Abstract
In the current study, we evaluated total phenolic content, antioxidant activity (by using β -carotene bleaching assay), and antibacterial activity of aqueous and methanol extracts of the aerial part of the Rumex crispus L. (Polygonaceae) naturally grown in the Eastern Anatolia region of Turkey. The aerial part of the plant had high total phenolic content (56.31 μ g/mg DW). The antioxidant activity of aqueous and methanol extracts of the aerial part of R. crispus L., BHA, and BHT were found to be 92.35%, 95.49%, 98.16%, and 96.66%, respectively. Although the antioxidant activity of the aqueous and methanol extracts of R. crispus was lower than that of the BHA and BHT, the difference between these was not statistically significant, p < 0.05. The methanol extract possessed strong antibacterial activity against Agrobacterium tumefaciens, Bacillus cereus, Bacillus subtilis, Pseudomonas corrugate, Pseudomonas syringae pv. tomato, Salmonella typhimurium, Serratia liquefaciens, Vibrio cholerae, Yersinia frederiksenii, and Yersinia pseudotuberculosis. Therefore, the aerial part of R. crispus can be used as an effective and safe source of antioxidants and antibacterial agent.
Introduction
Wild plants have been reported to have antimicrobial and antioxidant properties for centuries, and indigenous plants have been used in herbal medicine for curing various diseases (CitationBeuchat & Golden, 1989; CitationSokmen et al., 1999; CitationAlvarez-Castellanos et al., 2001; CitationTepe et al., 2005). Oxidative damage, as a result of normal metabolism or secondary to environmental pollutants, leads to free radical formation, which has been thought to play a central role in cancer and atherosclerosis. Therefore, antioxidants, which can neutralize free radicals, may be important in the prevention of these diseases (CitationDuh et al., 1999). Although there are some synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) that are commonly used in processed foods, it has been reported that these compounds have some side effects (CitationIto et al., 1983). Therefore, substitution of synthetic antioxidants by natural ones and the screening of plant species for identifying new antioxidants have become critically important in recent years (CitationMoure et al., 2001).
The development of bacterial resistance to currently available antibiotics has necessitated the search for new antibacterial agents. The Gram-positive bacteria such as Bacillus subtilis are rod-shaped aerobic bacteria and are reported to have some pathogenic role (CitationGorden et al., 1973). Pseudomonas is aerobic and nonfermentative and mainly causes urinary tract infection, wound or burn infection, chronic otitis media, septicemia, etc., in human (CitationBodey, 1983).
Wild plants, including Rumex (Polygonaceae), are widely used as medicines by populations that inhabit the rural parts of Eastern Anatolia of Turkey. The flowers, stems, leaves, and roots of these plants are usually prepared and consumed to treat a variety of ailments that include pain, edema, digestive problems, arthritis, colds and flu, fevers, and irritability (CitationBaytop, 1996).
The genus Rumex is widespread in the flora of Turkey and is represented by 22 species (CitationCullen, 1972), and the most common species of the genus in Turkey are R. patientia L, R. crispus L, R. acetosa L, R. caucasicus Rech., and R. alpinus L. (CitationBaytop, 1996). Many species of Rumex have been reported as having beneficial properties. The genus has been used extensively in traditional medicine in Turkey to treat a variety of disorders such as constipation, diarrhea, and eczema (CitationUlukanli et al., 2005). The genus also possesses laxative, diuretic, antipyretic, wound cure, and anti-inflammatory properties (CitationBaytop, 1996; CitationSuleyman et al., 1999). In the Eastern part of Turkey, young leaves of Rumex species are used as a vegetable or for meat stuffed with leaves.
R. crispus L. is a perennial wild plant, and it has length of 30–150 cm. Its basal leaves are acute and narrowly lanceolate to oblanceolate. Petioles are canaliculate above, and their inflorescence is dense. The pedicles are longer than the fruiting perianth segments and articulate below the middle. Fruiting perianth segments cordate to triangular, at least one tuberculate, 4–5 × 3–4 mm. It can grow on banks, marshes, and waste places and can be found at altitudes up to 2300 m (CitationCullen, 1972). Its young leaves are cultivated in spring and used as a vegetable, whereas the seeds of this plant are cultivated in the summer and used in Turkish folk medicine (CitationBaytop, 1996).
Though antimicrobial activity of some Rumex species has been previously reported (CitationGetie et al., 2003; CitationUlukanli et al., 2005), there are limited studies on the total phenolic content and antioxidant and antibacterial activity of R. crispus L. Hence, the current study is aimed at evaluating the phenolic content and antioxidant and antibacterial activity of the aerial parts of R. crispus L.
Materials and Methods
Plant material
Plant material (R. crispus) was collected in July 2006 from the Eastern part of Turkey. The plant was further identified by Irfan Coruh (Department of Plant Protection, Ataturk University, Erzurum, Turkey), and a voucher specimen was deposited in the herbarium of the Plant Protection Department of the Agricultural Faculty of Ataturk University. At least 30 separate plants were pooled to form a single plant sample. They were packed in a portable refrigerator during transportation to the laboratory (2–3 h). The samples from different locations were combined prior to analysis. Then sample was dried at 50°C in an oven, and dried leaves were ground to a fine powder with a mortar and pestle and kept at room temperature prior to extraction for antioxidant activity, total phenolics analysis, and antibacterial tests. The dried and powdered leaves of the plant (500 g) were extracted successively with 1 L methanol by Soxhlet for 72 h at a temperature not exceeding the boiling point of the solvent (CitationLin et al., 1999). Crude aqueous infusion was also prepared by adding 1 L of boiling water to 500 g of powdered plant material in a glass 2.5-L flask and incubating at room temperature for 2 h on a rotating shaker (200 rpm). The aqueous extracts were filtered using Whatman no. 1 filter paper and then concentrated in vacuo at 40°C using a rotary evaporator. The residues obtained were stored in a freezer at −80°C until use.
Determination of total phenolics and antioxidant activity
The concentration of total phenolics in methanol extract of R. crispus plants was determined by the Folin-Ciocalteu colorimetric method (CitationGulcin et al., 2002). Briefly, 1 mL of the solution (containing 1 mg) extract in methanol was pipetted into a flask. Then, 46 mL distilled water and 1 mL Folin-Ciocalteu reagent was added and mixed thoroughly. The mixture was left to stand for 3 min, and 3.0 mL of 2% sodium carbonate was added. After 120 min incubation at ambient temperature with shaking, the resulting absorbance was measured at 760 nm. Measurements were carried out in duplicate, the calibration curve was performed with gallic acid, and the results were expressed as μ g Gallic Acid Equivalent (GAE)/mg. The antioxidant activity of water and methanol extracts of R. crispus was determined according to the β -carotene bleaching method described by CitationKaur and Kapoor (2002). BHA and BHT were used as standards. All samples were assayed in duplicate. Degradation rate (DR) was calculated according to first-order kinetics, using the equation:
Where ln is natural log, a is the initial absorbance (470 nm) at time 0, b is the absorbance (470 nm) at 100 min, and t is time.
Antioxidant activity (AA) was expressed as percent of inhibition relative to the control, using the formula:
Determination of antibacterial activity
To determine antibacterial activity, Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. tomato, Bacillus subtilis, Bacillus cereus GC subgroup A, Yersinia enterocolita, Vibrio cholerae non 01, Corynebacterium diphtheriae, Yersinia frederiksenii, Yersinia pseudotuberculosis, Salmonella typhimurium GC subgroup A, Serratia liquefaciens, Pseudomonas corrugate, Xanthomonas compestris compestris, Agrobacterium tumafaciens, and Pseudomonas aeruginosa were used. The bacteria, maintained on Nutrient Agar (Merck, Darmstadt, Germany), were supplied by the Microbiology Laboratory of the Agricultural Faculty of Ataturk University. Identity of the bacteria used in this study was confirmed by the Microbial Identification System in Biotechnology Application and Research Center at Ataturk University. Antibacterial activities were determined by using the disk diffusion method using 100 μ L of suspension containing 108 CFU/mL of bacteria spread on nutrient agar (NA) medium. Sterile 6-mm-diameter filter paper disks were impregnated with 10 μ g of the sterile test material and placed onto nutrient agar. Negative controls were prepared using the same solvents employed to dissolve the plant extracts. Ofloxacin (5 μ g/disk), sulbactam (30 μ g) + cefoperazona (75 μ g) (105 μ g/disk) and/or netilmicin (30 μ g/disk) were used as positive standards. The inoculated plates with bacteria were incubated at 27°C for 24 h. The diameter of the clear zone around the disk was measured and expressed in millimeters as its antibacterial activity. Five disks per plate and three plates were used, and each test was run in triplicate (CitationDjipa et al., 2000). The minimal inhibition concentration (MIC) values were also studied. The inocula of bacteria were prepared from 12-h broth cultures, and suspensions were adjusted to 0.5 McFarland standard turbidity. R. crispus extracts dissolved in 0.5% dimethylsulfoxide (DMSO) were first diluted to the highest concentration (500 μ g/mL) to be tested, and then serial twofold dilutions were made in a concentration range from 3.90 to 500 μ g/mL in 10-mL sterile test tubes containing nutrient broth. MIC values of extracts against bacterial strains were determined based on a microwell dilution method (CitationOzturk & Ercisli, 2006). The 96-well plates were prepared by dispensing into each well 95 μ l of nutrient broth and 5 μ L of the inoculum. A 100 μ L aliquot from extracts initially prepared at the concentration of 500 μ g/mL was added into the first wells. Then, 100 μ L from their serial dilutions was transferred into six consecutive wells. The last well containing 195 μ L of nutrient broth without compound and 5 μ L of the inoculum on each strip was used as negative control. The final volume in each well was 200 μ L. Maxipime (Bristol-Myers Squibb, Cincinnati, USA) at the concentration range of 500–7.8 μ g/mL was prepared in nutrient broth and used as standard drug for positive control. Contents of each well were mixed on a plate shaker at 300 rpm for 20 s and then incubated at appropriate temperatures for 24 h. Microbial growth was determined by absorbance at 600 nm using the ELx 800 universal microplate reader (Biotek Instrument Inc., Highland Park, VT, USA) and confirmed by plating 5-μ L samples from clear wells on nutrient agar medium. The extract tested in this study was screened two times against each organism. The MIC of each extracts was taken as the lowest concentration that showed no growth (CitationOzturk & Ercisli, 2006).
Statistical analysis
The experiment was a completely randomized design with four replications. Data were subjected to analysis of variance (ANOVA), and means were separated by Duncan multiple range test at P < 0.05 significance level.
Results and Discussion
Total phenolic content and antioxidant activity
Total phenolics in aerial part of R. crispus were found to be 56.31 μ g/mg dry weight (DW). As is well-known, phenolic compounds are considered to be the most important antioxidative plant components, and antioxidant activity of plant materials, was well correlated with the content of their phenolic compounds (CitationElzaawely et al., 2005). Phenolics have also played an important role against chronic diseases (CitationSimopoulos, 2004). The Folin-Ciocalteu procedure is nonspecific because it detects all phenolics (phenolic acids, flavonoids, and tannins) (CitationNiklova et al., 2001), so it does not give details of the quantity and quality of the phenolic constituents of the extracts. Nevertheless, this widely used method provides a rapid and useful overall evaluation of the phenolic content of extracts (CitationLuximon-Ramma et al., 2003).
The results for total phenolics of aerial parts of R. crispus clearly outline it as a rich phenolics source. CitationElzaawely et al. (2005) previously reported that total phenolic contents of Rumex japonicus Houtt grown in Japan was 200 mgGAE/g extract. The variability could be due to plant species used, environmental factors, and collection period.
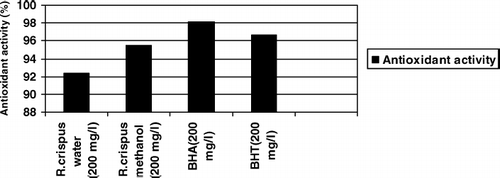
The antioxidant assay using the discoloration of β -carotene is widely used to measure the antioxidant activity of plant extracts, because β -carotene is extremely susceptible to free radical–mediated oxidation of linoleic acid (CitationKumazawa et al., 2002). Antioxidant activity of the aerial part of R. crispus is shown in . The differences among water, methanol extracts, BHA, and BHT were found statistically not important. Antioxidant activity was found to be 92.35% and 95.49% in R. crispus water and methanol extracts and 98.16% in BHA and 96.66% in BHT (). The water and methanol extracts had a little lower antioxidant activity than did BHA and BHT. This result suggested that aerial parts of R. crispus had strong antioxidant activity. This result can be important both for human diet and food safety.
Antibacterial activity
The in vitro antibacterial activities of R. crispus methanol and aqueous extracts against examined microorganisms in the current study and their potency were quantitatively assessed by the presence or absence of inhibition zones and zone diameters and MIC values ().
Table 1 Antibacterial activity of Rumex crispus extracts against a number of bacteria.
According to our results, the negative control and water extract had no antimicrobial activity against any of the bacterial species tested in the current study. However, methanol extracts of R. crispus has inhibition effects on the growth of a number of bacteria tested (). These results confirmed the evidence in previous studies reported that methanol is the better solvent for more consistent extraction of antimicrobial substances from medical plants compared with water (CitationAhmad et al., 1998; CitationLin et al., 1999). The most pronounced antibacterial activity was shown by the methanol extracts of R. crispus against Agrobacterium tumefaciens, Bacillus cereus, Bacillus subtilis, Pseudomonas corrugate, Pseudomonas syringae pv. tomato, Salmonella typhimurium, Serratia liquefaciens, Vibrio cholerae, Yersinia frederiksenii, and Yersinia pseudotuberculosis ().
MIC values show by the methanol extract and standard drug (Maxipime) were in the range of 15.62–125 and 7.81–500 μ g/mL, respectively (), with Xanthomonas compestris compestris being the most sensitive microorganism to the plant extract with a lower MIC value than that of standard drug. The other sensitive microorganisms were Pseudomonas aeruginosa, Pseudomonas syringae pv. syringae, and Serratia liquefaciens (). Vibrio cholerae had similar MIC values with standard antibiotic (Maxipime) ().
The results obtained in the course of the current study are in agreement to a certain degree with the traditional uses of Rumex evaluated (CitationBaytop, 1996). As far as our literature survey could ascertain, several studies were carried out for antibacterial activity of some Rumex species such as Rumex crispus (CitationUlukanli et al., 2005) and Rumex nervosus and Rumex abyssinicus (CitationGetie et al., 2003) and Rumex japonicus (CitationElzaawely et al., 2005) species demonstrated significant inhibitory effects against most microorganisms under test. The antibacterial activity of methanol extract can be attributed to the presence of several compounds. Phenolic compounds such as pyrogallol were toxic to microorganisms as they inhibit the enzymes through reaction with sulfhydryl group (CitationCowan, 1999). Our study indicated that methanol extract was a strong antibacterial inhibitor.
In conclusion, the results obtained in this work are noteworthy, not only with respect to the antioxidant and antibacterial activity of methanol extract of R. crispus aerial parts but also with respect to its high level of phenolic content. It can be suggest that the consumption of R. crispus could possibly offer some dietary benefits as it contains antioxidant constituents, which are able to protect against lipid peroxidation and to scavenge free radicals. The ability of edible plant extracts in inhibiting pathogenic bacteria in food matrices and the impact of plant materials on organoleptic properties of food require further studies.
References
- I Ahmad, Z Mehmood, and F Mohammad. (1998). Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 62:183–193.
- PP Alvarez-Castellanos, CD Bishop, and MJ Pascual-Villalobos. (2001). Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 57:99–102.
- T Baytop. Türkiye'de Bitkiler ile Tedavi. , , (1996) I.U. Yayinlari No. 3255, Eczacilik Fak, Istanbul University, Istanbul. No. 40, 444 pp.
- LR Beuchat, and DA Golden. (1989). Antimicrobials occurring naturally in foods. Food Technol 43:134–142.
- GP Bodey. (1983). Infection caused by Pseudomonas aeruginosa. Rev Infect Dis 5:279–283.
- MM Cowan. (1999). Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582.
- J Cullen. RumexFlora of Turkey and East Aegean Islands, Vol 2PH Davis. Edinburgh University Press, Edinburgh, (1972)281–293.
- CD Djipa, M Delmee, and P Quetin-Leclercq. (2000). Antimicrobial activity of bark extracts of Syzygium jambos L. J Ethnopharmacol 71:307–313.
- PD Duh, YY Tu, and GC Yen. (1999). Antioxidant activity of aqueous extract of harn jyur (Chyrsanthemum morifolium Ramat). Lebensmittel-Wissenschaft und Technologie 32:269–277.
- AA Elzaawely, TD Xuan, and S Tawata. (2005). Antioxidant and antibacterial activities of Rumex japonicus Houtt. aerial parts. Biol Pharm Bull 28:2225–2230.
- M Getie, T Gebre-Mariam, R Rietz, C Hohne, C Huschka, M Schmidtke, A Abate, and RH Neubert. (2003). Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa Rumex nervosus Rumex abyssinicus.. Fitoterapia 74:139–143.
- RE Gorden, WC Haynes, and CHN Pang. The Genus Bacillus. U.S. Dept. Agriculture, WashingtonDC, (1973) Agric. Hand Book No. 427.
- I Gulcin, M Oktay, I Kufrevioglu, and A Aslan. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–329.
- N Ito, S Fukushima, A Hassegawa, M Shibata, and T Ogiso. (1983). Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst 41:215–217.
- C Kaur, and HC Kapoor. (2002). Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 37:153–161.
- S Kumazawa, M Taniguchi, Y Suzuki, M Shimura, M Kwon, and T Nakayama. (2002). Antioxidant activity of polyphenols in carob pods. J Agric Food Chem 50:373–377.
- J Lin, AR Opoku, M Geheeb-Keller, AD Hutchings, SE Terblanche, AK Jager, and J van Staden. (1999). Preliminary screening of some traditional Zulu medicinal plants for anti-inflammatory and anti-microbial activities. J Ethnopharmacol 68:267–274.
- A Luximon-Ramma, T Bahorun, and A Crozier. (2003). Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J Sci Food Agric 83:496–502.
- A Moure, JM Cruz, D Franco, JM Domínguez, J Sineiro, H Domínguez, MJ Nunez, and JC Parajo. (2001). Natural antioxidants from residual sources. Food Chem 72:145–171.
- I Niklova, S Schmidt, K Habalova, and S Sekretar. (2001). Effect of evening primrose extracts on oxidative stability of sunflower and rapeseed oils. Eur J Lipid Sci Technol 103:299–306.
- S Ozturk, and S Ercisli. (2006). Chemical composition and in vitro antibacterial activity of Seseli libanotis. World J Microbiol Biotechnol 22:261–265.
- A Simopoulos. (2004). Omega-3 fatty acids and antioxidants in edible wild plants. Biol Res 37:263–277.
- A Sokmen, BM Jones, and M Erturk. (1999). The in vitro antibacterial activity of Turkish plants. J Ethnopharmacol 67:79–86.
- H Suleyman, LO Demirezer, A Kuruuzum, ZN Banoglu, F Gocer, G Ozbakir, and A Gepdiremen. (1999). Antiinflammatory effect of the aqueous extract from Rumex patientia L. roots. J Ethnopharmacol 65:141–148.
- B Tepe, D Daferera, A Sokmen, M Sokmen, and M Polissiou. (2005). Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90:333–340.
- Z Ulukanli, S Ulukanli, H Ozbay, A Ilcim, and M Tuzcu. (2005). Antimicrobial activities of some plants from the Eastern Anatolia region of Turkey. Pharm Biol 43:334–339.