Abstract
Objective
This study aimed to determine whether concentrations of testosterone and its main precursor after menopause, dehydroepiandrosterone (DHEA), are associated with lipoproteins and other lipids in community-dwelling older women.
Methods
The Sex Hormones in Older Women (SHOW) study was an observational study of 6358 Australian women, aged at least 70 years, with no prior major adverse cardiovascular event who had sex hormones measured by liquid chromatography–tandem mass spectrometry. Associations between hormones and lipids were examined using multilinear regression adjusted for potential confounders.
Results
The cross-sectional analyses included 3231 participants, median age 74.0 (interquartile range 71.7–77.9) years. Compared with concentrations in the lowest quartile (Q1), testosterone concentrations in the highest quartiles (Q3 and Q4) were positively associated with high-density lipoprotein cholesterol (HDL-C) (p = 0.002 and p < 0.001, respectively) while Q4 testosterone concentrations were positively associated with total cholesterol (p = 0.038). Q2, Q3 and Q4 testosterone concentrations were significantly inversely associated with triglycerides (TG) (p = 0.024, p = 0.003 and p < 0.001, respectively). For DHEA, Q4 concentrations was positively associated with non-HDL-C (p = 0.024).
Conclusions
In older women, higher endogenous testosterone concentrations are significantly associated with higher HDL-C and lower TG, indicating a less atherogenic profile. These findings suggest a neutral, or potentially protective, cardiovascular disease effect of testosterone in older women.
摘要
目的:本研究旨在确定社区老年妇女绝经后睾酮及其主要前体脱氢表雄酮(DHEA)的浓度是否与脂蛋白和其他脂质有关。
方法:老年女性性激素(SHOW)研究是一项观察性研究, 研究对象为6358名澳大利亚女性, 年龄至少70岁, 既往无重大心血管不良事件, 使用液相色谱-串联质谱法测量性激素。激素和脂质之间的关系采用校正潜在混杂因素的多元线性回归进行检验。
结果:横断面分析纳入3231名参与者, 中位年龄74.0岁(四分位数间距71.7-77.9)。与最低四分位数(Q1)的浓度相比, 最高四分位数(Q3和Q4)的睾酮浓度与高密度脂蛋白胆固醇(HDL-C)呈正相关(p = 0.002和p < 0.001), 而Q4的睾酮浓度与总胆固醇呈正相关(p = 0.038)。Q2、Q3和Q4睾酮浓度与甘油三酯(TG)呈显著负相关(p = 0.024、p = 0.003和p < 0.001)。对于脱氢表雄酮, Q4浓度与非HDL-C呈正相关(p = 0.024)。
结论:在老年妇女中, 较高的内源性睾酮浓度与较高的HDL-C和较低的TG显著相关, 表明动脉粥样硬化程度较低。这些发现表明, 睾酮对老年妇女心血管疾病的影响是中性的, 或可能具有保护作用。
Introduction
Atherosclerotic cardiovascular disease (CVD) is largely preventable. Among known risk factors, the concentrations of atherogenic, and possibly protective, lipid fractions have the largest population attributable risk for myocardial infarction in both women and men, although the associations may be slightly lower in older than younger individuals [Citation1]. Of the commonly assessed lipid parameters, high triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C) are important CVD risk factors in older women [Citation2–4], and may confer greater risk than elevated total cholesterol and low-density lipoprotein cholesterol (LDL-C) [Citation5].
Our study of community-dwelling women aged 70 years and older revealed that women with a blood testosterone concentration in the lowest quartile had a two-fold greater likelihood of experiencing a first ever ischemic major adverse cardiovascular event (MACE) [Citation6]. This is consistent with other studies that have reported inverse associations between blood testosterone and ischemic cardiac events [Citation7–9] and ischemic stroke in postmenopausal women [Citation10–12]. Further to this, low testosterone in women with heart failure and reduced ejection fraction has been associated with a 10-fold greater likelihood of a combined endpoint of all-cause mortality and cardiovascular hospitalization in women [Citation13].
A protective effect of testosterone against MACE and heart failure in postmenopausal women is biologically plausible. In postmenopausal women, exogenous testosterone exerts acute vasodilatory effects [Citation14] and at physiological concentrations enhances endothelial-dependent and independent vasodilation [Citation15, Citation16]. TG, along with non-HDL-C, contribute to the pathogenesis of atherosclerotic CVD [Citation17] and increase following natural menopause [Citation18, Citation19]. However, in a prospective study of postmenopausal women, with a mean age of 54 years, blood testosterone concentrations were not associated with lipid concentrations [Citation20].
Whether the lack of association between testosterone and lipid levels can be generalized to older postmenopausal women is questionable. This is because testosterone blood concentrations decline by approximately 25% across the reproductive years [Citation21], do not acutely change at menopause but do slowly decline to reach a nadir in women in the seventh decade of life [Citation22]. Subsequently, blood testosterone concentrations increase with age such that women in the eighth decade of life have similar levels to premenopausal women [Citation23]. Hence, the physiological role of testosterone in older postmenopausal women may differ from that in younger women, and the associations between testosterone and lipid variables may change with age.
We have measured testosterone and its primary adrenal precursor dehydroepiandrosterone (DHEA) by the gold standard liquid chromatography–tandem mass spectrometry (LCMS) in a large sample of women aged 70 years and older with no prior MACE [Citation23]. LCMS ensures precise measurement of testosterone at the low concentrations seen in women compared with men [Citation24]. Our hypothesis was that relative testosterone sufficiency is associated with a more favorable lipid profile compared with low testosterone concentrations (relative insufficiency) in older women.
Methods
Study design and population
The Sex Hormones in Older Women (SHOW) study comprised 6392 of the 9180 Australian women participating in the ASPirin in Reducing Events in the Elderly (ASPREE) clinical trial who provided biobank samples at recruitment [Citation25, Citation26]. The participants, recruited between March 2010 and December 2014, were aged 70 years and older and lived in the southern Australian states of Victoria, South Australia, New South Wales, Tasmania and the Australian Capital Territory. Women were excluded from the ASPREE trial if they had a prior MACE or life expectancy of less than 5 years, they were at risk of major bleeding, they had anemia, a contraindication to aspirin, uncontrolled hypertension, impaired cognition [Citation27] or severe difficulty in performing any of six basic activities of daily living [Citation28], or they were taking aspirin, antiplatelet or anticoagulant therapy.
Ethical approval for the SHOW study was obtained from the Monash Human Research Ethics Committee (CF16/10-2016000001) and the Alfred Hospital Human Research Ethics Committee (616/15). The ASPREE trial was also approved by the each of the ethics committees of the participating centers. Written informed consent for the ASPREE trial and the ASPREE Biobank was given by all participants.
Covariates
Demographic data were recorded at study entry. Clinical measurements included blood pressure, waist circumference, weight and height. Diabetes was defined as a fasting plasma glucose concentration of at least 126 mg/dl (≥7 mmol/l) or treatment for diabetes [Citation29]. Impaired renal function was defined as estimated glomerular filtration rate < 60 ml/min per 1.73 m2. Hypertension was defined as blood pressure > 140/90 mmHg or the use of anti-hypertensive agents at study entry.
Biochemical measurements
Baseline bloods were collected from ASPREE participants within the first year after study enrollment (the majority were within a month of enrollment) and plasma aliquots were kept under nitrogen vapor until hormone assays were performed. Testosterone and DHEA were measured in a single plasma sample, without derivatization, by LCMS (ANZAC Research Institute, University of Sydney, NSW, Australia) [Citation30, Citation31]. The assay limits of detection, limits of quantification, and within-run and between-run coefficients of variation for testosterone are 35 pmol/l (1.01 ng/dl), 0.09 nmol/l (2.59 ng/dl), 2.0% and 3.9–6.5%, respectively, and for DHEA are 0.07 nmol/l (2.02 ng/dl), 0.17 nmol/l (4.90 ng/dl), <10% and <10%, respectively [Citation32].
Outcomes
Fasting bloods were collected at ASPREE study enrollment for measurement of total cholesterol, HDL-C and TG, which was undertaken by National Association of Testing Authorities Australia (NATA)-approved laboratories convenient to the participants. Non-HDL-C was calculated as total cholesterol minus HDL-C.
Statistical analyses
Participants were excluded from the analysis if they were using any of the following at the time of recruitment: systemic or topical sex steroid therapy, tamoxifen or other selective estrogen receptor modulator, aromatase inhibitors, anti-androgen therapy, glucocorticosteroid therapy or lipid-lowering medications.
Descriptive statistics including the median (interquartile range), frequencies and percentages were used to describe the study population. The normality of the outcome data was checked using a histogram, and TG values were log-transformed as they were not normally distributed. For testosterone and DHEA, medians and inter-decile ranges are reported as descriptive statistics. Quartiles, with quartile 1 (Q1) as the reference, were used to investigate the associations, and reported as the β-coefficient with 95% confidence interval (CI). Simple linear regression was employed to determine the unadjusted associations between testosterone, DHEA and each of the lipid variables. Multiple linear regression was performed to adjust for factors potentially influencing lipid levels including age, body mass index, smoking (current, former, never), alcohol consumption (current, former, never), impaired renal function and diabetes. Multi-collinearity was assessed between independent variables by both the correlation coefficient and the variance inflation factor before entering into the final models. All statistical tests were two-sided, and a p < 0.05 was considered statistically significant. The analysis was performed using Stata Version 17.0 (Stata Corporation, College Station, TX, USA). This manuscript was written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies (STROBE) [Citation33].
Results
Characteristics of the study participants
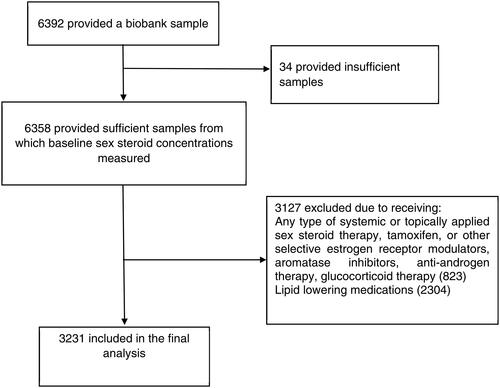
Sufficient biobank samples for measurement of sex steroids were available for 6358 study participants. After excluding 823 women who were taking sex hormones, anti-estrogens, anti-androgens or systemic glucocorticoids, and 2304 women who were on lipid-lowering medications, 3231 participants with a median age of 74.0 (interquartile range 71.7–77.9) years were included for the cross-sectional analysis (). Half (50.2%) of the participants were aged 70–74 years, and most (n = 3207; 99.3%) were of white/European ancestry. The majority (n = 2168; 67.3%) were overweight or obese, and 144 (4.5%) and 533 (17%) women had diabetes and impaired renal function, respectively ().
Table 1. Baseline characteristics of the study participants.
Associations between testosterone, DHEA and lipids
Multilinear regression showed that higher testosterone concentrations were significantly positively associated with total cholesterol and HDL-C (). For total cholesterol Q4 versus Q1, β = 0.09 (95% CI 0.01 to 0.17; p = 0.038) means that, on average, women with testosterone levels in Q4 had total cholesterol concentrations 0.09 nmol/l greater than women with testosterone concentrations in Q1. Testosterone concentrations in both Q3 and Q4 were associated with higher HDL-C compared with Q1 (Q3 vs. Q1: β = 0.07, 95% CI 0.03 to 0.11, p = 0.002; and Q4 vs. Q1: β = 0.10, 95% CI 0.06 to 0.14, p < 0.001).
Table 2. Association between testosterone, dehydroepiandrosterone and blood lipid concentrations.
Conversely, statistically significant inverse associations were seen for testosterone and TG (Q2 vs. Q1: β = −0.04, −0.08 to −0.01; p = 0.024; Q3 vs. Q1: β = −0.06, −0.10 to −0.02; p = 0.003; and Q4 vs. Q1: β = −0.09, −0.12 to −0.05; p < 0.001). There was no association between testosterone concentrations and non-HDL-C. Only 1% of the variation in any lipid measured was explained by testosterone.
In the multilinear regression analysis, a statistically significant positive association was seen for DHEA and non-HDL-C (Q4 vs. Q1: β = 0.10, 0.01–0.18; p = 0.024). DHEA alone accounted for no more than 1% of the variation in any of the measured lipids.
Discussion
In this prospective analysis of androgens and lipid profiles in older postmenopausal women, higher concentrations of testosterone were associated with a less atherogenic lipid profile, specifically higher HDL-C and lower TG. While earlier studies have demonstrated favorable vascular effects of testosterone after menopause, to our knowledge this study is the first to document the associations between testosterone and lipids in women aged 70 years and older.
Having documented that, after a postmenopausal nadir, testosterone blood concentrations in women aged 70 years and older do not differ from premenopausal counterparts [Citation23], we sought to understand the clinical significance of this observation. Our initial finding was that women in the SHOW study with testosterone concentrations in the lowest quartile had a two-fold greater risk of a first ever MACE [Citation6]. The highly statistically significant, positive association between testosterone and the two highest quartiles of HDL-C and the negative association with the two highest quartiles of TG in the present study further support the likelihood that testosterone may be cardioprotective in older women [Citation6, Citation34]. This should not be interpreted as a potential role for testosterone therapy to improve lipid profiles as blood testosterone concentrations explained no more than 1% of the variation in these lipid fractions.
While the contribution of testosterone to the variation in lipids appears small, it needs to be considered in the context of testosterone physiology in postmenopausal women. Following menopause, testosterone is made from adrenal pre-androgens in peripheral tissues where it acts, and blood levels primarily reflect spillover of testosterone that has escaped metabolism in the cells in which it has been made, notably fat tissue [Citation35]. Consequently, serum testosterone concentrations only provide an estimate of tissue testosterone exposure or action [Citation36]. Thus, the true contribution of testosterone to the variation in lipids may be greater than observed. In addition, the relatively narrow range of serum testosterone concentrations, as opposed to the much wider ranges of the other measured sex steroids, limits the likelihood of finding significant associations between testosterone and clinical outcomes. Therefore, our main finding is of a highly significant signal that testosterone is positively associated with a more favorable lipid profile.
Whether the associations between testosterone and each of HDL-C and TG are due to direct androgenic effects or mediated via obligatory metabolism of testosterone to estradiol, or whether testosterone is a biomarker but not causative of a more favorable lipid profile, is uncertain. Although the majority of older postmenopausal women have serum estradiol concentrations below the limit of detection [Citation23], serum estrone – the main postmenopausal estrogen – provides a proxy for estradiol in this context [Citation37]. We recently found blood estrone concentrations to be positively associated with HDL-C and inversely with TG in female SHOW study participants [Citation38], but the statistical strengths of these associations were less than for those of testosterone. An analysis adjusting for estrone would not be helpful as in postmenopausal women testosterone is a critical precursor for estrogen biosynthesis [Citation39]. Consequently, we can only conclude that testosterone and estrogens in concert are associated with the favorable lipid effects seen in this study.
The observed associations between DHEA and lipids were less robust than for testosterone, and conflict with previous studies reporting inverse associations between DHEA, and its sulfate (DHEAS), and coronary heart disease and ischemic stroke in postmenopausal women [Citation40–43]. Thus, the weak association between DHEA and non-HDL-C should be interpreted with caution.
Elevated TG is an independent risk factor for coronary heart disease in women aged 65 years and older [Citation5]. A causative role of high TG in atherogenesis and MACE is supported by Mendelian randomization studies [Citation4, Citation17]. There has been substantial debate as to the contribution of HDL-C to CVD risk and the potential benefit of increasing HDL-C [Citation17, Citation44]. However, hypertriglyceridemia and low serum HDL-C are closely related disorders of lipid metabolism and their concurrence is associated with increased CVD risk in women [Citation45, Citation46]. Indeed, an elevated TG/HDL-C ratio confers a significantly greater risk of coronary heart disease in women of all ages [Citation5]. In women suspected of having myocardial ischemia, the ratio of TG to HDL-C has been identified as a strong, independent predictor of all-cause mortality and cardiovascular death [Citation45]. A high TG to HDL-C ratio is also indicative of a greater proportion of small, dense pro-atherogenic LDL particles, even in healthy people without hyperlipidemia [Citation2,Citation47–49].
An association between the highest quartile of testosterone and total cholesterol was seen, with no association between testosterone and non-HDL-C. Thus, the association between high testosterone and total cholesterol could be due to the HDL-C fraction as high testosterone was associated with higher HDL-C, or this could be an isolated chance finding.
Study strengths include the large sample size, measurement precision of testosterone and DHEA with LCMS, and exclusion of women taking lipid-lowering therapy and medications that might influence their sex hormone concentrations. Exclusion of women taking lipid-lowering therapy, although a strength, also limited the inclusion of women with more severe dyslipidemia. The design of the ASPREE trial was such that study participants had their routine biochemistry, including lipid profiles, measured at a NATA-accredited laboratory convenient to them, as opposed to a single laboratory. The measurement of total cholesterol, HDL-C and TG has been internationally standardized [Citation50], and NATA accreditation ensures laboratories use assays traceable to internationally recognized standard reference materials. Therefore, we do not consider this a study limitation. While sex hormones do not change, there may be slight variations in lipids over time but with the large sample size this should not meaningfully influence our findings. Physical activity may also impact lipid parameters, but was not included as a covariate in analyses. Women with prior atherothrombotic cardiovascular events were excluded from the ASPREE trial and therefore also from this study, and study participants were predominantly of European ancestry, consistent with the composition of the Australian population of this age. Therefore, our findings cannot be generalized to women with prior MACE or to women of other ethnicities. The cross-sectional design of our study allows us to report associations, but not causation.
Conclusion
In older women, higher endogenous testosterone concentrations are significantly associated with a potentially less atherogenic lipid profile. This provides further support for a possible protective effect of testosterone against CVD in older women.
Potential conflict of interest
S.R.D. reports honoraria from Besins Healthcare, Abbott, Mayne Pharma, Health Ed, BioSyent, Lawley Pharmaceuticals and Que Oncology; has served on Advisory Boards for Mayne Pharma, Astellas Pharmaceuticals, Theramex and Gedeon Richter; and has been an institutional investigator for Que Oncology and Ovoca Bio.
A.M.T. reports serving on Advisory Boards or receiving honoraria for lectures from Amgen, Bayer, Boehringer-Ingelheim, Merck, Novartis and Pfizer; and serving as data safety monitoring Board Member for Medicines Group and Novartis.
Data availability statement
The ASPREE study protocol is available online (www.aspree.org). De-identified participant data for this analysis will be made available on application. Request for data access is via the ASPREE Principal Investigators with details for applications provided online (https://aspree.org/aus/for-researchers/ or https://aspree.org/usa/for-researchers/), and SHOW sub-study data on sex hormones can be requested through this system with approval by the sub-study Chief Investigator.
Data will be made available to investigators whose proposed use of the data, registered as a project through the ASPREE Access Management Site (https://ams.aspree.org/public/), has been approved by a review committee. Access will be through a secure web-based data portal (the ASPREE Safe Haven system), based at Monash University, Australia.
Additional information
Funding
References
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): a case controlled study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9.
- Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438–447. doi: 10.1056/NEJMoa1400283.
- LaRosa JC. Triglycerides and coronary risk in women and the elderly. Arch Intern Med. 1997;157(9):961–968. doi: 10.1001/archinte.1997.00440300051004.
- Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, et al. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027.
- Dugani SB, Moorthy MV, Li C, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 2021;6(4):437–447. doi: 10.1001/jamacardio.2020.7073.
- Islam RM, Bell RJ, Handelsman DJ, et al. Associations between blood sex steroid concentrations and risk of major adverse cardiovascular events in healthy older women in Australia: a prospective cohort substudy of the ASPREE trial. Lancet Healthy Longev. 2022;3(2):e109–e118. doi: 10.1016/S2666-7568(22)00001-0.
- Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95(2):740–747. doi: 10.1210/jc.2009-1693.
- Sievers C, Klotsche J, Pieper L, et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur J Endocrinol. 2010;163(4):699–708. doi: 10.1530/EJE-10-0307.
- Naessen T, Sjogren U, Bergquist J, et al. Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab. 2010;95(4):1889–1897. doi: 10.1210/jc.2009-1722.
- Debing E, Peeters E, Duquet W, et al. Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy. Eur J Endocrinol. 2007;156(6):687–693. doi: 10.1530/EJE-06-0702.
- Bernini GP, Sgro’ M, Moretti A, et al. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab. 1999;84(6):2008–2012. doi: 10.1210/jcem.84.6.5824.
- Golden SH, Maguire A, Ding J, et al. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155(5):437–445. doi: 10.1093/aje/155.5.437.
- Marra AM, D’Assante R, Salzano A, et al. Testosterone deficiency independently predicts mortality in women with HFrEF: insights from the T.O.S.CA. registry. ESC Heart Fail. 2022;10(1):159–166.
- Davison S, Thipphawong J, Blanchard J, et al. Pharmacokinetics and acute safety of inhaled testosterone in postmenopausal women. J Clin Pharmacol. 2005;45(2):177–184. doi: 10.1177/0091270004269840.
- Worboys S, Kotsopoulos D, Teede H, et al. Evidence that parenteral testosterone therapy may improve endothelium-dependent and-independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab. 2001;86(1):158–161. doi: 10.1210/jcem.86.1.7103.
- Montalcini T, Gorgone G, Gazzaruso C, et al. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis. 2007;18(1):9–13. doi: 10.1097/01.mca.0000236290.79306.d1.
- Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118(1):138–145. doi: 10.1016/j.amjcard.2016.04.004.
- Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009.
- Derby CA, Crawford SL, Pasternak RC, et al. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health across the Nation. Am J Epidemiol. 2009;169(11):1352–1361. doi: 10.1093/aje/kwp043.
- Worsley R, Robinson PJ, Bell RJ, et al. Endogenous estrogen and androgen levels are not independent predictors of lipid levels in postmenopausal women. Menopause. 2013;20(6):640–645. doi: 10.1097/GME.0b013e318279bd4a.
- Skiba MA, Bell RJ, Islam RM, et al. Androgens during the reproductive years, what’s normal for women? J Clin Endocrinol Metab. 2019;104(11):5382–5392. doi: 10.1210/jc.2019-01357.
- Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847–3853. doi: 10.1210/jc.2005-0212.
- Davis SR, Bell RJ, Robinson PJ, et al. Testosterone and estrone increase from the age of 70 years: findings from the sex hormones in older women study. J Clin Endocrinol Metab. 2019;104(12):6291–6300. doi: 10.1210/jc.2019-00743.
- Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95(10):4542–4548. doi: 10.1210/jc.2010-1314.
- Robman LD, Guymer RH, Wolfe R, et al. Baseline characteristics and age-related macular degeneration in participants of the “ASPirin in Reducing Events in the Elderly”(ASPREE)-AMD trial. Contemp Clin Trials Commun. 2020;20:100667. doi: 10.1016/j.conctc.2020.100667.
- A.I. Group. Study design of ASPirin in reducing events in the elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36(2):555–564.
- Teng E, Chui H. The modified mini-mental state examination (3MS). Can J Psychiatry. 1987;41(2):114–121.
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K.
- Wolfe R, Murray AM, Woods RL, et al. The aspirin in reducing events in the elderly trial: statistical analysis plan. London (UK): SAGE Publications Sage UK; 2018.
- Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography–tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1-2):78–84. doi: 10.1016/j.cca.2009.09.003.
- Keski-Rahkonen P, Desai R, Jimenez M, et al. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem. 2015;87(14):7180–7186. doi: 10.1021/acs.analchem.5b01042.
- Hsu B, Cumming RG, Hirani V, et al. Temporal trend in androgen status and androgen-sensitive outcomes in older men. J Clin Endocrinol Metab. 2016;101(4):1836–1846. doi: 10.1210/jc.2015-3810.
- Vandenbroucke JP, Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010.
- Spoletini I, Vitale C, Pelliccia F, et al. Androgens and cardiovascular disease in postmenopausal women: a systematic review. Climacteric. 2014;17(6):625–634. doi: 10.3109/13697137.2014.887669.
- Rosano GM. Androgens and coronary artery disease. A sex-specific effect of sex hormones? Eur Heart J. 2000;21(11):868–871. doi: 10.1053/euhj.1999.2050.
- Labrie F, Bélanger A, Pelletier G, et al. Science of intracrinology in postmenopausal women. Menopause. 2017;24(6):702–712. doi: 10.1097/GME.0000000000000808.
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78(3):C113–C118. doi: 10.1016/0303-7207(91)90116-a.
- Davis SR, Martinez-Garcia A, Robinson PJ, et al. Estrone is a strong predictor of circulating estradiol in women age 70 years and older. J Clin Endocrinol Metab. 2020;105(9):e3348–e3354.
- Azene Z, Davis SR, McNeil JJ, et al. Estrone, sex hormone binding globulin, and lipid profiles in older women: an observational study. Climacteric. 2023;26(2):114–120. doi: 10.1080/13697137.2023.2165908.
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology. 2001;142(11):4589–4594. doi: 10.1210/endo.142.11.8547.
- Shufelt C, Bretsky P, Almeida CM, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab. 2010;95(11):4985–4992. doi: 10.1210/jc.2010-0143.
- Cappola AR, Xue Q-L, Walston JD, et al. DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61(9):957–962. doi: 10.1093/gerona/61.9.957.
- Jiménez MC, Sun Q, Schürks M, et al. Low dehydroepiandrosterone sulfate is associated with increased risk of ischemic stroke among women. Stroke. 2013;44(7):1784–1789. doi: 10.1161/STROKEAHA.111.000485.
- Hemachandra C, Bell RJ, Islam MR, et al. The association between testosterone and depression in postmenopausal women: a systematic review of observational studies. Maturitas. 2022;68:62–70.
- Siddiqi HK, Kiss D, Rader D. HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol. 2015;30(5):536–542. doi: 10.1097/HCO.0000000000000211.
- Bittner V, Johnson BD, Zineh I, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2009;157(3):548–555. doi: 10.1016/j.ahj.2008.11.014.
- Wan K, Zhao J, Huang H, et al. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PLoS One. 2015;10(4):e0123521. doi: 10.1371/journal.pone.0123521.
- Bhalodkar NC, Blum S, Enas EA. Accuracy of the ratio of triglycerides to high-density lipoprotein cholesterol for predicting low-density lipoprotein cholesterol particle sizes, phenotype B, and particle concentrations among Asian Indians. Am J Cardiol. 2006;97(7):1007–1009. doi: 10.1016/j.amjcard.2005.10.036.
- Maruyama C, Imamura K, Teramoto T. Assessment of LDL particle size by triglyceride/hdl-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J Atheroscler Thromb. 2003;10(3):186–191. doi: 10.5551/jat.10.18614564088
- Hanak V, Munoz J, Teague J, et al. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94(2):219–222. doi: 10.1016/j.amjcard.2004.03.069.