leung RW, et al. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325(3):147–152. doi: 10.1056/NEJM199107183250302.
- Elavsky S. Longitudinal examination of the exercise and self-esteem model in Middle-aged women. J Sport Exerc Psychol. 2010;32(6):862–880. doi: 10.1123/jsep.32.6.862.
- Maseroli E, Rastrelli G, Di Stasi V, et al. Physical activity and female sexual dysfunction: a lot helps, but not too much. J Sex Med. 2021;18(7):1217–1229. doi: 10.1016/j.jsxm.2021.04.004.
- Liu T, Chen S, Mielke GI, et al. Effects of exercise on vasomotor symptoms in menopausal women: a systematic review and meta-analysis. Climacteric. 2022;25(6):552–561. doi: 10.1080/13697137.2022.2097865.
- Fausto DY, Leitão AE, Silveira J, et al. An umbrella systematic review of the effect of physical exercise on mental health of women in menopause. Menopause. 2023;30(2):225–234. doi: 10.1097/GME.0000000000002105.
- Carcelén-Fraile MDC, Aibar-Almazán A, Martínez-Amat A, et al. Effects of physical exercise on sexual function and quality of sexual life related to menopausal symptoms in peri- and postmenopausal women: a systematic review. Int J Environ Res Public Health. 2020;17(8):2680. doi: 10.3390/ijerph17082680.
- Gerber JR, Johnson JV, Bunn JY, et al. A longitudinal study of the effects of free testosterone and other psychosocial variables on sexual function during the natural traverse of menopause. Fertil Steril. 2005;83(3):643–648. doi: 10.1016/j.fertnstert.2004.08.028.
- Hess R, Conroy MB, Ness R, et al. Association of lifestyle and relationship factors with sexual functioning of women during midlife. J Sex Med. 2009;6(5):1358–1368. doi: 10.1111/j.1743-6109.2009.01225.x.
- Yim G, Ahn Y, Chang Y, et al. Prevalence and severity of menopause symptoms and associated factors across menopause status in Korean women. Menopause. 2015;22(10):1108–1116. doi: 10.1097/GME.0000000000000438.
- Castillo-Hernández IM, Ward-Ritacco CL, Evans EM. Depressive symptoms, but not moderate-to-vigorous physical activity, impacts sexual well-being and menopause-specific quality of life in Middle-aged women. Women Health. 2023;63(5):346–358. doi: 10.1080/03630242.2023.2195959.
- Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10(4):1024–1033. doi: 10.1111/jsm.12069.
- Chakhtoura M, Haber R, Ghezzawi M, et al. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine. 2023;58:101882. doi: 10.1016/j.eclinm.2023.101882.
- Jensterle M, Podbregar A, Goricar K, et al. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr Connect. 2019;8(3):195–202. doi: 10.1530/EC-18-0514.
- La Vignera S, Condorelli RA, Calogero AE, et al. Sexual and reproductive outcomes in obese fertile men with functional hypogonadism after treatment with liraglutide: preliminary results. J Clin Med. 2023;12(2):672. doi: 10.3390/jcm12020672.
- Saad B. A review of the anti-obesity effects of wild edible plants in the mediterranean diet and their active compounds: from traditional uses to action mechanisms and therapeutic targets. Int J Mol Sci. 2023;24(16):12641. doi: 10.3390/ijms241612641.
- Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8(2):156–164. doi: 10.1007/s13679-019-00335-3.
- Imprialos KP, Stavropoulos K, Doumas M, et al. Sexual dysfunction, cardiovascular risk and effects of pharmacotherapy. Curr Vasc Pharmacol. 2018;16(2):130–142. doi: 10.2174/1570161115666170609101502.
- Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, et al. Sexual functioning and depressive symptoms in women with diabetes and prediabetes receiving metformin therapy: a pilot study. Exp Clin Endocrinol Diabetes. 2017;125(1):42–48. doi: 10.1055/s-0042-116594.
- Liao J, Yin Y, Zhong J, et al. Bariatric surgery and health outcomes: an umbrella analysis. Front Endocrinol (Lausanne). 2022;13:1016613. doi: 10.3389/fendo.2022.1016613.
- Oliveira CFA, Dos Santos PO, de Oliveira RA, et al. Changes in sexual function and positions in women with severe obesity after bariatric surgery. Sex Med. 2019;7(1):80–85. doi: 10.1016/j.esxm.2018.10.001.
- Olivera CK, Herron DM, Kini SU, et al. Long-term quality of life and pelvic floor dysfunction after bariatric surgery. Am J Obstet Gynecol. 2012;207(5):431.e1-4–431.e4. doi: 10.1016/j.ajog.2012.06.041.
- Loh HH, Shahar MA, Loh HS, et al. Female sexual dysfunction after bariatric surgery in women with obesity: a systematic review and meta-analysis. Scand J Surg. 2022;111(1):14574969211072395. doi: 10.1177/14574969211072395.
- World Health Organization. 1st world report on ageing and health. Geneva: WHO; 2015.

