Abstract
Objective
This study aimed to examine physicians’ and patients’ perceptions regarding symptom burden and impact in women experiencing natural vasomotor symptoms (nVMS) or vasomotor symptoms induced by endocrine therapy for breast cancer (iVMS).
Methods
The cross-sectional survey based on real-world clinical consultations was conducted in the USA and five European countries. Obstetrician–gynecologists, primary-care physicians and oncologists provided demographic and symptom data for patients experiencing VMS; patients optionally self-reported their experiences via questionnaires, including their symptom profile and work/activity burden through the Menopause Quality of Life (MENQOL) and Work Productivity and Activity Impairment (WPAI) tools.
Results
Physicians completed survey forms on 2451 consulting patients; patients completed 1029 questionnaires. nVMS and iVMS severity was significantly associated with the severity of mood symptoms and sleep disturbances (p < 0.0001). However, around half of the patients with mild nVMS/iVMS also experienced moderate–severe mood changes (55.4%/43.7%) or sleep disturbances (42.4%/40.4%). Presence of mood/sleep disturbances alongside nVMS increased MENQOL vasomotor scores (p = 0.004/p < 0.001). Presence of sleep disturbances increased WPAI activity impairment (p < 0.001) but mood changes did not. Similar findings were reported for iVMS patients.
Conclusion
Significant burden from the triad of natural or induced menopausal symptoms, sleep disturbances and mood changes affected women’s daily activities, work and quality of life more than vasomotor symptoms alone.
Introduction
In the complex tapestry of women’s health, the menopausal transition represents a profound biological milestone that can significantly impact a woman’s quality of life (QoL) [Citation1]. Approximately 47 million women around the world enter natural menopause each year, and almost a third of their lives are spent in the postmenopause period [Citation2].
Menopausal symptoms may manifest during the natural transition into menopause as a result of estrogen reduction or may be induced via antihormonal endocrine therapies (ET) taken by breast cancer survivors (BCS) to prevent cancer recurrence. An estimated 2.3 million women worldwide are diagnosed with breast cancer annually, meaning that there have been up to 7.8 million BCS diagnosed in the last 5 years [Citation3]. Around 80% of women experiencing natural menopause and 30–95% of BCS taking ET report menopausal symptoms, classically including vasomotor symptoms (VMS) and night-time awakenings secondary to VMS (night-time VMS) [Citation1,Citation4,Citation5].
Whether occurring naturally (nVMS) or induced by ET (iVMS), VMS are among the most prevalent and distressing manifestations of menopause, affecting women across diverse geographical, cultural and health-care landscapes [Citation1,Citation6]. VMS can also interfere with sleep, concentration, mood, energy, work, social activities and sexual relationships [Citation7–9]. The interactions between VMS, sleep and mood changes during menopause are complex and underexplored in real-world settings; however, the prevalence of both sleep and mood disturbances appear to have close links with menopausal status [Citation9]. Further, few studies examining menopausal symptoms have included both women with nVMS and BCS with iVMS [Citation10].
This study elicited perspectives and data from US and European physicians and patients to explore the real-world experiences of women with nVMS or iVMS. Our research comprised two linked publications: the current article reports the present results, including demographics and clinical characteristics, as well as the burden and impact of VMS, sleep and mood symptoms on women’s QoL and activities; and a second article evaluates the alignment between physicians and patients regarding their perceptions of natural and induced menopause-related symptoms and their impacts [Citation11].
Methods
Study design and participants
The observational REAL-world evIdence on vasomotor and other Symptoms in menopausal womEn (REALISE) study was an analysis of a secondary dataset, the Adelphi 2020 Vasomotor Disease Specific Programme (DSP™) [Citation12,Citation13], using a large, quantitative, cross-sectional methodology. The VMS DSP™ captured data from multiple cross-sectional surveys and retrospective chart reviews by obstetrician/gynecologists, primary care physicians and oncologists, conducted in the USA and five European countries (EUR5: the UK, France, Germany, Italy and Spain) between February and October 2020.
Physicians were recruited via publicly available lists of health-care providers selected from geographically diverse regions and screened by field-based interviewers. Physicians were included if they saw three or more women experiencing nVMS or iVMS per week.
The patient sample included women who were either naturally postmenopausal (nVMS group, aged 40–65 years) or BCS taking the ET tamoxifen and/or aromatase inhibitors to prevent recurrence (iVMS group, aged 18+ years) and were experiencing physician-confirmed VMS. Primary care physicians and obstetrician/gynecologists completed information on women with nVMS; oncologists reported on BCS with iVMS. Patients were seen by the aforementioned physicians for clinical consultations in ambulatory care settings.
Measurements
Through an electronic patient record form, physicians recorded demographic and clinical information – including clinically assessed VMS severity (mild, moderate, severe) and concomitant menopausal symptoms – for eight consecutively consulting patients who met the inclusion criteria. Physicians used a checkbox list of 26 menopause-related symptoms to record concomitant symptoms either being treated or currently experienced by the patient.
Each included patient was invited to complete a self-completion form (PSC) regarding their menopausal symptoms and associated impacts on their daily lives. Patients rated the impact of VMS over the past week on a list of 10 QoL domains, including work/study, social activities, leisure activities (collectively described as activities of daily living [ADLs]), self-confidence, sleep, mood, concentration, relations with others, sexual intimacy, enjoyment of life, and overall QoL. These were rated on a 7-point Likert-type scale from 1 (‘no problem’) to 7 (‘problem as bad as can be’) or ‘not applicable’. A substantial symptom impact was recorded if patients rated the impact as ‘moderate’ or worse. Patients also completed two validated patient-reported outcome tools – the Menopause Quality of Life (MENQOL) and the Work and Activity Impairment Questionnaire: Specific Health Problem (WPAI: SHP V2.0) – to assess health-related QoL and work absenteeism/presenteeism, respectively (see Supplementary Methods for details of patient-reported outcomes and scoring) [Citation14–17]. MENQOL symptoms endorsed by patients denoted the presence of those concomitant symptoms.
Patient and physician participation was voluntary and anonymous, and informed consent was given. The survey received ethical exemption from the Western Institutional Research Board (reference 1-1258281-1).
Statistical analysis
Physicians’ reports on demographic and symptom data were analyzed, and patients were categorized by physician-reported VMS severity: mild, moderate or severe. The presence of physician-reported sleep disturbances or mood changes/combined mood symptoms (defined as any of depression, mood swings/anxiety/irritability and lack of zest in life) was clinically determined. To assess VMS impact on ADLs, QoL and work/study, patients were divided into two groups based on self-rated VMS severity: mild or moderate-to-severe, using US Food and Drug Administration (FDA) criteria [Citation18] and patient-reported presence/absence of sleep disruption or combined mood symptoms (derived from the MENQOL; see Supplementary Methods). We then stratified patients into four groups based on the presence/absence of concomitant symptoms (physician-reported except for the MENQOL and ADL analyses): ‘VMS only’ (no mood and no sleep symptoms), ‘SM’ (both sleep and mood symptoms), ‘SI’ (sleep impairment without mood changes) and ‘MC’ (mood changes without sleep impairment).
Data analysis was descriptive. Statistical analyses included the χ2 test for categorical variables, the Kruskal–Wallis H-test for continuous/ordinal dependent variables and double-selection LASSO regressions on QoL and work productivity, with mood or sleep as the main predictor. Covariates selected differed for each regression and included age, time since menopause onset, body mass index, VMS risk factors and VMS severity (see Supplementary Methods for details). Two-sided p-values with a 0.05 significance level were selected. Stata Statistical Software Release 17 was used for the analyses [Citation19]. The current article reports the aggregated (US and EUR5) findings. Unless stated otherwise, all results were based on physician-reported data.
Results
Demographics and comorbidities
Overall, 310 physicians (118 obstetrician/gynecologists, 115 primary care physicians, 77 oncologists) provided data on 1816 nVMS patients and 635 iVMS patients; 846 nVMS patients and 183 iVMS patients completed the PSC form. Around one-third (34.2%) of patient record forms were completed by US physicians, and 41.1% of PSC forms were from US women. The number of completed EU5 PSC forms ranged from 186 (18.1%, Germany) to 62 (6.0%, UK) (Supplementary Table 1).
Participants with nVMS/iVMS had a mean (standard deviation [SD]) age of 53.9 (5.4)/53.9 (9.5) years and a mean (SD) body mass index of 25.6 (4.6)/25.2 (4.0) kg/m2. The majority (82.5%/80.3%) of women in this sample were White/Caucasian. The most common non-oncological comorbid conditions among women with nVMS/iVMS were hypertension (24.0%/20.6%), anxiety (16.0%/7.7%), hyperlipidemia (13.7%/7.1%) and depression (10.2%/5.2%) (). Family history of breast cancer was 13.5% (nVMS patients) and 30.7% (iVMS patients). One in 10 (10.1%) nVMS participants and 5.5% of iVMS participants had undergone a hysterectomy; and 4.2% (nVMS) and 6.9% (iVMS) of participants had undergone an oophorectomy. Participant characteristics were similar across VMS severity groups and strata () and countries (data not shown).
Table 1. Demographics and clinical characteristics.
VMS severity, frequency and duration
In the week before the survey, physicians classified 40.3%/31.7% of nVMS/iVMS patients with mild VMS, 43.8%/52.3% with moderate VMS and 15.9%/16.1% with severe VMS, respectively. nVMS patients experienced a mean (SD) of 2.6 (3.3) VMS and 1.9 (2.0) night-time VMS in the week before the survey. Patients with nVMS had a mean (SD) duration of 8.1 (11.5) min for VMS and 9.7 (16.9) min for night-time VMS (data not shown). The frequency and duration of VMS and night-time VMS increased with severity (Supplementary Figure 1a and 1b). Physicians reported similar findings for patients with iVMS (Supplementary Figure 1a and 1b).
Concomitant symptoms beyond VMS
Overall, the most common concomitant symptoms were combined mood symptoms (nVMS: 50.1%; iVMS: 48.4%), vaginal dryness (44.6%; 38.7%), sleep disturbances (34.7%; 35.9%), loss of libido (32.2%; 26.0%) and weight gain (21.9%; 23.9%) (see Supplementary Table 2 for the top 10 physician-reported concomitant symptoms).
Concomitant symptoms and VMS severity
Sleep disturbances (severe nVMS: 47.9%; mild nVMS: 24.9%), combined mood symptoms (67.4%; 36.5%) and vaginal dryness (59.0%; 36.8%) were reported for nearly a quarter to two-thirds of patients, with proportions generally higher in the severe nVMS group. Similar findings were observed for patients with iVMS (). Nevertheless, up to 37% of patients with mild nVMS and 32% with mild iVMS experienced concomitant symptoms ().
Figure 1. Proportions of nVMS patients and iVMS patients experiencing (a) top five concomitant symptoms excluding VMSa and (b) top five most bothersome symptoms in addition to VMS; stratified by VMS severityb.
aPhysician-reported VMS severity and concomitant symptoms based on case-note review; VMS and night-time VMS not shown as patients were included based on having VMS in this study.
bPhysician-reported VMS severity, concomitant symptoms and bother based on case-note review.
iVMS, induced vasomotor symptoms; nVMS, natural vasomotor symptoms; VMS, vasomotor symptoms.
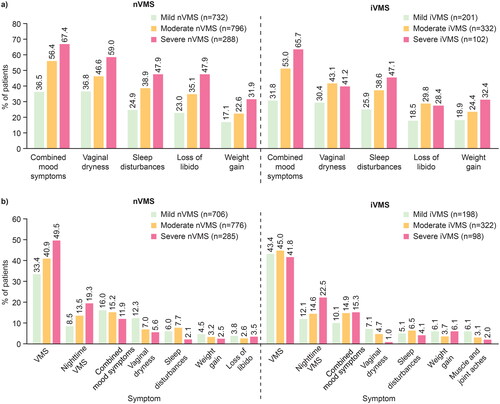
Most bothersome symptoms and VMS severity
The proportion of patients with mild, moderate and severe nVMS/iVMS with VMS as the most bothersome symptom was 33.4%/43.4%, 40.9%/45.0% and 49.5%/41.8%, respectively. Similar patterns were seen with night-time nVMS/iVMS (). Physicians reported combined mood changes as the most bothersome symptom in 10.1–16.0% of patients. Vaginal dryness (1.0–12.3%) and sleep disturbances (2.1–7.7%) were most bothersome in smaller proportions of patients ().
Severity of VMS and concomitant symptoms
The relationship between VMS severity and concomitant symptoms (mild, moderate or severe by clinical assessment) was examined. Approximately 55.4% of mild nVMS patients also experienced moderate–severe mood symptoms, and 42.4% experienced moderate–severe sleep disturbances (Supplementary Table 3). Similarly, 43.7% and 40.4% of mild iVMS patients experienced moderate–severe mood symptoms and sleep disturbances, respectively. Furthermore, nVMS/iVMS severity was significantly associated with the severity of mood symptoms and sleep disturbances (Kruskal–Wallis H-test, all p < 0.0001) (Supplementary Table 3).
Activities of daily living, relationships and quality of life (patient-reported)
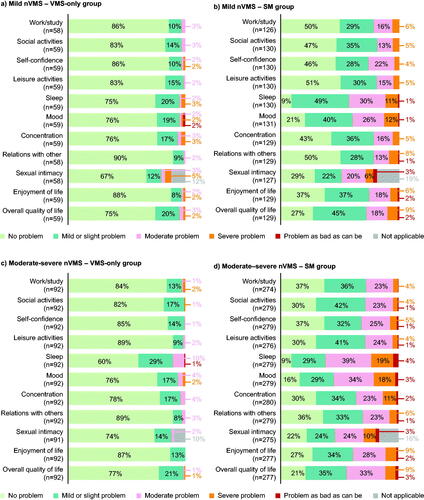
Few patients with mild or moderate-to-severe nVMS/iVMS only (with no concomitant sleep/mood symptoms) reported an impact of VMS on their ADLs, relationships and QoL (nVMS substantial problem range: 0–10.9% []; iVMS substantial problem range: 0–10.5% [Supplementary Figure 2a and 2c]). In the SMS group, a more significant proportion of patients reported a substantial impact on work/study (mild nVMS: 21.4%; mild iVMS: 10.5%), social activities (18.5%; 10.5%), concentration (21.7%; 26.3%), sexual intimacy (29.9%; 33.3%), self-confidence (26.2%; 10.5%) and overall QoL (27.9%; 15.8%) over the past week independent of VMS severity (for nVMS patients, see ; for iVMS patients, see Supplementary Figure 2b and 2d).
Figure 2. Impact of VMS on daily activities, other symptoms and quality of life by VMS severity with and without sleep and mood symptoms (nVMS).
Patient-reported data. Substantial impact was defined as ‘moderate problem’ or worse by patients on a 7-point Likert-type scale from 1 (‘no problem’) to 7 (‘problem as bad as can be’), or ‘not applicable’. Where data were 0%, these were omitted from the chart.
nVMS, natural vasomotor symptoms; SM, sleep and mood symptoms; VMS, vasomotor symptoms; VMS only, no mood or sleep symptoms.
Quality of life and sleep/mood symptoms (patient-reported)
Patients in the SM group, regardless of self-rated nVMS/iVMS severity, reported the highest mean (SD) overall MENQOL scores (higher score = greater impact on QoL) (mild nVMS: 3.1 [1.4]; moderate–severe nVMS: 3.4 [1.4]; mild iVMS: 2.5 [0.8]; moderate–severe iVMS: 3.2 [1.1]) (Supplementary Table 4). In contrast, patients with nVMS or iVMS only reported the lowest mean (SD) overall MENQOL scores (mild nVMS only: 1.3 [0.3]; moderate–severe nVMS only: 1.4 [0.4]; mild iVMS only: 1.5 [0.3]; moderate–severe iVMS only: 1.4 [0.4]) (Supplementary Table 4).
Using regression analysis, patients with nVMS and mood changes had significantly increased overall mean MENQOL (p < 0.001), vasomotor (p = 0.004) and psychosocial (p < 0.001) domain scores compared to those without mood changes, indicating a negative impact of mood disturbances on these domains. Similarly, patients with nVMS and sleep disturbances had significantly higher mean MENQOL vasomotor (p < 0.0001) and physical (p < 0.001) domain scores compared to those without sleep disturbances (Supplementary Table 5). Mood changes in iVMS patients were significantly associated with increased psychosocial and physical MENQOL domain scores (p < 0.001 and p = 0.035, respectively) but not overall MENQOL scores. An increased vasomotor domain score (p < 0.001) was observed in the iVMS group with sleep disturbances (Supplementary Table 5).
nVMS patients who experienced moderate to severe bother from their sleep/mood disturbances also had significantly higher MENQOL scores overall and in all domains (p < 0.001), with similar findings for iVMS patients in the overall MENQOL and psychosocial domains (both p < 0.001), physical domain (p = 0.016) and sexual domain (p = 0.04), compared to those with mild or no bother from these symptoms (Supplementary Table 6).
Work and activity impairment (patient-reported)
WPAI-derived absenteeism due to VMS was low overall (percent of work time missed due to nVMS, range: 0–2.6%; due to iVMS, range: 0–1.3%) but highest in the mild nVMS SM group (5.2% [SD 16%]). Comparable findings were observed for presenteeism (mild nVMS only: 14.1% [SD 18.7%]; SM: 23.3% [23.7%]), non-work activity impairment (mild nVMS only: 12.5% [15.8%]; SM: 27.1% [23.5%]) and overall work impairment (mild nVMS only: 15.0% [18.2%]; SM: 26.0% [27.5%]). Similar results were found in the moderate–severe group (Supplementary Table 7). Patients with mild iVMS showed no such pattern; however, overall work impairment was again higher in the moderate–severe iVMS SM group (27.8% [SD 21.0%]) than in the iVMS-only group (13.3% [10.5%]) (Supplementary Table 7).
Patients with nVMS reporting sleep disturbances reported significantly increased work and activity impairment in most WPAI domains (presenteeism: coefficient [C] = 6.859, p = 0.003, 95% confidence interval [CI] [2.391, 11.328]; overall work impairment: C = 7.402, p = 0.003, 95% CI [2.452, 12.352]; activity impairment: C = 8.053, p ≤ 0.001; 95% CI [4.341, 11.765]), except for absenteeism (C = 0.466, p = 0.243, 95% CI [−0.317, 1.249]), compared to those not reporting sleep disturbances and when controlling for VMS severity and other confounders (Supplementary Table 5). No association was found between mood changes or sleep disturbances and any WPAI domain score in patients with iVMS (Supplementary Table 5).
When controlling for nVMS severity and other confounders, patients reporting moderate or severe bother caused by mood changes had significantly increased work and activity impairment across all WPAI domains (except for absenteeism) compared to those with no or mild bother (presenteeism: C = 8.476, 95% CI [3.119, 13.831], p = 0.002; overall work impairment: C = 7.321, 95% CI [1.347, 13.294], p = 0.016; activity impairment: C = 14.223, 95% CI [10.222, 18.224], p < 0.001), with similar findings for those with moderate-to-severe bother caused by sleep disturbances (presenteeism: C = 9.425, 95% CI [3.005, 15.844], p = 0.004; overall work impairment: C = 8.3, 95% CI [0.257, 16.344], p = 0.043; activity impairment: C = 16.181, 95% CI [11.771, 20.590], p < 0.001). However, for iVMS patients, no comparable correlation was observed (Supplementary Table 6).
Discussion
This real-world study found that substantial proportions of patients with nVMS or iVMS experience concomitant symptoms beyond VMS that affect their QoL, such as sleep disturbances, mood changes, weight gain and vaginal dryness. While our data supported previous findings that VMS are the most bothersome menopausal symptom for most women [Citation1,Citation9,Citation20,Citation21], stratification of patients by VMS severity and the presence/absence of concomitant sleep and/or mood symptoms helped delineate the impact of these commonly co-occurring symptoms. More than half of the patients with mild nVMS also experienced moderate–severe mood symptoms and more than 40% experienced moderate–severe sleep disturbances. Moreover, nVMS severity was significantly associated with the severity of mood and sleep disturbances, with similar findings evident in patients with mild iVMS. This speaks to the unrecognized non-VMS burden experienced by women with mild VMS who are typically excluded from VMS clinical trials and treatment indications. While the causes of sleep and mood disturbances in mid-life women are multiplex, these findings further supported the concept of a triad of menopausal symptoms (VMS, sleep disturbances and mood changes) that interact with each other rather than a simple domino effect of VMS-induced sleep/mood issues. Considering that sleep and mood symptoms were consistently within the top three most bothersome symptoms after VMS, even in patients with mild VMS, the current study underlined the importance of considering the full spectrum of menopause-related symptoms, including and beyond VMS.
Sleep, mood, cognitive issues and their effects on work and social functioning are increasingly recognized as essential areas affecting women’s QoL during menopause [Citation9,Citation22,Citation23]. In this study, sleep and mood symptoms showed a more substantial impact on ADLs and QoL than VMS severity. Additionally, patients reported that VMS alone, even when severe, had minimal impact on their work, study, concentration, enjoyment of life and other important ADL domains. While our findings added to the understanding of their interrelationship, the causal directionality between the triad of VMS, sleep disturbances and mood changes has yielded inconsistent results in the literature and warrants further research [Citation24–27].
While the focus of this analysis was not a direct comparison between iVMS and nVMS, our data showed surprising consistency between women’s experiences in both the natural and induced VMS groups. VMS, concomitant symptoms and their associated burden (as reported by physicians from three different specialties), and their impact on QoL and activities (as reported by women themselves) were broadly similar, irrespective of cause. Previous work based on patient surveys has suggested that BCS have more severe menopausal symptoms and impact on QoL than naturally menopausal women [Citation5,Citation10]. Differences between our data and these studies may be due to differences in methodology, sampling and geography. Nevertheless, our findings support the assertion that all menopausal symptoms, irrespective of cause, are highly prevalent and have considerable impacts on women’s lives.
Despite their similar symptom profiles, BCS taking ET to prevent cancer recurrence have unique needs compared to women in natural menopause. In addition to the physical and emotional burden of cancer diagnosis and treatment, ET commonly induces or exacerbates menopausal symptoms in premenopausal and postmenopausal women [Citation5]. This study confirmed that BCS in real-world settings suffer from the effects of VMS and concomitant symptoms, which affect their QoL and functioning. Side effects of ET, particularly menopausal symptoms, are linked to poor ET medication adherence, early discontinuation, increased recurrence risk, reduced disease-free survival, worse QoL and higher medical costs [Citation28,Citation29]. Hence, it is critical to address modifiable factors, including menopausal symptoms, affecting ET compliance and duration of use [Citation29–31].
Physician education and training should raise awareness of the synergistic burden of a broader range of menopausal symptoms experienced by women beyond VMS, including in women with mild VMS, to optimize effective communication, shared decision-making and personalized menopause care [Citation32–36]. Bridging knowledge gaps through the education and training of clinicians across relevant specialties, including primary care, is crucial to improving physician competence and confidence when assessing and managing menopausal symptoms [Citation32]. Training should also aim to develop the skills to ask sensitively useful questions so that women are empowered to discuss their symptoms freely [Citation37].
To these ends, we recommend developing a validated menopause language that covers a broader range of symptoms and their impacts beyond VMS; this should also include women experiencing induced menopausal symptoms [Citation38]. The language should be co-created by women and clinicians as equal partners using consensus methods, as has been successful in developing the Core Outcomes in Menopause initiative [Citation38,Citation39].
Study strengths and limitations
Strengths of the study include the large sample size, consistent methodology across countries and real-world data collected from patients and their physicians. Further, a mix of specialties captured clinician perspectives across relevant but differing fields. Finally, examining both nVMS and iVMS allowed for a more holistic evaluation of the menopause landscape, as the latter patient group is often neglected in menopause clinical trials. Limitations included cross-sectional surveys being limited by data temporality and unknown direction of causation; both physician-reported and patient-reported data may introduce biases (however, alignment between physicians and patients was evaluated in a separate paper on the same dataset [Citation11]); MENQOL overall scores were affected by domain scores; and limited sampling only of women with access to, and seeking, health care. As physician specialty was not included as a potential covariate, it was not pulled through and therefore not confounded for. Additionally, our data did not include whether the oophorectomies recorded in ≤6.9% of patients were unilateral or bilateral; we can therefore not examine the impact of bilateral oophorectomy causing surgical menopause from our data. Finally, our study did not differentiate new-onset sleep/mood symptoms (which may be related or unrelated to menopause [Citation24]) from pre-existing conditions.
Conclusions
Menopausal symptoms, whether natural or induced, are not limited to VMS alone. Mood and sleep disturbances, among many other concomitant symptoms, have detrimental impacts on women’s QoL and functioning. BCS taking ET also experience similar symptom profiles and impacts to those with natural menopause. Through patient-reported and physician-reported outcomes, this study offered real-world evidence for the synergistic effects of VMS and concomitant sleep and mood symptoms on women’s daily lives; however, the direction of causality remains unclear. Nonetheless, we advocate a more personalized approach to menopause care via effective communication and shared decision-making between patients and physicians. This, in turn, requires physician education and training to raise awareness of the various menopausal symptoms beyond VMS and their burden, even in women with mild VMS.
Supplemental Material
Download MS Word (483.7 KB)Acknowledgements
The authors thank the patients and physicians who participated in this study. They would also like to acknowledge Adelphi Real World for their assistance in processing and analyzing the data and Highfield (Oxford, UK) for their assistance with producing this article.
Disclosure statement
S.K. is an employee of University Hospitals Cleveland Medical Center; has received consulting fees or honoraria from Astellas, Alloy, Bayer, Daré, Freya, Reunion Neuroscience, Materna Medical, Madorra, Ms. Medicine, Palatin Technologies, Pfizer, Sprout, Strategic Science Technologies and TherapeuticsMD; and has received stock options from Reunion Neuroscience, Alloy and Materna Medical. N.S. and C.M. are employees of Bayer AG, Germany. C.C. and C.J. are employees of Bayer Consumer Care, Switzerland. M.S. and L.L. are employees of Adelphi Real World, UK.
R.E.N has past financial relationships (lecturer, member of advisory boards and/or consultant) with Boehringer Ingelheim, Eli Lilly, Endoceutics, Exeltis, Palatin Technologies, Pfizer, Procter & Gamble, Teva Women’s Health and Zambon; has ongoing relationships with Abbott, Astellas, Bayer HealthCare, Besins Healthcare, Fidia, Gedeon Richter, HRA Pharma, Merck & Co, Novo Nordisk, Organon, Shionogi, Theramex and Viatris; and serves as President-Elect of the International Menopause Society (IMS).
Data availability statement
The data collection materials and datasets generated for the purposes of this study were made fully available to all authors but are not publicly available and remain the intellectual property of Adelphi Real World. Requests to access the data used for this publication should be addressed to Adelphi Real World.
Additional information
Funding
References
- Nappi RE, Kroll R, Siddiqui E, et al. Global cross-sectional survey of women with vasomotor symptoms associated with menopause: prevalence and quality of life burden. Menopause. 2021;28(8):875–882. doi: 10.1097/GME.0000000000001793.
- Hill K. The demography of menopause. Maturitas. 1996;23(2):113–127. doi: 10.1016/0378-5122(95)00968-x.
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010.
- Stearns V, Ullmer L, López JF, et al. Hot flushes. Lancet. 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0.
- Cole KM, Clemons M, Alzahrani M, et al. Vasomotor symptoms in early breast cancer – a “real world” exploration of the patient experience. Support Care Cancer. 2022;30(5):4437–4446. doi: 10.1007/s00520-022-06848-3.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3(1):47. doi: 10.1186/1477-7525-3-47.
- Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32–43. doi: 10.1080/13697130701744696.
- Williams RE, Levine KB, Kalilani L, et al. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62(2):153–159. doi: 10.1016/j.maturitas.2008.12.006.
- English M, Stoykova B, Slota C, et al. Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS sleep disturbance and sleep-related impairment measures for assessment of VMS impact on sleep. J Patient Rep Outcomes. 2021;5(1):42. doi: 10.1186/s41687-021-00322-0.
- Marino JL, Saunders CM, Emery LI, et al. Nature and severity of menopausal symptoms and their impact on quality of life and sexual function in cancer survivors compared with women without a cancer history. Menopause. 2014;21(3):267–274. doi: 10.1097/GME.0b013e3182976f46.
- Kingsberg S, Nappi RE, Scott M, et al. Physician and patient alignment on menopause-associated symptom burden and treatment decisions: RWE from Europe and the US. Climacteric. 2024 [Manuscript submitted for publication].
- Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: disease-specific programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040.
- Babineaux SM, Curtis B, Holbrook T, et al. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352.
- Radtke JV, Terhorst L, Cohen SM. The menopause-specific quality of life questionnaire: psychometric evaluation among breast cancer survivors. Menopause. 2011;18(3):289–295. doi: 10.1097/gme.0b013e3181ef975a.
- Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 2008;61(1–2):107–121. doi: 10.1016/j.maturitas.2008.09.014.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006.
- Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the menopause-specific quality of life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005;50(3):209–221. doi: 10.1016/j.maturitas.2004.06.015.
- Food and Drug Administration. Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms: recommendations for clinical evaluation. In: Administration UFaD, editor; 2003.
- StataCorp. Stata statistical software: release 17. College Station (TX): StataCorp LLC; 2021.
- Nappi RE, Siddiqui E, Todorova L, et al. Prevalence and quality-of-life burden of vasomotor symptoms associated with menopause: a European cross-sectional survey. Maturitas. 2023; Jan167:66–74. doi: 10.1016/j.maturitas.2022.09.006.
- Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: Who is most bothered by vasomotor symptoms? Menopause. 2008;15(5):841–847. doi: 10.1097/gme.0b013e318168f09b.
- DePree B, Shiozawa A, King D, et al. Association of menopausal vasomotor symptom severity with sleep and work impairments: a US survey. Menopause. 2023;30(9):887–897. doi: 10.1097/GME.0000000000002237.
- Tomida M, Otsuka R, Tange C, et al. Vasomotor symptoms, sleep problems, and depressive symptoms in community‐dwelling Japanese women. J Obstet Gynaecol Res. 2021;47(10):3677–3690. doi: 10.1111/jog.14937.
- Baker FC, De Zambotti M, Colrain IM, et al. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73–95. doi: 10.2147/NSS.S125807.
- Vousoura E, Spyropoulou AC, Koundi KL, et al. Vasomotor and depression symptoms may be associated with different sleep disturbance patterns in postmenopausal women. Menopause. 2015;22(10):1053–1057. doi: 10.1097/GME.0000000000000442.
- Baker FC, Willoughby AR, Sassoon SA, et al. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96–104. doi: 10.1016/j.psyneuen.2015.06.005.
- Caruso D, Masci I, Cipollone G, et al. Insomnia and depressive symptoms during the menopausal transition: theoretical and therapeutic implications of a self-reinforcing feedback loop. Maturitas. 2019;123:78–81. doi: 10.1016/j.maturitas.2019.02.007.
- Collin LJ, Cronin-Fenton DP, Ahern TP, et al. Early discontinuation of endocrine therapy and recurrence of breast cancer among premenopausal women. Clin Cancer Res. 2021;27(5):1421–1428. doi: 10.1158/1078-0432.CCR-20-3974.
- Rosso R, D’Alonzo M, Bounous VE, et al. Adherence to adjuvant endocrine therapy in breast cancer patients. Curr Oncol. 2023;30(2):1461–1472. doi: 10.3390/curroncol30020112.
- Sella T, Zheng Y, Rosenberg SM, et al. Extended adjuvant endocrine therapy in a longitudinal cohort of young breast cancer survivors. NPJ Breast Cancer. 2023;9(1):31. doi: 10.1038/s41523-023-00529-y.
- Chang C-H, Huang C-W, Huang C-M, et al. The duration of endocrine therapy and breast cancer patients’ survival. Medicine. 2019;98(43):e17746. doi: 10.1097/MD.0000000000017746.
- Macpherson BE, Quinton ND. Menopause and healthcare professional education: a scoping review. Maturitas. 2022;166:89–95. doi: 10.1016/j.maturitas.2022.08.009.
- Allen JT, Laks S, Zahler-Miller C, et al. Needs assessment of menopause education in United States obstetrics and gynecology residency training programs. Menopause. 2023;30(10):1002–1005. doi: 10.1097/GME.0000000000002234.
- Faubion SS, Shufelt C. The menopause management vacuum. Cancer J. 2022;28(3):191–195. doi: 10.1097/PPO.0000000000000594.
- Kling JM, MacLaughlin KL, Schnatz PF, et al. Menopause management knowledge in postgraduate family medicine, internal medicine, and obstetrics and gynecology residents: a cross-sectional survey. Mayo Clin Proc. 2019;94(2):242–253. doi: 10.1016/j.mayocp.2018.08.033.
- Manson JE, Kaunitz AM. Menopause management – getting clinical care back on track. N Engl J Med. 2016; 2016/03/03374(9):803–806. 806. doi: 10.1056/NEJMp1514242.
- Harper JC, Phillips S, Biswakarma R, et al. An online survey of perimenopausal women to determine their attitudes and knowledge of the menopause. Womens Health. 2022;18:174550572211068. doi: 10.1177/17455057221106890.
- Lensen S, Archer D, Bell RJ, et al. A core outcome set for vasomotor symptoms associated with menopause: the COMMA (core outcomes in menopause) global initiative. Menopause. 2021;28(8):852–858. doi: 10.1097/GME.0000000000001787.
- Peng Y, Wu T, Chen Z, et al. Value cocreation in health care: systematic review. J Med Internet Res. 2022;24(3):e33061. doi: 10.2196/33061.
