 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
A better understanding of patient non-adherence to type 2 diabetes medication is needed to design effective interventions to address this issue.
Objectives
(1) To estimate the prevalence of non-adherence to diabetes medication; (2) to examine its impact on glycemic control and insulin initiation; (3) to develop and validate a prediction model of non-adherence.
Methods
We conducted a longitudinal cohort study based on data from electronic health records. We included adult patients registered within the Health Service of the Balearic Islands (Spain) starting a new prescription of a non-insulin glucose-lowering drug between January 2016 and December 2018. We calculated non-adherence at 12 months follow-up, defined as medication possession ratio (MPR) ≤ 80%. We fitted multivariable regression models to examine the association between non-adherence and glycemic control and insulin initiation and identified predictors of non-adherence.
Results
Of 18,119 patients identified, after 12 months follow-up, 5,740 (31.68%) were non-adherent. Compared with non-adherent, adherent patients presented lower HbA1c levels (mean difference = −0.32%; 95%CI = −0.38%; −0.27%) and were less likely to initiate insulin (aOR = 0.77; 95%CI = 0.63; 0.94). A predictive model explained 22.3% of the variation and presented a satisfactory performance (AUC = 0.721; Brier score = 0.177). The most important predictors of non-adherence were: non-Spanish nationality, currently working, low adherence to previous drugs, taking biguanides, smoker and absence of hypertension.
Conclusion
Around one-third of the patients do not adhere to their non-insulin glucose-lowering drugs. More research is needed to optimise the performance of the predicting model before considering its implementation in routine clinical practice.
KEY MESSAGES
One-third of patients do not adhere to glucose-lowering drugs 12 months after its initial prescription
Non-adherence is associated with worse glycemic control and a higher likelihood of starting insulin
The most important predictors of non-adherence are: non-Spanish nationality, currently working, taking biguanides, smoker, without hypertension and shorter time with diabetes.
Introduction
Type 2 diabetes mellitus (T2DM) is a life-long condition which, in 2021 affected around 537 million adults worldwide [Citation1]. Medicines are frequently prescribed alongside lifestyle changes to lower blood glucose and prevent long-term complications [Citation2].
Adherence has been defined as ‘the process by which patients take their medications as prescribed’ [Citation3]. Non-adherence to glucose-lowering drugs (GLD) is a common problem worldwide [Citation4,Citation5] that contributes to disease progression, increased risk for hospitalisation and mortality [Citation6], and healthcare costs [Citation7]. In Spain, non-adherence rates to GLD range from 48 to 55% [Citation8–12].
Identifying patients who are less likely to follow their medication can help providers intervene to improve adherence. Factors contributing to non-adherence can be classified as condition-related (concomitant diseases), medication-related (insulin use, primary non-adherence, and early non-persistence, class of medication and other prescriptions), healthcare system (insurance, medication cost) and socioeconomic and patient-related factors (age, race, health beliefs, health literacy, physician trust) [Citation13,Citation14].
The evidence on adherence to GLD in Spain is insufficient because it is mostly based on cross-sectional studies (not suitable to determine causality) using self-reported measures of medication adherence [Citation15–17] (while objective measures could provide more reliable estimations). The impact of non-adherence on glycemic control has not been studied in Spain.
The general aim of this study was to investigate the phenomenon of non-adherence to GLD in Spain. The specific objectives were: (1) to estimate the prevalence of non-adherence to GLD; (2) to examine the impact of non-adherence on glycemic control and insulin initiation and; (3) to develop and validate a prediction model of non-adherence.
Methods
Study design
We carried out a longitudinal cohort study based on data extracted from the electronic health records of the Balearic Islands (Spain) between 2016 and 2018. We extracted the data from two clinical databases: eSIAP and RELE. eSIAP (established in 2002) is a repository of routine clinical data from over 1,350,000 patients registered with primary care services, with data from all 58 primary care centres in the Balearic Islands. It includes demographic data, diagnostic codes, and biochemistry results. RELE contains information about all primary and outpatient care prescriptions dispensed in community pharmacies, including dispensation date, pharmaceutical product, dose, and prescription duration.
Ethics
Approval for the study was granted by the Ethical Research Committee of the Balearic Islands (IB3949/19 PI). Given that this study is based on anonymized data from a large sample of patients, Informed Consent was deemed unnecessary.
Eligibility criteria
We included patients aged ≥18, registered within the Public Health Service of the Balearic Islands, who started a first-time prescription (i.e. never prescribed to them before in their life) of a non-insulin GLD (hereinafter called ‘index’) between 1 January 2016 and 31 December 2018. The index prescription had to be kept active (i.e. not stopped by their doctors) for at least 12 consecutive months to qualify. The non-insulin GLD considered in this study are listed in Online Supplementary Appendix 1.
We excluded patients with a diagnosis of gestational diabetes, a prescription of biguanides without a diagnosis of T2DM, or a prescription of insulin at the time of initiating the index GLD. We excluded patients with a prescription for an index GLD initiated during the follow-up period but that had also been prescribed earlier in their life. Furthermore, we excluded patients who died during the follow-up period (as we believed that MPR data spanning less than 12 months would lead to less reliable and consistent estimations of adherence).
Data management
The data from both sources (eSIAP and RELE) was linked based on the patient’s unique identifier. Data extraction was validated for accuracy by two clinicians with considerable expertise in diabetes and by a medical coder (see acknowledgements). For patients with >1 eligible prescription during the study period, we only kept the earliest one.
Defining and calculating medication adherence
We calculated adherence in terms of medication possession ratio (MPR) [Citation18], defined as the number of days with treatment as medication being dispensed from the pharmacy to the patient (numerator), out of the total days of treatment prescribed by the doctor (denominator) (Online Supplementary Appendix 2).
We used MPR as a continuous variable. Additionally, we dichotomised MPR according to the previously defined cut-off, settled in >80% for good adherence [Citation4].
Exploring predictors of adherence
We identified potential predictors of adherence based on previous research [Citation14]. We selected those available in our data sources (Box 1).
Statistical analysis
Initially, we conducted descriptive statistics to characterise our sample. Subsequently, we carried out specific analyses for each of our three objectives.
Objective #1: we determined the prevalence of non-adherence to the index GLD (MPR ≤80%), overall and according to ATC Code-Group.
Objective #2: we examined the impact of non-adherence on HbA1c response after one year. These analyses were restricted to 9,025 subjects (since 50.2% of the overall sample presented missings in HbA1c values). We fitted linear regression models where HbA1c after follow-up was used as dependent variable, and adherence to the index prescription (categorized as MPR ≤80%, MPR >80%; and per 10% increment), overall and by treatment group, as independent variable. Models were adjusted by baseline HbA1c (%) and the following potential confounding factors: age, sex, smoking habit, treatment group (only for overall treatment), duration of diabetes, socioeconomic deprivation index, and prescription of insulin or new GLD during 12 months of follow-up. We also examined the impact of non-adherence on insulin initiation using a multivariable logistic regression model, adjusting for potential confounding factors. We applied multiple imputations to deal with missing data concerning baseline HbA1c, smoking status, and socioeconomic deprivation [Citation19]. The same analyses were replicated without applying multiple imputation, as sensitivity analyses.
Objective #3: we split the database into two sub-databases, randomly allocating patients to a derivation dataset (with two-thirds of the patients) or to a validation dataset (with one-third of the patients). For model derivation, we used logistic regression models to identify predictors of non-adherence (MPR ≤80%) and to estimate the coefficients and Odds Ratios associated with each potential predictor. We used the Akaike and Bayes information criteria (AIC and BIC) to compare models [Citation20,Citation21]. We used multiple imputation with chained equation to replace missing values for HbA1c, occupational status, alcohol consumption and smoking status. Ten imputed samples were generated, and estimates were combined using Rubin rules [Citation22]. Predictors associated with non-adherence (based on a conservative p < 0.1) were selected to be included in the multivariate model. Potential predictors were selected following a backward approach in the imputed prediction dataset, using ‘stacked’ imputed data sets with weighted regression [Citation23]. For model validation, we tested the performance of the final model in the validation dataset. We evaluated the model’s discriminative ability using the area under the curve (AUC). We calculated the mean predicted risk of ‘good’ adherence at baseline in the final model and compared it to the 12 months adherence. We computed the Brier score [Citation24], which measures disagreement between the forecasted predictions and the observed outcome and carries benchmark values of 0 for no disagreement, 1 for complete disagreement and 0.25 for predictions no better than chance [Citation25]. We used the R2 statistic to measure explained variation. We calculated the mean predicted risk and the observed risk of non-adherence at one year and compared these by 10th of the predicted risk.
All analyses were carried out in Stata 17 (StataCorp) and we used an α of 5% throughout.
Results
Cohort characteristics
Overall, 18,119 patients met our selection criteria. Their characteristics are shown in and . The mean (SD) age was 64 (13). Around six of ten patients were male. The majority had Spanish nationality (83.16%). Biguanides was the most frequent type of prescription (37.39%). The mean (SD) HbA1c was 7.77% (1.58).
Table 1. Sociodemographic characteristics of the patients included (n = 18,119).
Table 2. Clinical characteristics of the patients included (n = 18,119).
Adherence to GLD
Of 18,119 patients, 31.68% were non-adherent (MPR ≤ 80%) to their prescribed GLD after 12 months follow-up (mean (SD) MPR 80.86 (24.35)). By medication type, the highest non-adherence rate was found for biguanides (44.63%), whereas the lowest was for GLP-1 analogues (17.12%) ().
Table 3. Adherence to non-insulin glucose-lowering drugs (GLDs, overall and by treatment).
Impact of non-adherence on glycemic control and insulin initiation
When comparing adherent vs non-adherent patients, no differences were observed in mean HbA1c at baseline (p = 0.084). After 12 months follow-up, mean HbA1c was significantly (p < 0.001) lower in adherent (mean 6.87% (SD 0.02%)) than in non-adherent patients (7.06% (0.03%)) (data not shown). The adjusted mean (95% CI) difference overall between adherent and non-adherent groups in one-year HbA1c was −0.32% (−0.38 to −0.27%) (). Sensitivity analyses without imputing the missing values in covariables yielded similar results (Online Supplementary Appendix 5).
Table 4. Association between adherence to non-insulin GLDs (glucose-lowering drugs, overall and by type of drug) and HbA1c response after one year (multiple imputations for covariables*).
A 10% increase in MPR was independently associated with a significant (p < 0.001) reduction in HbA1c of −0.080% (Online Supplementary Appendix 6a). Results were very similar without imputing the missing values in covariables for sensitivity analyses (Online Supplementary Appendix 6b).
Adherence, compared with non-adherence, was associated with a lower risk of initiating insulin after one year (aOR = 0.77; 95%CI = 0.63–0.94) (Online Supplementary Appendix 7a). By type of treatment, a statistically significant association was observed for SGLT2i (0.55 (0.33–0.94)), but not for the other drugs examined. When examining MPR as a continuous variable (Online Supplementary Appendix 8a), a 10% increase in MPR was associated with a lower risk of starting insulin (0.94 (0.90–0.96)). Similar results were obtained in our sensitivity analyses without multiple imputation (Online Supplementary Appendices 5b and 8b).
Prediction of non-adherence to GLD
Non-adherence (MPR ≤80%) to the index drug was independently associated (p < 0.05) with socioeconomic and patient-related factors (non-Spanish nationality, currently working); medication-related factors (low adherence to previous drugs, lower number of active prescriptions, taking biguanides), and; condition-related factors (smoker, diagnosis of diabetes concurrent to the index prescription, shorter time of diagnosis of diabetes, obesity, absence of diagnosis of hypertension) ( and Online Supplementary Appendix 9).
Table 5. Association between adherence and patient characteristics (predictors retained in the final multivariate logistic regression model).
The predictive model explained 22.2% (95%CI = 18.6–25.8%) of the variation. The AUC () was 0.722 (95%CI = 0.706–0.737) and considered satisfactory. The Brier score was 0.177 (95%CI = 0.171–0.183), lower than the 0.25 threshold, indicating that predictions were significantly better than chance.
Figure 1. Receiver-operating characteristic curve showing the discrimination between adherent (MPR >80%) and non-adherent (MPR ≤80%) patients for the index GLD when using predicted values from the full logistic regression model*. MPR: medication possession ratio. *Validation statistics: R squared (higher values indicate better discrimination) = 0.222 (95%CI = 0.186–0.258); ROC statistic (higher values indicate better discrimination) = 0.728 (95%CI = 0.706–0.737); Brier score (lower values indicate better performance) = 0.177 (95%CI = 0.171–0.183)
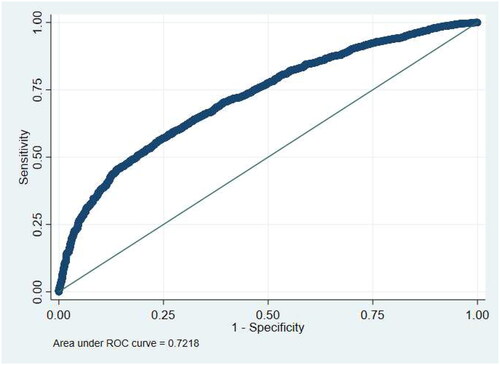
We observed a close correspondence between predicted and observed one year adherence within each model 10th (RR = 0.997), suggesting that the model was well calibrated (Online Supplementary Appendix 10).
Discussion
Main findings
We observed that one-third (32%) of the patients (n = 18,119) did not adequately adhere to their GLD prescription. Adherence, compared to non-adherence, was associated with better glycemic control (mean difference= −0.32%) and lower likelihood of starting insulin (aOR = 0.77). Non-adherence was independently associated with socioeconomic and patient-related factors (non-Spanish nationality, currently working), medication-related factors (low adherence to previous drugs, lower number of active prescriptions, taking biguanides), and; condition-related factors (smoker, diagnosis of diabetes concurrent to the index prescription, shorter time of diagnosis of diabetes, obesity, absence of hypertension).
Comparison with existing literature
One previous study in Spain measured adherence to non-insulin GLD based on objective data [Citation26], estimating a non-adherence rate of 28%. The prevalence of non-adherence in our study (32%) is similar to that figure; and the 32% observed in a meta-analysis [Citation5]. As in ours, these two studies defined medication non-adherence as MPR ≤80%.
Non-adherence differed according to medication class, with biguanides presenting the highest non-adherence rates (44.6%), supporting previous studies [Citation27,Citation28]. This remarkably high non-adherence to biguanides may be partially due to some doctors recommending their patients gradually increasing the dose until reaching the agreed target in case they experience gastrointestinal side effects. In our study, DPP4i presented the highest adherence rate (87.9%). This is substantially higher than the 56.9% observed in a recent meta-analysis [Citation29]. Most studies in this meta-analysis were from the USA, a country with a different health system from the Spanish one in terms of medication cost-sharing. In a recent study in a primary care population from Catalonia, Spain, the adherence rate to DPP4i was 53.6%. However, this study was restricted to adherence to a second add-on treatment and focused on patients with poor glycemic control [Citation30]. Thus, comparisons of the results in different countries and populations should be interpreted cautiously.
Compared with adherent patients, the non-adherent had an overall HbA1c reduction of 0.32%. This reduction is similar to the 0.4% observed in a previous study in the UK [Citation27]. Although this reduction was statistically significant and may be considered relevant at the population/public health level, it is not clinically relevant at the individual level [Citation31]. However, we studied reduction for 12 months; as GLDs are often prescribed for longer periods, the reduction may become clinically relevant over time.
The reductions in HbA1c were observed in all drug classes except for ‘Other blood GLDs’ (which included alpha-glucosidase inhibitors, thiazolidinediones, guar gum, glinides, and other combinations different from biguanides + DPP-4 inhibitors, and biguanides + SGLT-2 inhibitors) suggesting that suboptimal adherence does not lead to a lower efficacy of these GLDs due to a weaker hypoglycaemic effect among them. Furthermore, an association between adherence and a lower risk of insulin initiation was only observed for SGLT2i – which may be due to their stronger hypoglycaemic effect [Citation32]. As far as we know, no previous studies have examined the association between non-adherence and insulin initiation.
Based on the predictors of non-adherence identified in our study, it may be argued that reasons for non-adherence could be related to the patient’s lack of insight into the illness, as well as by the asymptomatic nature of T2DM in its early stages, as already observed by a previous meta-synthesis [Citation33]. The factor most strongly associated with adherence was adherent to a previous diabetes medication - in line with the findings from a recent study in UK [Citation34].
Strengths and limitations
Particular strengths of our study are the use of representative population from a validated database and robust analytic methods.
Our study has several limitations. First, although the adherence process entails three separate phases (initiation, implementation, and persistence), our analyses specifically targeted the implementation phase. Second, we used MPR to measure adherence. Medication possession does not necessarily imply medication taking. However, no gold standard exists and using MPR is more reliable [Citation35]. Third, we did not identify patients based on a diagnosis code but rather on the prescription of a GLD. Therefore, we excluded patients with biguanides but without a registered diagnosis of T2DM (1.5%). Fourth, we excluded 13% of the initial sample because prescriptions were issued for less than 12 months. This could have resulted in an underestimation of the prevalence of non-adherence if the reason for the prescribers to stop the prescription was that patients did not adequately adhere to it. Fifth, the association between non-adherence and HbA1c was estimated based on a subsample of the overall cohort. Patients not closely followed by their providers may be more likely to self-manage their condition sub-optimally, and therefore more likely to be non-adherent and with worse glycemic control. This potential selection bias could have limited our ability to identify a stronger association between non-adherence and worse glycemic control. Sixth, the validation of the predictive model within the same cohort as the one used for its development might have resulted in an overestimation of its performance, primarily due to the similarity or resemblance of the patient population. Finally, we could not examine healthcare system-related predictors because this data was unavailable.
Implications for clinical practice
Although more research is needed to optimise the performance of our predicting model, the predictors identified (socioeconomic and patient-related, medication-related, and condition-related) may help clinicians to be aware of potential non-adherence when they issue a new GLD prescription.
Conclusion
In a representative primary care sample, we observed that (i) one-third of patients do not adhere to glucose-lowering drugs 12 months after its prescription; (ii) non-adherence is associated with worse glycemic control and higher likelihood of starting insulin, and (iii) most important predictors of non-adherence are: non-Spanish nationality; currently working; taking biguanides; smoker; without hypertension; and shorter time with diabetes.
Box 1. List of predictors considered†.
Socioeconomic and patient-related factors: sex, age, nationality, occupational status, and social deprivation index.
Medication-related factors: medication class, number of drugs at the time of initiation of the index GLD, number of GLD when starting the index GLD, number of psychoactive drugs when starting the index GLD, mean adherence (%) to chronic prescriptions (including codes C01, C02, C03, C04, C07, C08, C09, C10), and mean adherence to previous GLD during the 13 months prior to the initiation of the index GLD.
Condition-related factors: time from T2DM diagnosis to prescription of the index GLD, clinical complexity (adjusted morbidity group), smoking status, alcohol consumption, diagnosis of hypertension, hypercholesterolaemia, asthma, neoplasm, heart diseases, mental diseases, chronic kidney insufficiency, obesity, and retinopathy.
†Predictors classified according to the framework proposed by Peh et al. 2021 [Citation14]
GLD: Glucose‐Lowering Drug, T2DM, type 2 diabetes mellitus
Author contributions
IRC conceived the study design. ALR, JR and JK performed all analyses. RZC and IRC wrote the initial draft. All the authors aided interpretation of findings, edited and commented on the manuscript, as well as approved the final article.
Supplemental Material
Download MS Word (28.7 KB)Supplemental Material
Download MS Word (39.8 KB)Supplemental Material
Download MS Word (28.9 KB)Supplemental Material
Download MS Word (29.3 KB)Supplemental Material
Download MS Word (29.2 KB)Supplemental Material
Download MS Word (29.2 KB)Supplemental Material
Download MS Word (28.4 KB)Supplemental Material
Download MS Word (28.2 KB)Supplemental Material
Download MS Word (28.8 KB)Supplemental Material
Download MS Word (29.4 KB)Supplemental Material
Download MS Word (35.6 KB)Supplemental Material
Download MS Word (28.3 KB)Supplemental Material
Download MS Word (26.4 KB)Acknowledgements
We thank Dr. Antonio Colom, for his support with the obtention of patients’ deprivation index data. We thank Pau Pericás and Juan José Tomás Ivorra from the Research in Health Information Platform (Health Research Institute of the Balearic Islands (IdISBa)) for their support with the extraction of the data used in this study. We thank Dr. Escarlata Angullo and Dr. Isabel Socias (primary care doctors with large expertise in diabetes) and Rosa Ortuño for their support in validating the data extraction. We are grateful to Professor Andrew Farmer (University of Oxford) for his ideas and insight in the initial development of this work.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- IDF. International Diabetes Federation. 10th ed. Brussels, Belgium: IDF Diabetes Atlas; 2021. [cited 2023 Oct 4]. Available from: https://www.diabetesatlas.org.
- Draznin B, Aroda VR, Bakris G, et al. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–s143. doi: 10.2337/dc22-S009.
- De Geest S, Zullig LL, Dunbar-Jacob J, et al. ESPACOMP medication adherence reporting guideline (EMERGE). Ann Intern Med. 2018;169(1):30–35. doi: 10.7326/m18-0543.
- Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32(6):725–737. doi: 10.1111/dme.12651.
- Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–1296. doi: 10.1185/03007995.2015.1053048.
- Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836.
- Brunton SA, Polonsky WH. Medication adherence in type 2 diabetes mellitus: real-world strategies for addressing a common problem. J Fam Pract. 2017;66(4 Suppl):S46–S51.
- De León AC, Del Castillo Rodrí Guez JC, Coello SD, et al. Lifestyle and treatment adherence of type 2 diabetes mellitus people in the Canary Islands. Rev Esp Salud Publica. 2009;83(4):567–575.
- Gutiérrez-Angulo ML, Lopetegi-Uranga P, Sánchez-Martín I, et al. Cumplimiento terapéutico en pacientes con hipertensión arterial y diabetes mellitus 2. Rev Calid Asist. 2012;27(2):72–77. doi: 10.1016/j.cali.2011.09.008.
- López-Simarro F, Brotons C, Moral I, et al. Inertia and therapeutic compliance in patients with type 2 diabetes mellitus in primary care. Med Clin. 2012;138(9):377–384. doi: 10.1016/j.medcli.2011.07.023.
- Márquez Contreras E, Martell Claros N, Gil Guillén V, et al. Therapeutic compliance with insulin in the treatment of type 2 diabetes mellitus: CUMINDIAB study. Aten Primaria. 2012;44(2):74–81. doi: 10.1016/j.aprim.2010.11.013.
- Piñeiro F, Gil V, Donis M, et al. Relationship between medical treatment compliance and the degree of control in patients with high blood pressure, non-insulin dependent diabetes mellitus and dyslipidemia. Med Clin. 1998;111(15):565–567.
- Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016;42(1):34–71. doi: 10.1177/0145721715619038.
- Peh KQE, Kwan YH, Goh H, et al. An adaptable framework for factors contributing to medication adherence: results from a systematic review of 102 conceptual frameworks. J Gen Intern Med. 2021;36(9):2784–2795. doi: 10.1007/s11606-021-06648-1.
- Barba EL, Mijares AH, Moreno FA, et al. Medication adherence and persistance in type 2 diabetes mellitus (T2dm): patients perspective in the Spanish health care system. Value Health. 2016;19(7):A351.
- Gomez-Peralta F, Fornos Pérez JA, Molinero A, et al. Adherence to antidiabetic treatment and impaired hypoglycemia awareness in type 2 diabetes mellitus assessed in Spanish community pharmacies: the ADHIFAC study. BMJ Open Diabetes Res Care. 2021;9(2):e002148. doi: 10.1136/bmjdrc-2021-002148.
- Llorca CV, Cortés Castell E, Ribera Casado JM, et al. Factors associated with non-adherence to drugs in patients with chronic diseases who go to pharmacies in Spain. Int J Environ Res Public Health. 2021;18(8):4308. doi: 10.3390/ijerph18084308.
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218.
- Duque I, Domínguez-Berjón MF, Cebrecos A, et al. Deprivation index by enumeration district in Spain, 2011. Gac Sanit. 2021;35(2):113–122. doi: 10.1016/j.gaceta.2019.10.008.
- Akaike H. Information theory and the maximum likelihood principle. In: Petrov BN, Csäki F, editors. 2nd International Symposium on information theory. Budapest: akailseoniai-Kiudo.
- Schwarz G. Estimating the dimension of a model. Ann. Statist. 1978;6(2):461–464. doi: 10.1214/aos/1176344136.
- Rubin DB. Multiple imputation for survey nonresponse. New York: John Wiley and Son; 1987.
- Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med. 2008;27(17):3227–3246. doi: 10.1002/sim.3177.
- Brier GW. Verification of forecasts expressed in terms of probability. Mon Wea Rev. 1950;78(1):1–3. doi: 10.1175/1520-0493(1950)078<0001:VOFEIT>2.0.CO;2.
- Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2.
- Moreno Juste A, Gimeno Miguel A, Poblador Plou B, et al. Adherence to treatment of hypertension, hypercholesterolaemia and diabetes in an elderly population of a Spanish cohort. Med Clin. 2019;153(1):1–5. doi: 10.1016/j.medcli.2018.10.023.
- Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose-Lowering therapies and associations with 1-year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258–263. doi: 10.2337/dc15-1194.
- McGovern A, Tippu Z, Hinton W, et al. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(4):1040–1043. doi: 10.1111/dom.13160.
- Ogundipe O, Mazidi M, Chin KL, et al. Real-world adherence, persistence, and in-class switching during use of dipeptidyl peptidase-4 inhibitors: a systematic review and meta-analysis involving 594,138 patients with type 2 diabetes. Acta Diabetol. 2021;58(1):39–46. doi: 10.1007/s00592-020-01590-w.
- Vlacho B, Mata-Cases M, Mundet-Tudurí X, et al. Analysis of the adherence and safety of second oral glucose-lowering therapy in routine practice from the Mediterranean area: a retrospective cohort study. Front Endocrinol. 2021;12:708372. doi: 10.3389/fendo.2021.708372.
- Dankers M, Nelissen-Vrancken M, Hart BH, et al. Alignment between outcomes and minimal clinically important differences in the Dutch type 2 diabetes mellitus guideline and healthcare professionals’ preferences. Pharmacol Res Perspect. 2021;9(3):e00750. doi: 10.1002/prp2.750.
- Monami M, Liistro F, Scatena A, et al. Short and medium-term efficacy of sodium glucose co-transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(5):1213–1222. doi: 10.1111/dom.13221.
- McSharry J, McGowan L, Farmer AJ, et al. Perceptions and experiences of taking oral medications for the treatment of type 2 diabetes mellitus: a systematic review and meta-synthesis of qualitative studies. Diabet Med. 2016;33(10):1330–1338. doi: 10.1111/dme.13152.
- Shields BM, Hattersley AT, Farmer AJ. Identifying routine clinical predictors of non-adherence to second-line therapies in type 2 diabetes: a retrospective cohort analysis in a large primary care database. Diabetes Obes Metab. 2020;22(1):59–65. doi: 10.1111/dom.13865.
- Clifford S, Perez-Nieves M, Skalicky AM, et al. A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Curr Med Res Opin. 2014;30(6):1071–1085. doi: 10.1185/03007995.2014.884491.
