Abstract
The leaves of Gynura procumbens (Merr.) Compositae, commonly called “sambung nyawa” in Malaysia, are often eaten raw with rice. The methanol extract was prepared from the dried leaves using a Soxhlet apparatus. The methanol extract was then fractionated into chloroform, ethyl acetate, n-butanol, and aqueous fractions using a separating funnel. In the current study, the antioxidant potency of G. procumbens extract and fractions were investigated, employing various established in vitro systems, such as trolox equivalent antioxidant capacity, β -carotene–linoleic acid model system, 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging, reducing power, and xanthine oxidase inhibitory activity. Based on the results obtained, the extract and fractions showed different antioxidant potential. Among the fractions, the ethyl acetate fraction displayed higher antioxidant properties. The contents of the potential antioxidant component of the extract and fractions were also determined using HPTLC densitometric and spectrophotometric (using Folin-Ciocalteu reagent) methods. HPTLC study revealed that the methanol extract and the ethyl acetate and n-butanol fractions contain 0.74% and 2.9%, 7.76% and 12.75%, and 4.52% and 0.33% of kaempferol-3-O-rutinoside and astragalin, respectively. The total phenolic content of the extract and fractions varied from 4.37% to 23.43% of dry weight, expressed as gallic acid equivalents (GAE). With further data analysis, it was found there was a significant correlation (p < 0.05) between the total phenolic content of the sample and its DPPH scavenging activity and reducing power with correlation coefficients (r) of 0.891 and 0.926, respectively. These results suggest that phenolics in these plants provide substantial antioxidant activity.
Introduction
Oxidation is an important process for living organisms. The uncontrolled production of oxygen free radicals and the unbalanced mechanism of antioxidant protection result in the onset of many diseases, namely cancer, diabetes, Alzheimer's disease, coronary heart diseases, and aging (CitationHertog et al., 1995; CitationKeli et al., 1996; CitationDeng et al., 1998; CitationGeleijnse et al., 1999, Citation2000; CitationErlund et al., 2001; CitationYang et al., 2004). Antioxidants are regarded as possible protection agents reducing oxidative damage in the human body. Therefore, there is a growing interest in the substances exhibiting antioxidant properties that are available to human and animal organisms as food components or as specific pharmaceutics. There are two basic categories of antioxidants, namely, synthetic and natural ones. Restriction on the use of synthetic antioxidants is being imposed because of their carcinogenicity (CitationGrice, 1986, Citation1988). Recently, natural antioxidants have become a major area of scientific research (CitationDemo et al., 1998; CitationSanchez-Moreno et al., 1999). Owing to safety concerns of synthetic antioxidants and effectiveness of natural antioxidants, the public prefers to take natural antioxidant sources from edible materials such as fruits, spices, herbs, and vegetables. Therefore, the development and use of more effective antioxidants of natural origin is desired.
Gynura procumbens (Merr.) (Compositae) is an annual evergreen shrub with a fleshy stem and purple tint. In Southeast Asia, especially Indonesia, Malaysia, and Thailand, the plant has been traditionally used for treatment of eruptive fevers, rash, kidney disease, migraines, constipation, hypertension, diabetes mellitus, and cancer (CitationPerry, 1980). Recently, pharmacologic studies reported that G. procumbens has anti–Herpes simplex virus, anti–hyperglycemic, anti–hyperlipidemic, anti–inflammatory, analgesic, and reduced blood hypertension properties (CitationLam et al., 1998; CitationNawawi et al., 1999; CitationAkowuah et al., 2001, Citation2002; CitationIskander et al., 2002). However, by reviewing the current literature, we know of no previous research on the antioxidant study of this plant. Therefore, experiments were carried out to test the antioxidant potential of G. procumbens. In the experiment, antioxidant properties of G. procumbens were assayed in terms of inhibitory ability on xanthine oxidase and β -carotene, scavenging abilities on 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′azino-bis(3-ethybenzthiazoline-6-sulfonic acid) (ABTS), and reducing power. The contents of the potential antioxidant components (kaempferol-3-O-rutinoside and astragalin) of the extract and fractions were also determined using HPTLC densitometric and spectrophotometric methods.
Materials and Methods
Chemicals and reagents
Polyoxyethylene sorbitan monopalmitate, β -carotene, xanthine oxidase, xanthine, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), ferric chloride (FeCl3), allopurinol, sodium phosphate (dibasic), sodium phosphate (monobasic), potassium persulfate, linoleic acid, 2,2′azino-bis(3-ethybenz-thiazoline-6-sulfonic acid), 1,1-diphenyl-2-picrylhydrazyl, Folin-Ciocalteu reagent, phosphate-buffered saline (PBS) (pH 7.4), quercetin, gallic acid, and sodium carbonate were purchased from Sigma (St. Louis, MO, USA). Trolox was purchased from Calbiochem (Darmstadt, Germany). Chloroform, petroleum ether (60–80°C), methanol, ethyl acetate, and n-butanol were purchased from Merck (Darmstadt, Germany). Potassium ferricyanide was purchased from R&M Marketing (Essex, UK). Kaempferol-3-O-rutinoside and astragalin (kaempferol-3-O-glucoside) were purchased from ChromaDex Inc. (California, USA).
Extraction and fraction procedure
The Gynura procumbens was obtained from Penang Island, Malaysia in (December 2004). A voucher specimen (no. 10833) was deposited at the herbarium of the School of Biological Sciences, Universiti Sains Malaysia, by a botanist. The leaves of G. procumbens were dried in an oven at 45°C and ground into powder. The dried powdered leaves (500 g) were first defatted with 5 L petroleum ether (60–80°C) and then further extracted with methanol in a Soxhlet apparatus. The methanol extract (ME) was concentrated by a rotary evaporator (Büchi-RE121, Flawil, Switzerland) under vacuum (Büchi-B169), and the concentrated extract was dried in a freeze-dryer (HETO-Hetovac VR-1, Birkerød, Denmark) (yield 12.2% of dried leaf). The ME was then resuspended in water and fractionated to chloroform fraction (CF), ethyl acetate fraction (EF), n-butanol fraction (BF), and aqueous fraction (AF) using a separating funnel. The yields of CF, EF, BF, and AF were 20.4%, 6.7%, 11.8%, and 60.8% of ME, respectively.
HPTLC procedure
Chromatography was performed on a preactivated (100°C) silica gel 60F254 TLC plate (20 × 10 cm; 0.25 mm layer thickness; Merck). The Camag densitometry (Camag Model-3 TLC scanner equipped with Camag CATS 4 software; Mutten, Switzerland) and a reflectance spectrometer (190–700 nm) were employed for the analysis. The slit was set to 8 × 0.4 mm, and data acquisition and processing were performed using winCATS software. Four-microliter samples were applied to the layer at 8-mm-width bands, positioned 10 mm from the the bottom of the plate, using a Camag Linomat IV automated TLC applicator with nitrogen flow providing delivery from the string at a speed of 10 μ L/s that of was maintained for all analyses. The HPTLC plates were developed in a Camag twintrough glass tank presaturated with the mobile phase [ethyl acetate:methanol:water (100:13.5:10)] for 2 h at room temperature (22–24°C). Solvent was allowed to run the plate to a height of 8 cm. After development, the TLC plate was dried, and the components were visualized by UV light at 365 and 254 nm for kaempferol-3-O-rutinoside and astragalin, respectively.
The quantitative determination was performed by winCATS software program using the external calibration method. The calibration curve was prepared with kaempferol-3-O-rutinoside and astragalin in the range from 1000 to 15.63 μ g/mL in methanol. The G. procumbens extract and fractions (10 mg/mL) in methanol were subjected to HPTLC analysis.
Assessment of total antioxidant activity
The total antioxidant activity (TAA) value was estimated by the trolox equivalent antioxidant capacity (TEAC) test (CitationRe et al., 1999; CitationYam et al., 2007). 2,2′Azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) was dissolved in deionized water to a 7 mM concentration. ABTS radical cation (ABTS. +) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature (22–24°C) for 12–16 h before use. The concentrated ABTS+ solution was diluted with PBS pH 7.4 to a final absorbance of 0.70 ± 0.02 at 734 nm at 30°C. Stock solution of trolox (0.0625, 0.125, 0.25, 0.5, 1, 2, and 4 mM) and G. procumbens extract and fractions were prepared in methanol. The spectrophotometer (Hitachi U-2000, Tokyo, Japan) was first blanked with PBS. Ten microliters of antioxidant-containing solution was added to 2 mL ABTS. + solution. The decrease of absorbance was measured at 734 nm 6 min after addition of the trolox and G. procumbens extract and fractions. All determinations were carried out in triplicate. The TEAC of the G. procumbens extract and fractions was calculated by relating this decrease in absorbance to that of a trolox solution on a molar basis.
Determination of antioxidant assay using the β -carotene–linoleic acid method
The procedure described by CitationSun and Ho (2005) was used for evaluating the antioxidant activity of the G. procumbens leaf extract and fractions. One milliliter of β -carotene (1 mg in 1 mL chloroform) was added to a conical flask with 20 mg linoleic acid and 200 mg polyoxyethylene sorbitan monopalmitate (Tween-40). Chloroform was removed under vacuum at 40°C (using a rotary evaporator), and the resultant mixture was diluted with 10 mL of water, mixed well, and followed by addition of oxygenated water (40 mL) to form a solution. The aliquots (4 mL) were pipetted into different test tubes containing 0.2 mL of extracts, quercetin, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and quercetin (0.2 mg/mL, in ethanol), respectively, and the absorbance was measured with a spectrometer (PerkinElmer Lambda 45, Massachusetts, USA) at 470 nm immediately and to 120 min (15-min intervals), against a blank solution without the β -carotene. All determinations were carried out in triplicate. The antioxidant activity (AA) was evaluated following the formula
where A0 and A00 are the absorbance value measured at zero time of the incubation for test sample and control, respectively, and At and At0 are the absorbance value measured after incubation for test sample and control, respectively. The results were expressed in percentage.
Determination of xanthine oxidase inhibition
The xanthine oxidase activities with xanthine as the substrate were measured spectrophotometrically, based on the procedure reported by CitationNoro et al. (1983) and CitationSweeney et al. (2001), with minimum modification. The extract, fractions (500 μ L of 0.1 mg/mL), and allopurinol (in methanol) were mixed with 1.3 mL phosphate buffer (pH 7.5) and 0.2 mL 0.2 unit/mL xanthine oxidase solution. After 10 min of incubation at room temperature (22–24°C), the mixture was added with 1.5 mL 0.15 M xanthine substrate solution. The mixture was incubated for 30 min at room temperature (22–24°C), and then the absorbance was measured at 293 nm using a spectrometer (PerkinElmer Lambda 45) against a blank (0.5 mL methanol, 1.3 mL phosphate buffer, 0.2 mL xanthine oxidase). The solution of 0.5 mL methanol, 1.3 mL phosphate buffer, 0.2 mL xanthine oxidase, and 1.5 mL xanthine substrate solution was used as a control. Each sample was measured in triplicate and was averaged. Percentage of inhibition was calculated using the formula
Where As and Ac are the absorbance value of test sample and control, respectively.
Reducing capacity
Reducing capacity was determined through a method used by CitationOyaizu (1986). One milliliter of different concentration of G. procumbens extract and fractions (0.25, 0.125, and 0.0625 mg/mL) (in methanol) was mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm (Eppendorf 5403, Engelsdorf, Germany) for 10 min. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 0.1%), and the absorbance was measured at 700 nm. Increased absorbance of the reaction indicated increased reducing power.
Determination of DPPH scavenging activity
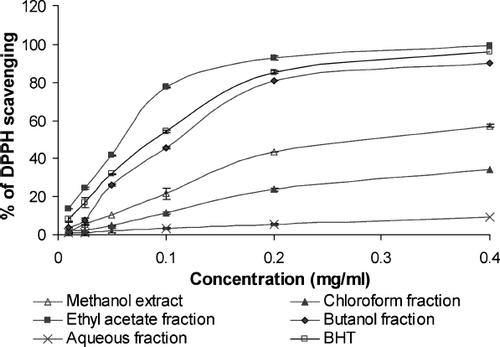
The free radical scavenging activity of Gynura procumbens extract, fractions, and BHT were measured in terms of hydrogen donating or radical scavenging ability using the stable DPPH (CitationKumaran & Karunakaran, 2006). One milliliter each of 0.025, 0.05, 0.1, 0.2, and 0.4 mg/mL Gynura procumbens extract, fractions, and BHT (in methanol) were placed in different tubes. To this mixture, 2 mL 0.1 mM DPPH was added. After 60 min of incubation at room temperature (22–24°C), absorbance was measured at 517 nm by using a spectrometer (PerkinElmer Lambda 45) against methanol as the blank. A control contained 1 mL methanol and 2 mL 0.1 mM DPPH methanol. Free radical scavenging activity of the extract and fractions was determined according to the following formula: Free radical scavenging activity (%) = (Ac - As)/Ac × 100 Where As is the absorbance of DPPH and sample, and Ac is the absorbance of control.
Determination of total phenolic content
The total phenolic content of the methanol extract and its fractions was determined by the Folin-Ciocalteu method with some modification (CitationSlinkard & Singleton, 1977). Five hundred microlitres of 1 mg/mL extract and fractions (in methanol) was added to 500 μ L 2 N Folin-Ciocalteu reagent. After 4 min of incubation at room temperature (22–24°C), 1 mL 20% (w/v) sodium carbonate was added followed by 6 mL distilled water. After 2 h of incubation at room temperature (22–24°C), absorbance of the mixture was measured at 760 nm using a spectrometer (PerkinElmer Lambda 45). A calibration curve, using gallic acid in a concentration range of 0.001–0.1 mg/mL, was prepared. The total phenolic content of the samples was expressed as gallic acid equivalents (GAE), which reflected the phenolic content as amount of gallic acid in sample. Experiments were performed in triplicate.
Statistical analysis
The experimental data were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by least significant difference (LSD) multiple range test was carried out to determined significant differences (p < 0.05), and correlation coefficients (r) were quantified by SPSS (version 10.01).
Results and Discussion
HPTLC analysis
The quantitative analysis of the G. procumbens extract and fractions, made using high performance thinlayer liquid chromatography coupled with Camag densitometry, is presented in , and representative chromatograms are presented in . Phenolic compounds were identified and quantified at 365 and 254 nm as kaempferol-3-O-rutinoside and astragalin, respectively. The results revealed that ME, EF, and BF contain 0.74%, and 2.9%, 7.76%, and 12.75%, and 4.52%, and 0.33% kaempferol-3-O-rutinoside and astragalin respectively. The chromatogram showed the kaempferol-3-O-rutinoside and astragalin with symmetrical peak, at Rf= 0.43 and Rf = 0.72, respectively.
Figure 1 HPTLC profiles (overly chromatograms) of methanol extract, ethyl acetate fraction, and butanol fraction. Eluent: ethyl acctate:methanol:water (100:13.5:10) (v/v). (a) Detection, 365 nm; (b) detection, 254 nm.
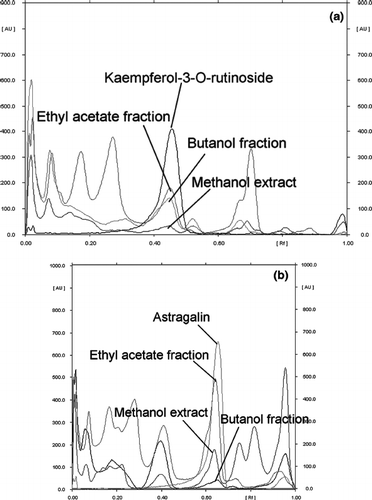
Table 1 Percentage of kaempferol-3-O-rutinoside and astragalin in G. procumbens extract and fractions.
Total antioxidant capacity
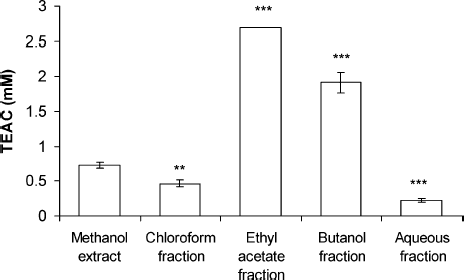
Reaction of the G. procumbens extract and fractions with ABTS radicals was examined. The TEAC assay is applied to assess the total amount of ABTS radical that can be scavenged by G. procumbens extract and fractions. The total antioxidant activity of G. procumbens extract and fractions was expressed as mM trolox equivalents. As shown in , EF and BF were 2.69 ± 0.004 and 1.92 ± 0.15 mM of trolox equivalent, respectively, resulting in significantly higher ABTS radical scavenging than that of methanol extract (p < 0.001). The antioxidant activity of putative antioxidants have been attributed to various mechanisms, namely, prevention of chain initiation, binding of transition metal ion catalyst, decomposition of peroxides, prevention of hydrogen abstraction, and radical scavenging (CitationDiplock, 1997; CitationYilidirim et al., 2001).
β-Carotene–linoleic acid antioxidant capacity
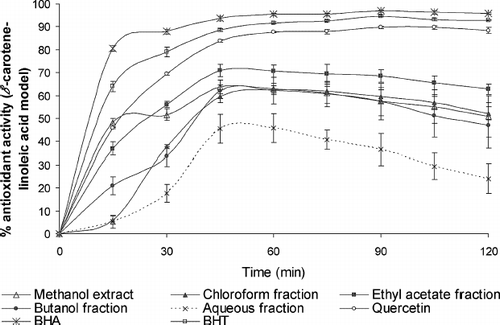
The antioxidant activity of G. procumbens extract and fractions, BHA, BHT, and quercetin, as measured by the bleaching of β -carotene, is presented in . It can be seen that G. procumbens extract and fractions exhibited varying degrees of antioxidant activity. The mechanism of bleaching of β -carotene is a free radical–mediated phenomenon resulting from the hydroperoxides formed from linoleic acid. β -Carotene, in this model system, undergoes rapid discoloration in the absence of antioxidant. The linoleic acid free radical, formed upon the abstraction of a hydrogen atom from one of its diallylic methylene groups, attacks the highly unsaturated β -carotene molecule. As β -carotene molecules lose their double bonds by oxidation, the compound loses it's chromophore (orange color). The antioxidant of the extract and fractions of G. procumbens and standard increased in the order BHA > BHT > quercetin > EF > CF > ME > BF > AF.
Xanthine oxidase inhibition
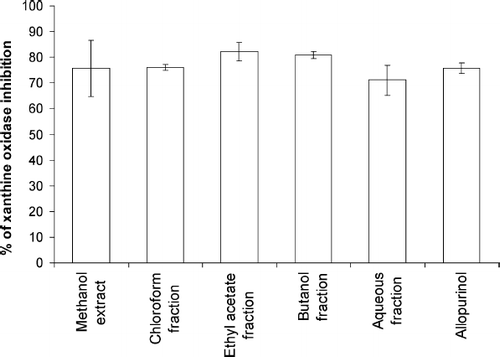
Xanthine oxidase is a highly versatile enzyme and widely distributed within the various tissues of mammals. It is an important source of oxygen free radicals. Xanthine oxidase catalyzes the reduction of O2, leading to the formation of superoxide (O2. −) and hydrogen peroxide (H2O2), and it has been proposed as a central mechanism of oxidative injury (CitationMassey et al., 1969; CitationMcCord, 1985; CitationZweier et al., 1988). The xanthine oxidase inhibitory ability of G. procumbens extract, fractions, and known standard compound, viz., allopurinol, is presented in . The extract and fractions of G. procumbens exhibited xanthine oxidase inhibitory activity. This activity increased up to 82.2%, 80.9%, and 76.0% after the crude extract was fractionated with ethyl acetate, n-butanol, and chloroform, respectively. There were no significant differences found between xanthine oxidase inhibition ability of G. procumbens extract or fractions and allopurinol. Xanthine oxidase inhibitory activity of the samples followed the order EF > BF > CF > allopurinol > ME > AF.
Reducing power
Reducing power assay is often used to evaluate the ability of an antioxidant to donate an electron (CitationDorman et al., 2003). The reducing capacity of a plant extract or compound may serve as a significant indicator of its potential antioxidant activity (CitationMeir et al., 1995). Previous studies also reported that there is a direct correlation between the antioxidant effect and reducing capacity of some plant extracts (CitationYilidirim et al., 2001).
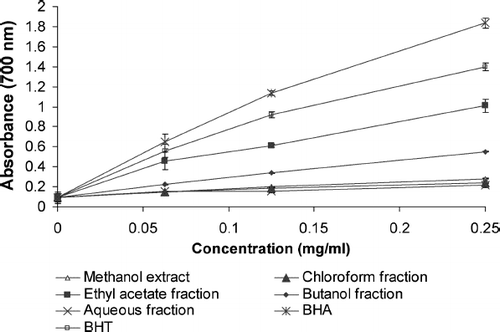
shows the reductive capabilities of G. procumbens extract and fractions compared with BHA and BHT. Reducing power of G. procumbens extract and fractions and standard compounds followed the order BHA > BHT > EF > BF > CF > ME > AF. According to the results in the current study, it is suggested that EF is a good electron donor and has a potency to convert the free radicals to nonreactive form and terminate the free radical reaction.
Free radical scavenging activity
DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (CitationSoares et al., 1997). The reduction in DPPH radical was determined by the decrease of its absorbance at 517 nm by antioxidants. All the extract and fractions of G. procumbens showed DPPH scavenging effect at all amounts (). The DPPH radical scavenging effects of the extract and fractions ofG. procumbens and standard compounds increased in the order EF > BHT > BF > ME > CF > AF. From , we observe that a dose-response relationship is found in DPPH radical scavenging activity; the activity increased as the concentration increased for each extract and fraction of G. procumbens. These results were consistent with total antioxidant and reducing power.
Total phenolic compounds
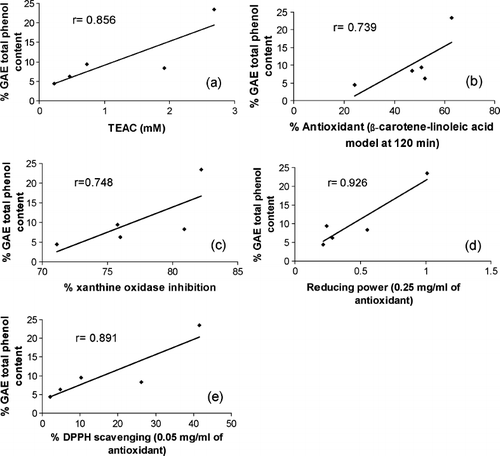
The phenolic compounds are rich in hydroxyl groups and are believed to have the ability of free radical scavenging and antioxidation. Polyphenols are multifunctional and can act as reducing agents, as hydrogen atom–donating antioxidants, chelating metal ions and as singlet oxygen quenchers. The results for phenolic content in the studied G. procumbens extract and fractions are presented in . The data clearly outlines the phenolics sources: EF [23.4 ± 0.26% GAE (w/w)], ME [9.4 ± 0.08% GAE (w/w)], BF [8.3 ± 0.87% GAE (w/w)], CF [6.25 ± 0.5% GAE (w/w)], and AF [4.4 ± 0.79% GAE (w/w)]. However, in this study, the fractionation product may be fractionated to different amounts of total phenolic content. Our results demonstrated a significantly higher (p < 0.001) amount of phenolics when the ethyl acetate fraction was used compared with crude extract. The ability of phenolic compounds of G. procumbens extract and fractions to scavenge free radicals and the reducing power were in direct agreement with earlier reported literature (CitationBehera et al., 2006). Correlation coefficients (r) of total phenolic content and reducing power and scavenging ability of DPPH were 0.926 (p < 0.05) and 0.891 (p < 0.05), respectively ().
Figure 7 Gallic acid equivant total phenolic contents of the Gynura procumbens extract and fraction. Each value represents a mean ± SD (n = 3).*** indiactes significat differences comared with the methanol extract at p < 0.05.
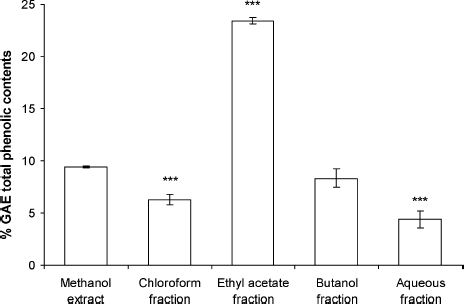
Figure 8 Linear correlation between the total phenolic contents and (a) trolox equivant antioxidant captacity (TEAC), (b) β-carotene–linoleic acid antioxidant model, (c) xanthine oxidase inhibition, (d) reducuing power, and (e) DPPH seaveing activites of G. procumbents extract and fractions.
Overall, EF and BF were better in reducing power, total antioxidant capacity, xanthine oxidase inhibition, and DPPH scavenging activities, and higher in total phenol content. Kaempferol-3-O-rutinoside and astragalin are the most representative phenolic compounds in EF and BF (); 58.4% and 87.6% of total phenolic compound in EF and BF, respectively. It seems that contents of total phenol, especially kaempferol-3-O-rutinoside and astragalin were highly associated with antioxidant properties (CitationChiang et al., 1994; CitationKim et al., 1999; CitationPlumb et al., 1999; CitationHan et al., 2004).
Conclusions
In conclusion, all the extract and fractions of G. procumbens in this research exhibited different extents of antioxidant activity. The results of the current study showed that the ethyl acetate fractions, which contain the highest amount of phenolic compounds, exhibited the greatest antioxidant activity. The high scavenging property of G. procumbens may be due to hydroxyl groups existing in the chemical structure of the phenolic compound that can provide the necessary component as a radical scavenger and antioxidant. Research is still going on in our laboratory to isolate and verify the mechanism of antioxidative effect of kaempferol-3-O-rutinoside, astragalin and other phenolic compounds from the ethyl acetate fraction of G. procumbens methanol extract.
References
- GA Akowuah, S Amirin, A Mariam, and I Aminah. (2001). Blood sugar lowering activity of Gynura procumbens leaf extracts. J Trop Med Plant 2:5–10.
- GA Akowuah, A Sadikun, and A Mariam. (2002). Flavonoid identification and hypoglycaemic studies of butanol fraction from Gynura procumbens. Pharm Biol 40:405–410.
- BC Behera, N Verma, A Sonone, and U Makhija. (2006). Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT-Food Sci Technol 39:80–85.
- HC Chiang, YJ Lo, and FJ Lu. (1994). Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (Hook) Tryon. J Enzyme Inhib 8:61–71.
- A Demo, C Petrakis, P Kefalas, and D Boskou. (1998). Nutrient antioxidants in some herbs and Mediterranean plant leaves. Food Res Int 31:351–354.
- Z Deng, B Tao, X Li, J He, and Y Chen. (1998). Effect of green tea and black tea on the blood glucose, the blood triglycerides, and antioxidation in aged rats. J Agr Food Chem 46:3875–3878.
- AT Diplock. (1997). Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease?. Free Radic Res 27:511–532.
- HJD Dorman, A Peltoketo, R Hiltunen, and MJ Tikkanen. (2003). Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem 83:255–262.
- I Erlund, G Alfthan, J Mäenpää, and A Aro. (2001). Tea and coronary heart disease: The flavonoid quercetin is more bioavailable from rutin in women than in men. Arch Intern Med 161:1919–1920.
- JM Geleijnse, LJ Launer, A Hofman, HA Pols, and JC Witteman. (1999). Tea flavonoids may protect against atherosclerosis: The Rotterdam study. Arch Intern Med 159:2170–2174.
- JM Geleijnse, JC Witteman, LJ Launer, SW Lamberts, and HA Pols. (2000). Tea and coronary heart disease: protection through estrogen-like activity?. Arch Intern Med 160:3328–3329.
- HC Grice. (1986). Safety evaluation of butylated hydroxyltoluene (BHT) in the liver, lung, and gastrointestinal tract. Food Chem Toxicol 24:1127–1130.
- HC Grice. (1988). Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–723.
- JT Han, MH Bang, OK Chun, DO Kim, CY Lee, and NI Baek. (2004). Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch Pharm Res 27:390–395.
- MG Hertog, D Kromhout, C Aravanis, H Blackburn, R Buzina, F Fidanza, S Giampaoli, A Jansen, A Menotti, S Nedeljkovic, M Pekkarinen, BS Simic, H Toshima, EJM Feskens, PCH Hollman, and MB Katan. (1995). Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155:381–386.
- MN Iskander, Y Song, IM Coupar, and W Jiratchariyakul. (2002). Antiinflammatory screening of the medicinal plant Gynura procumbens. Plant Food Hum Nutr 57:233–244.
- SO Keli, MG Hertog, EJ Feskens, and D Krombout. (1996). Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Arch Intern Med 156:637–642.
- SY Kim, JJ Gao, WC Lee, KS Ryu, KR Lee, and YC Kim. (1999). Antioxidative flavonoids from the leaves of Morus alba. Arch Pharm Res 22:81–85.
- A Kumaran, and RJ Karunakaran. (2006). Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem 97:109–114.
- SK Lam, A Idris, ZAA Bakar, and R Ismail. (1998). Gynura procumbens and blood pressure in the rat: Preliminary study. Asia Pac J Pharmacol 13:S14–15.
- V Massey, PE Brumby, H Komai, and G Palmer. (1969). Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Bio & Chem 244:1682–1691.
- JM McCord. (1985). Oxygen-derived free radicals in postichemic tissue injury. New Engl J Med 312:159–163.
- S Meir, J Kanner, B Akiri, and SP Hadas. (1995). Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agr Food Chem 43:1813–1817.
- A Nawawi, N Nakamura, M Hattori, M Kurokawa, and K Shiraki. (1999). Inhibitory effects of Indonesian medicinal plants on the infection of Herpes simplex virus type 1. Phytother Res 13:37–41.
- T Noro, Y Oda, T Miyase, A Ueno, and S Fukushima. (1983). Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull 31:3984–3987.
- M Oyaizu. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315.
- LM Perry. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses. MIT Press, CambridgeMA, (1980)94.
- GW Plumb, KR Price, and G Williamson. (1999). Antioxidant properties of flavonol glycosides from green beans. Redox Rep 4:123–127.
- R Re, N Pellegrini, A Proteggente, A Pannala, M Yang, and C Rice-Evans. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med 26:1231–1237.
- C Sanchez-Moreno, JA Larrauri, and F Saura-Calixto. (1999). Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int 32:407–412.
- K Slinkard, and VL Singleton. (1977). Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic 28:49–55.
- JR Soares, TC Dinis, AP Cunha, and LM Almeida. (1997). Antioxidant activity of some extracts of Thymus zygis. Free Radic Res 26:469–478.
- T Sun, and CT Ho. (2005). Antioxidant activities of buckwheat extracts. Food Chem 90:743–749.
- AP Sweeney, SC Wyllie, RA Shalliker, and JL Markham. (2001). Xanthine oxidase inhibitory activity of selected Australian native plants. J Ethnopharmacol 75:273–277.
- YC Yang, FH Lu, JS Wu, CH Wu, and CJ Chang. (2004). The protective effect of habitual tea consumption on hypertension. Arch Intern Med 164:1534–1540.
- MF Yam, R Basir, MZ Asmawi, and Z Ismail. (2007). Antioxidant and hepatoprotective effects of Orthosiphon stamineus Benth standardized extract. Am J Chin Med 35:117–128.
- A Yilidirim, M Oktay, and V Bialoglu. (2001). The antioxidant activity of the leaves of Cydonia vulgaris. Turkish J Med Sci 31:23–27.
- JL Zweier, P Kuppusamy, and GA Lutty. (1988). Measurement of endothelial cell free radical generation: Evidence for central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA 85:4046–4050.