ABSTRACT
Aging is associated with a number of alterations to cerebrovascular function. We aimed to investigate the effect of age on cerebrovascular responses to cognitive stimulation using an objective two-parameter method.
Previously derived from a large data-set (135 healthy participants) were applied to a task-activated dataset of 69 healthy participants in five different task conditions. Cumulative response rate (CRR) was calculated as the sum of responses across tasks and hemispheres.
There was a significant effect of age (adjusted odds ratio: 1.02 (95% confidence interval: 1.01, 1.04), p = 0.016). There was also a significant effect of task (p = 0.002), but there was no significant interaction between age and task (p = 0.37). Increasing age was associated with increased CRR (adjusted odds ratio: 1.04 (95% confidence interval: 1.01, 1.07), p = 0.009).
Using an objective two-parameter method, healthy older adults had increased cerebrovascular responses to cognitive testing.
1. Introduction
The world population is aging and by 2050 almost 25% of individuals will be aged over 60 years (Organisation, W. H., Citation2018). Given this rise, the prevalence of age-related diseases is also anticipated to increase significantly in the coming years (Organisation, W. H., Citation2018). In particular, the number of individuals living with dementia is projected to increase significantly (Prince et al., Citation2015). Thus, understanding the normal age-related physiological changes in brain function is integral to developing strategies to promote and sustain healthy brain aging.
Healthy aging is associated with a number of key changes in cerebral function (Murman, Citation2015). Healthy older adults demonstrate reduced hemispheric lateralization to cognitive tasks (Cabeza, Citation2002), posterior-anterior shift of cognitive processes (McCarthy et al., Citation2014), and variable changes to the magnitude of the response to cognitive stimulation (Beishon et al., Citation2018; Jamadar, Citation2020; Nowak-Flück et al., Citation2018; Vermeij et al., Citation2014). These changes were initially thought to be compensatory, given that cognitive function was comparable to younger adults despite these differences (Berlingeri et al., Citation2013; Reuter-Lorenz & Cappell, Citation2008). However, recent studies contradict this traditional view and posit that changes may actually be maladaptive, reflecting loss of efficiency and specialization of cognitive processes (Morcom & Henson, Citation2018; Myrum, Citation2019). In the longer term, this may be counter-productive, where prolonged exposure to an excitatory environment can result in neuronal damage and loss (Merlo et al., Citation2019). Therefore, understanding the changes in cerebral physiology that occur with aging are important to determine whether these features (e.g. hyperactivation, reduction in asymmetric activation, posterior-anterior shift) should be enhanced or reduced to protect brain health and function in the longer term. The Scaffolding Theory of Aging and Cognition Revised (STAC-r), suggests that cognitive function is modulated over the life-span by a series of enriching or depleting factors (neural challenges) which will modulate cognitive function, and alter the susceptibility to aging and disease processes (Reuter-Lorenz & Park, Citation2014). By increasing the number of enriching factors (e.g. education, exercise, stimulation or engagement), and reducing the depleting factors (e.g. atrophy, white matter changes, depression and stress), compensatory scaffolding can be built through neurogenesis, additional neurone recruitment, and reorganization of brain processes (structurally and functionally) to improve cognitive performance (Reuter-Lorenz & Park, Citation2014).
Neurovascular coupling (NVC) is a key process which maintains cerebral perfusion at times of increased neuronal activity, and thus meeting metabolic demand (Hosford & Gourine, Citation2019; Iadecola, Citation2017). Disruption of NVC processes will result in hypoperfusion, particularly at critical moments of increased cognitive activity where function is required to be maximal (Iadecola, Citation2017). Studies have thus far found conflicting effects of aging on NVC processes with increased (Beishon et al., Citation2018; Csipo et al., Citation2019; Grinband et al., Citation2017; Jamadar, Citation2020), reduced (Fabiani et al., Citation2014; Lipecz et al., Citation2019; Nowak-Flück et al., Citation2018; West et al., Citation2019) and comparable (Madureira et al., Citation2017; Stefanidis et al., Citation2019) levels of activation and perfusion with younger individuals. However, this may be as a result of variations in methodologies and techniques, lack of correction for brain volume loss, and different cognitive paradigms used to stimulate responses.
Recently, we developed a novel, objective method to classify the presence or absence of a cerebral response to cognitive testing using a large, healthy normative dataset based on cerebral blood flow velocity (CBFv) responses from transcranial Doppler ultrasonography (TCD) measured task-activation studies (Beishon et al., Citation2020). This method uses two parameters: cross correlation function peak (CCF) and the variance ratio (VR) (Beishon et al., Citation2020). CCF describes the correlation of the individual CBFv signal with the population coherent average in response to task activation (Beishon et al., Citation2020). The CCF approach one represents highly correlated individual and population signals, indicating the presence of a robust CBFv response to stimulation (Beishon et al., Citation2020). VR describes the ratio of variance in the data pre- and post-stimulus (Beishon et al., Citation2020). Where a CBFv response is present, the variation post-stimulation will be significantly higher than that before the stimulus. Importantly, the combined CCF and VR model provides a number of advantages over traditional methods of NVC with fewer partial volume effects, and a reduced susceptibility to the effects of CO2, blood pressure, and baseline artifacts (Beishon et al., Citation2020).
To date, no study has utilized this novel method to characterize age-related effects on task-activated CBFv responses. Therefore, the aim of this study was to apply this two-criteria method to determine age-related effects on CBFv response rates to cognitive stimulation.
2. Materials and methods
2.1 Participants
Data were extracted on 69 healthy volunteers (age range: 21–83 years) from the Cerebral Hemodynamics in Aging and Stroke Medicine (CHiASM) research database (Patel et al., Citation2016). Healthy volunteers were free from comorbidities or medications that would adversely affect cognitive function, but stable or well-controlled comorbidities common in otherwise healthy and active older people (e.g. mild hypertension) were accepted for inclusion. All studies had ethics committee approval and volunteers provided written informed consent.
2.2 Data collection protocols
All studies were conducted in the CHiASM research space: a temperature controlled laboratory free from noise and distraction. Studies used similar methods and procedures for data collection (L. C. Beishon et al., Citation2017; Williams et al., Citation2017). All participants underwent bilateral continuous TCD-monitoring of CBFv in the middle cerebral artery (Vyasis Companion III or DWL Doppler Box), heart rate (3-lead ECG), blood pressure (BP, Finometer – Finapres Medical Systems; Amsterdam, the Netherlands), end tidal CO2 (nasal capnography, Capnocheck Plus). All participants completed a five-minute recording of resting parameters. Sixty-nine participants underwent a further task-activation protocol using five cognitive tasks from the Addenbrooke’s Cognitive Examination III (ACE-III) targeting attention, verbal fluency, language, visuospatial, and memory domains. There was a minute rest between each task to allow signals to return to baseline prior to the next task, and task initiation was marked with an event recorder. Data were stored in the PHYSIDAS acquisition system for offline analysis after data collection was completed. Signals were sampled at 500 samples/second. Data were analyzed offline using software developed by this group. Large, non-physiological spikes were removed by interpolation, and smaller spikes using a median filter, followed by a zero-phase low-pass Butterworth filter. Data were extracted on bilateral CBFv, heart rate, blood pressure and ETCO2. Resting and task activated data from this data-set have been published previously (Beishon et al., Citation2018).
2.3 Data analysis
The methods have been published in full previously (Beishon et al., Citation2020). CCF and VR thresholds were determined previously using 135 resting baseline files. Resting data from both right and left hemispheres were extracted on 270 hemispheres (age range: 19–83 years). These files represent resting, unstimulated baseline data, and therefore responses consistent with task activation should not be present. However, naturally occurring random fluctuations and variability in resting data will affect CCF and VR values. Therefore, in order to determine the amount of variability present at rest, we randomly marked resting data and extracted CCF and VR values for each of the random marks. For each 120s segment of data, with the random mark placed at the 60th second. Data segments of 40s (200 samples) were used, where 20s of data occurred prior to the mark, and 20s after the mark. CCF was calculated as the correlation between the individual and the population coherent average, and the peak value was detected in the first 4s after the mark. For task-activated data this is the point at which maximal stimulation typically occurs in the CBFv response. VR was calculated as the ratio of variance pre- and post-point of synchronization (mark). CCF90 and VR90 thresholds were determined from the upper 90th confidence limits of the randomly marked resting files. Although the 95th limit is more commonly used, here we used the 90th confidence limit to adopt a compromise to increase the yield of detection for smaller task-activated responses. Normative threshold (CCF≥0.53 and VR≥2.59) derived from a previous analysis (Beishon et al., Citation2020) were applied to the task-activated data-set. Where CCF or VR exceeded the threshold to a given task, a response was considered present. CCF and VR were calculated for each task as described above using the event-recorded mark as the point of synchronization. The cumulative response rate (CRR) was calculated as the sum of responses to each task in each hemisphere, giving a total score out of 10, to provide a summary of an individual’s response status.
2.4 Statistical analyses
Data were tested for normality. Nominal data are reported as number and percentage with statistical significance by Chi-square testing. Continuous data are presented as mean and standard deviation (parametric) or median and interquartile range (non-parametric). Statistical analyses were performed using Mann–Whitney U or Kruskal–Wallis tests for non-parametric data. To examine the effect of age, sex, and BP on response to each task (repeated measures across five tasks), logistic regression with generalized estimating equations (GEE) was used. The data was clustered within participants, with a logit link for binomial distribution. An exchangeable correlation matrix was used to account for cluster level confounding. Age and BP were included as covariates, and task, hemisphere and sex as factors. Data are presented as odds ratio (OR) with 95% confidence interval (CI). Significance testing was by Wald Chi-square. Tests were significant where p < 0.05. Bonferroni correction was applied to multiple comparisons for post-hoc tests of task and hemisphere. An interaction term was included in the model to examine for an interaction between age and task. To examine the potential confounding effect of BP, the results were also analyzed in normotensive subjects and resting BP (averaged over 5 minutes from beat-to-beat noninvasive BP and calibrated with brachial BP) was included as a co-variate in the adjusted analyses. Multiple linear regression methods were used to investigate the predictive effect of BP and age on resting CBFv. Ordinal logistic regression (OLR) was used to determine the association of age with CRR, corrected for BP and sex. The association between cognitive function and CRR by age was tested using Spearman correlation. Where age could not be considered as a continuous variable (correlation of CRR with cognitive test scores and the distribution by age group), we have divided age into three groups as follows: younger (39 years and under, n = 26), mid-life (40–59 years, n = 18), and older (60 years and over, n = 25).
3. Results
3.1 Demographics
The median age of the task-activated data-set was older than the resting data set (53 vs 34 years), but comparable between the task-activated and normotensive data-sets (). The proportion of females was higher amongst the resting data-set (69%) than the task-activated data-set, but comparable between task-activated and normotensive data-sets (). Total ACE-III score did not differ between task-activated and normotensive data-sets ().
Table 1. Demographics of the baseline, task-activated, and normotensive only datasets. Data are median [range], mean (standard deviation), or number (percentage). Significance testing by Chi-square (nominal data) or Mann–Whitney U (continuous data) between task-activated and normotensive only data-sets. Significant where p < 0.05
On multiple linear regression, age and blood pressure were not associated with resting CBFv in either hemisphere ().
Table 2. Multiple linear regression of age and blood pressure (BP) on cerebral blood flow velocity (CBFv) in the non-dominant and dominant hemispheres. CI = confidence interval. Statistically significant where p < 0.05
Table 3. Correlation (Spearman) between cumulative response rate (CRR) and cognitive test score by age group. Cognitive test scores are Median [inter-quartile range]
Table 4. Median cumulative response rate (CRR) and [inter-quartile range] by age group. Significance testing across groups by Kruskal-Wallis
3.2 Age-related differences in response rate
3.2.1 Whole-group analyses
On GEE analysis, there was a significant effect of age (OR:1.02 (95% CI: 1.01, 1.04), X2(1, 5.81), p = 0.016), but not BP (OR: 1.01 (95% CI: 0.98, 1.03), X2(1, 0.44), p = 0.51), or sex (OR: 1.09 (95% CI: 0.52, 2.25), X2(1, 0.05), p = 0.74) on response to task activation. There was also a significant effect of task (X2(4, 16.79), p = 0.002), but not hemisphere (X2(1, 1.65), p = 0.20). On post-hoc tests, there were significant differences between attention and memory (p < 0.005) tasks. When an interaction term for task and age was included in the model, there was no significant interaction between age and task (X2(4, 4.20), p = 0.38).
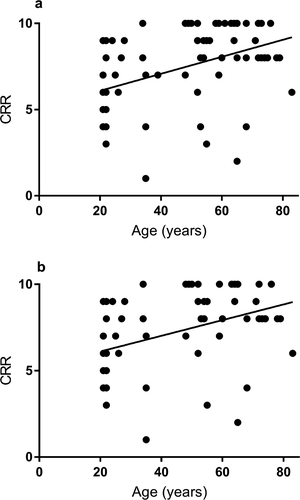
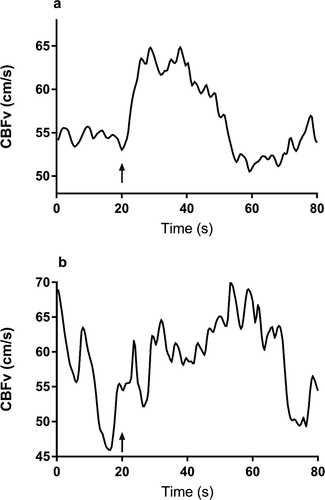
Age was moderately correlated with CRR on Spearman correlation (r = 0.41, p < 0.0005) (). The difference in CRR by age group is shown in . On OLR analysis, an increase in age (years) was associated with higher CRR to task activation (OR: 1.04 (95% CI: 1.02, 1.07), p < 0.005). This finding remained significant after adjustment for sex and BP (OR: 1.04 (95% CI: 1.01, 1.07), p = 0.009). Thus, for every year increase in age there is a 4% increase in CRR, equating to an increase in 2.4 task-activated responses over 60 years. demonstrates a typical “present” response in an older adult and “absent” response in a younger participant to task-activation.
3.2.2 Normotensive-only group analyses
On GEE analysis, there was a significant effect of age (OR:1.02 (95% CI: 1.00, 1.04), X2(1, 5.00), p = 0.025), but not BP (OR: 1.01 (95% CI: 0.98, 1.04), X2(1, 0.38), p = 0.54), or sex (OR: 0.94 (95% CI: 0.45, 1.96), X2(1, 0.03), p = 0.86) on response to task activation. There was also a significant effect of task (X2(4, 14.1), p = 0.007), but not hemisphere (X2(1, 1.10), p = 0.29). On post-hoc tests, there were significant differences between the attention and fluency (p = 0.007) and attention and memory tasks (p < 0.005). When an interaction term for task and age was included in the model, there was no significant interaction between age and task (X2(4, 4.84), p = 0.30).
Age was moderately correlated with CRR in the normotensive group (r = 0.39, p = 0.002), . On OLR analysis, an increase in age (years) was associated with higher CRR to task activation (OR: 1.4 (95% CI: 1.01, 1.06), p = 0.002). This finding remained significant after adjustment for sex and BP (OR: 1.04 (95% CI: 1.004, 1.07), p = 0.026).
3.2.3 Distribution of CRR by age groups
CRR was significantly different when compared across three age groups: young (under 40 years), median 6 responses [interquartile range: 4.25–8], mid-life (40–59 years), median 9 response [interquartile range: 7.25–10], and older (over 59 years), median 8 responses [interquartile range: 8–10], p < 0.005) (). Distribution remained the same in the normotensive cohort (p = 0.002).
3.4 Correlation of response rate with cognitive performance
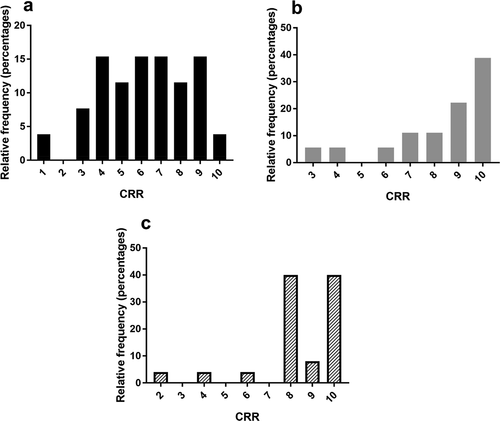
Language was positively correlated with CRR in the whole cohort (r = −.30, p = 0.013) (). On sub-group analysis, only performance in the memory task was significantly different across the three groups (p = 0.032). In the sub-group analysis, only performance in the memory task was moderately, negatively correlated with CRR in younger (under 40 years) adults (r = −0.42, p = 0.033) (). In adults in mid-life (40–59 years), performance in attention, language, and visuospatial tasks were moderately positively correlated with CRR (). In older adults (aged over 59 years), there were no significant correlations between CRR and cognitive test score ().
4. Discussion
4.1 Main findings
In summary, there was a significant effect of age on the response rate to cognitive stimulation. For every one-year increase in age, there was a 4% rise in response rate as measured by CRR, resulting in an increase of 2.4 responses between the ages of 20 and 80. These results were not influenced by effects of age-related rises in BP on adjusted analyses. Furthermore, there was a significant difference between attention and memory tasks, although this was not modulated by age. Finally, correlation analyses showed that in younger participants (aged under 40 years), reduced response rate to task-activation was associated with improved performance in memory tasks. Where-as in mid-life (aged 40–59 years), this association was reversed for attention, language, and visuospatial tasks, with loss of these associations in the older age group (aged over 60 years). Below we consider the mechanisms by which age may modulate vascular responses to cognitive stimulation.
4.2 Context of literature
This is the first study to apply a novel two-parameter method to examine age-related effects on response rate to task-activation. The association of increased vascular response rate with increasing age to cognitive activation is consistent with a number of previous studies (Beishon et al., Citation2018; Csipo et al., Citation2019; Jamadar, Citation2020). Studies investigating age-related effects using TCD-measured task activation have produced mixed findings. In a previous study by this group examining peak percentage change in CBFv response to similar cognitive tasks, CBFv responses to memory and visuospatial tasks were increased in older participants (age ≥50 years) compared to younger adults (Beishon et al., Citation2018). Similarly, Sorond et al. found increased CBFv responses to word stem and visual search tasks, with loss of regional activation selective to the language task (Sorond et al., Citation2008). However, two studies of working memory (Madureira et al., Citation2017) and reading (Stefanidis et al., Citation2019) based tasks did not find any age-related differences on CBFv response. Several TCD-based studies investigating motor (Orlandi & Murri, Citation1996), visual (Flück et al., Citation2014; Nowak-Flück et al., Citation2018; Zaletel et al., Citation2005) and working memory (Vermeij et al., Citation2014) found reduced rather than increased responses.
There are a number of reasons for these conflicting results between studies. Firstly, studies used different cognitive tasks, targeting different brain regions which may be differentially affected by aging processes. For example, studies using predominantly visual paradigms, or those targeting the posterior cortex, may be more likely to identify reduced CBFv responses due to posterior-anterior shift in processes that occurs in older relative to younger adults (McCarthy et al., Citation2014). Similarly, tasks which focus on the frontal cortex (executive function, working memory) may be more likely to identify increased CBFv responses as a result of this phenomenon (McCarthy et al., Citation2014). In the results reported here, there was no significant interaction between task and age. On pairwise comparisons, the differences in response to stimulation were most pronounced between attention and memory tasks. Interestingly, behavioral performance was only different amongst the three age groups in the memory tasks, and not in other cognitive domains. Therefore, the difference in CBFv response to task-activation may be due to declining performance in the memory tasks in older adults. Certainly, the correlation analysis suggests that in younger adults, improved performance in cognitive tasks is associated with a lower CBFv response rate to testing, and this pattern is reversed in mid-life and lost in later life.
However, different tasks may be differentially susceptible to aging processes. The ability to perform simple attention tasks (as in this study) is maintained up to 80 years of age, and age-related declines are only seen in more complex tasks requiring selective or divided attention (Murman, Citation2015). In contrast, memory is more susceptible to changes with increasing age, in particular, for retrieval of newly encoded information (as used in this study) (Murman, Citation2015). Thus, differences in CBFv response to different cognitive tasks may be as a result of selective susceptibility of certain cognitive processes to the effects of age.
The significance of the increased response rate in older, compared to younger adults, remains unclear. Early theories of cognitive aging suggested that age-related changes in brain function were compensatory, allowing older adults to recruit additional neural circuits to maintain performance in line with that of younger adults (Berlingeri et al., Citation2013; Cabeza, Citation2002; Reuter-Lorenz & Cappell, Citation2008). In the results reported here, increased response rate was only associated with better cognitive performance in mid-life. An inverse relationship existed between response rate and cognitive performance in younger adults, and there was no relationship between performance and CBFv response in older adults. In younger adults, a negative relationship between response rate and performance may indicate improved processing efficiency, and reduced resource requirement (Reuter-Lorenz & Park, Citation2014). Whereas, in mid-life, increased blood flow responses could be compensatory given the association with better cognitive performance in three of the ACE-III sub-domains (Cabeza et al., Citation2018). In this study, there was complete loss of all association between CBFv response and cognitive performance in older adults, which could suggest failure of these compensatory mechanisms utilized in mid-life. This was further indicated by differences in memory performance across the three groups, and it has been suggested that an over-reliance on compensatory mechanisms in mid-life may be associated with poorer cognitive function in older age (Reuter-Lorenz & Park, Citation2014). In a study by Jamadar et al, older adults demonstrated increased responses to functional magnetic resonance imaging (fMRI) measured activation to a working memory task (Jamadar, Citation2020). The increased responses were only apparent at the higher difficulty levels when compared to younger adults (Jamadar, Citation2020), contradictory to the widely accepted Compensation-Related Utilisation of Neural Circuits Hypothesis (CRUNCH) (Jamadar, Citation2020). CRUNCH proposes that hyperactivation will be seen at lower levels of cognitive load until the compensatory capacity is exceeded, at which point younger adults will show increased activation relative to older adults (Berlingeri et al., Citation2013; Jamadar, Citation2020). Similarly, Morcom et al. demonstrated increased prefrontal activation in response to memory tasks in older adults was consistent with reduced processing efficiency rather than compensation (Morcom & Henson, Citation2018). Delineating compensation from impaired efficiency is critical to understand whether age-related changes are beneficial, or potentially harmful in the longer term. Certainly, hyperactivated states are associated with increased levels of excitatory neurotransmitters which can result in neurotoxicity in the longer term (Merlo et al., Citation2019). Longitudinal studies of aging would be beneficial to determine whether hyperactivation is predictive of future cognitive risk.
4.3 Strengths, limitations and future directions
The strength of this study is the application of an objective two-parameter method to classify responses as “present” or “absent” to cognitive stimulation. This novel method is advantageous as it is less susceptible to artifacts and confounding by concomitant changes in blood pressure and CO2. A strength of this study is the derivation of thresholds from a large (270 hemispheres), normative sample. Although 95th confidence limits are typically used, we used the 90th confidence limit of CCF and VR to improve the sensitivity of detecting smaller CBFv responses, although this will incorrectly classify some participants as responders which is due to variability rather than a CBFv response. This may have resulted in the classification of artifact or “noise” as a CBFv response. The distribution of the included ages was limited and was not equally distributed across the decades. The 69 participants included in the task-activation study were derived from the larger sample used to set the thresholds and therefore represent dependent rather than independent samples. TCD is limited by poor spatial resolution, and age-related effects could not be localized to specific regions. Future studies should include measurements with higher spatial resolution to investigate this further. Finally, it remains unclear whether age-related increases in brain activation and vascular responses are compensatory or maladaptive. Further work is required to determine this, and longitudinal studies may be able to better identify the longer term associations of cognitive function with specific patterns of brain activation.
5. Conclusions
In conclusion, age-related differences were demonstrated in vascular response rate to cognitive stimulation using a novel method to classify response status, which is independent of changes occurring in blood pressure with age. Improved cognitive performance was associated with reduced response rate in younger adults, but the reverse was true in mid-life, and loss of all association in older age. This could reflect differences in brain efficiency and compensation occurring at different stage of the life-span. Future work is required to investigate the regional differences using this method, and to determine whether age-related increases in vascular response are beneficial or harmful to cognitive function.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Beishon, L., Minhas, J. S., Patrick, K., Shanmugam, I., Williams, C. A. L., Panerai, R. B., … Haunton, V. J. (2018). The effects of healthy ageing on cerebral blood flow responses to cognitive testing. Current Aging Science, 11(4), 226–235. https://doi.org/10.2174/1874609812666190131165310
- Beishon, L. C., Williams, C. A., Intharakham, K., Batterham, A. P., Barnes, S. C., Haunton, V. J. & Panerai, R. B. (2020). An objective method to identify non-responders in neurovascular coupling testing. J Neurosci Methods, 341, 108779. doi: 10.1016/j.jneumeth.2020.108779
- Beishon, L. C., Williams, C. A. L., Panerai, R. B., Robinson, T. G., & Haunton, V. J. (2017). The assessment of neurovascular coupling with the addenbrooke’s cognitive examination: A functional Transcranial Doppler Ultrasonographic Study. J Neurophysiol. 2018 Mar 1;119(3):1084-1094. https://doi.org/10.1152/jn.00698.2017
- Berlingeri, M., Danelli, L., Bottini, G., Sberna, M., & Paulesu, E. (2013). Reassessing the HAROLD model: Is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 224(3), 393–410. https://doi.org/10.1007/s00221-012-3319-x
- Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. https://doi.org/10.1037/0882-7974.17.1.85
- Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., Lindenberger, U., Nyberg, L., Park, D. C., Reuter-Lorenz, P. A., Rugg, M. D., Steffener, J., & Rajah, M. N. (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. https://doi.org/10.1038/s41583-018-0068-2
- Csipo, T., Mukli, P., Lipecz, A., Tarantini, S., Bahadli, D., Abdulhussein, O., Owens, C., Kiss, T., Balasubramanian, P., Nyúl-Tóth, Á., Hand, R. A., Yabluchanska, V., Sorond, F. A., Csiszar, A., Ungvari, Z., & Yabluchanskiy, A. (2019). Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience, 41(5), 495–509. https://doi.org/10.1007/s11357-019-00122-x
- Fabiani, M., Gordon, B. A., Maclin, E. L., Pearson, M. A., Brumback-Peltz, C. R., Low, K. A., McAuley, E., Sutton, B. P., Kramer, A. F., & Gratton, G. (2014). Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. Neuroimage, 85, 592–607. https://doi.org/10.1016/j.neuroimage.2013.04.113
- Flück, D., Beaudin, A., Steinback, C., Kumarpillai, G., Shobha, N., McCreary, C., Peca, S., Smith, E. E., & Poulin, M. (2014). Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front Physiol. 2014 Feb 19;5:49. https://doi.org/10.3389/fphys.2014.00049
- Grinband, J., Steffener, J., Razlighi, Q. R., & Stern, Y. (2017). BOLD neurovascular coupling does not change significantly with normal aging. Human Brain mapping, 38(7), 3538–3551. https://doi.org/10.1002/hbm.23608
- Hosford, P. S., & Gourine, A. V. (2019). What is the key mediator of the neurovascular coupling response? Neurosci Biobehav Rev. 2019 Jan; 96:174-181. https://doi.org/10.1016/j.neubiorev.2018.11.011
- Iadecola, C. (2017). The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron, 96(1), 17–42. https://doi.org/10.1016/j.neuron.2017.07.030
- Jamadar, S. D. (2020). The CRUNCH model does not account for load-dependent changes in visuospatial working memory in older adults. Neuropsychologia, 2020 May;142:107446. https://doi.org/10.1016/j.neuropsychologia.2020.107446
- Lipecz, A., Csipo, T., Tarantini, S., Hand, R. A., Ngo, B. T. N., Conley, S., Nemeth, G., Tsorbatzoglou, A., Courtney, D. L., Yabluchanska, V., Csiszar, A., Ungvari, Z. I., & Yabluchanskiy, A. (2019). Age-related impairment of neurovascular coupling responses: A dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience, 41(3), 341–349. https://doi.org/10.1007/s11357-019-00078-y
- Madureira, J., Castro, P., & Azevedo, E. (2017). Demographic and systemic hemodynamic influences in mechanisms of cerebrovascular regulation in healthy adults. Journal of Stroke and Cerebrovascular Diseases, 26(3), 500–508. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.12.003
- McCarthy, P., Benuskova, L., & Franz, E. A. (2014). The age-related posterior-anterior shift as revealed by voxelwise analysis of functional brain networks. Neurosci. 2014 Nov 7;6:301. https://doi.org/10.3389/fnagi.2014.00301
- Merlo, S., Spampinato, S. F., & Sortino, M. A. (2019). Early compensatory responses against neuronal injury: A new therapeutic window of opportunity for alzheimer’s disease? CNS Neuroscience & Therapeutics, 25(1), 5–13. https://doi.org/10.1111/cns.13050
- Morcom, A. M., & Henson, R. N. A. (2018). Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. The Journal of Neuroscience, 38(33), 7303–7313. https://doi.org/10.1523/jneurosci.1701-17.2018
- Murman, D. L. (2015). The impact of age on cognition. Seminars in Hearing, 36(3), 111–121. https://doi.org/10.1055/s-0035-1555115
- Myrum, C. (2019). Is PASA passé?: Rethinking compensatory mechanisms in cognitive aging. The Journal of Neuroscience, 39(5), 786–787. https://doi.org/10.1523/jneurosci.2348-18.2018
- Nowak-Flück, D., Ainslie, P. N., Bain, A. R., Ahmed, A., Wildfong, K. W., Morris, L. E., Phillips, A. A., & Fisher, J. P. (2018). Effect of healthy aging on cerebral blood flow, CO2 reactivity, and neurovascular coupling during exercise. Journal of Applied Physiology, 125(6), 1917–1930. https://doi.org/10.1152/japplphysiol.00050.2018
- World Health Organisation. (2018). Ageing and health. World Health Organisation, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- Orlandi, G., & Murri, L. (1996). Transcranial doppler assessment of cerebral flow velocity at rest and during voluntary movements in young and elderly healthy subjects. International Journal of Neuroscience, 84(1–4), 45–53. https://doi.org/10.3109/00207459608987249
- Patel, N., Panerai, R. B., Haunton, V., Katsogridakis, E., Saeed, N. P., Salinet, A., Brodie, F., Syed, N., D’Sa, S., & Robinson, T. G. (2016). The leicester cerebral haemodynamics database: Normative values and the influence of age and sex. Physiological Measurement, 37(9), 1485–1498. https://doi.org/10.1088/0967-3334/37/9/1485
- Prince, M., Wimo, A., Guerchet, M., Ali, G. C., Wu, Y., & Prina, M. (2015). World alzheimer report 2015 the global impact of dementia alzheimer’s disease international. Alzheimer's Disease Internationa.
- Reuter-Lorenz, P. A., & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. https://doi.org/10.1111/j.1467-8721.2008.00570.x
- Reuter-Lorenz, P. A., & Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 24(3), 355–370. https://doi.org/10.1007/s11065-014-9270-9
- Sorond, F. A., Schnyer, D. M., Serrador, J. M., Milberg, W. P., & Lipsitz, L. A. (2008). Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex, 44(2), 179–184. https://doi.org/10.1016/j.cortex.2006.01.003
- Stefanidis, K. B., Askew, C. D., Klein, T., Lagopoulos, J., Summers, M. J., & Mogi, M. (2019). Healthy aging affects cerebrovascular reactivity and pressure-flow responses, but not neurovascular coupling: A cross-sectional study. PLOS ONE, 14(5), e0217082. https://doi.org/10.1371/journal.pone.0217082
- Vermeij, A., Meel-van Den Abeelen, A. S. S., Kessels, R. P. C., Van Beek, A. H. E. A., & Claassen, J. A. H. R. (2014). Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. Neuroimage. 2014 Jan 15;85 Pt 1:608-615. https://doi.org/10.1016/j.neuroimage.2013.04.107
- West, K. L., Zuppichini, M. D., Turner, M. P., Sivakolundu, D. K., Zhao, Y., Abdelkarim, D., Spence, J. S., & Rypma, B. (2019). BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage. 2019 Mar;188:198-207. https://doi.org/10.1016/j.neuroimage.2018.12.012
- Williams, C. A. L., Panerai, R. B., Robinson, T. G., & Haunton, V. J. (2017). Transcranial Doppler ultrasonography in the assessment of neurovascular coupling responses to cognitive examination in healthy controls: A feasibility study. J Neurosci Methods. 2017 Jun 1;284:57-62. https://doi.org/10.1016/j.jneumeth.2017.04.013
- Zaletel, M., Strucl, M., Pretnar-Oblak, J., & Zvan, B. (2005). Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Functional Neurology, 20(3), 115–120. DOI: 10.1016/j.neuroimage.2004.04.019.