Abstract
Moringa oleifera L. (Moringaceae) leaves were examined for their effect on human platelet aggregation in vitro. The aqueous extract of Moringa oleifera leaves significantly (p ≤ 0.05) inhibited platelet aggregation induced by agonists such as adenosine diphosphate, collagen, and epinephrine. The degree of inhibitory activity varied depending on the agonist used, concentration of extract and duration of incubating the extract with platelets. Heat treatment reduced the inhibitory activity of extract against platelet aggregation. In addition, the extracts significantly (p ≤ 0.05) decreased the amount of malonaldehyde formed in agonist challenged platelets. This study is the first report on the effect of aqueous extract of Moringa oleifera leaves against human platelet aggregation. Overall, Moringa oleifera leaves have potential to protect platelets against aggregation.
Introduction
Platelet aggregation is a physiological process proved to have defined roles in cardiovascular diseases. Normally, in response to a vascular injury, platelets rapidly undergo the processes of adhesion, shape change, secretion, and aggregation through a series of exquisitely coordinated responses (CitationKroll & Schafer, 1989) that result in the formation of homeostatic plugs or arterial thrombi at the sites of vessel injury to stop blood loss. These thrombi are the source of thromboembolic complications of atherosclerosis, heart attacks, stroke, and peripheral vascular disease (CitationSon et al., 2004). Therefore, compounds that counter aggregation have a protective role regarding thromboembolic disorders (CitationSubramoniam & Ana, 1988).
It is realized that prevention of thrombotic events by dietary means rather than commonly used antithrombotic drugs (e.g., tolmetin, aspirin, heparin, warfarin) would be more effective and economic in large populations. This has led to interest in exploring the anti-platelet effects of various dietary components including fruits, vegetables, spices, and herbs, and their active constituents (CitationSrivastava, 1989). The anti-platelet effects of several herbs and spices including garlic, onion, ginger, turmeric, cloves, and their active principles have been established (CitationRattan, 1988).
Moringa oleifera L. (Moringaceae), or drumstick tree, is a rapidly growing perennial soft wood tree that is native to the Indo-Bangla subcontinent and cultivated throughout the tropical belt (CitationSastri, 1962; CitationFahey, 2005). All parts of the tree are edible and have long been consumed by humans (CitationFahey, 2005). The leaves are tri-pinnate, feathery, with many small leaflets, and are rich in β-carotene, vitamin C, vitamin B, calcium, iron, and essential amino acids. Thus, they have been advocated as an outstanding indigenous source of nutrients suitable for utilization in many of the so-called “developing” regions of the world to combat malnutrition (CitationFreiberger et al., 1998; CitationNambiar & Seshadri, 2001; CitationFahey, 2005). In the Indian indigenous system of medicine, M. oleifera leaves are used for the treatment of a variety of common ailments e.g., anemia, anxiety, dry cough, bronchitis, joint pain, scurvy, and psoriasis (CitationSastri, 1962; CitationMathur, 2005). A wide range of pharmacological properties such as anti-inflammatory, anti-tumor, antimicrobial, hypotensive, hypocholestrolemic, and hypoglycemic have been reported for M. oleifera leaves in the scientific literature (CitationFahey, 2005). This study is the first report on the effect of aqueous extract of M. oleifera leaves against human platelet aggregation.
Materials and methods
Chemicals
Adenosine 5’-diphosphate (ADP), collagen, and epinephrine were purchased from Sigma Chemicals (St. Louis, MO). Malonaldehyde-bis-dimethyl acetal was obtained from Merck-Schuchardt (Germany) and thiobarbituric acid (TBA) from HiMedia Lab (Bombay, India).
Materials
Moringa oleifera leaves were obtained in bulk from a local market in Mysore, India. The taxonomic identity of the plant was confirmed by comparison of the plant material with those of the known identity in the Herbarium specimen identified by Dr. G. R. Janardhana, Department of Studies in Botany, University of Mysore, Mysore, India.
Preparation of aqueous extracts of raw and heat-treated M. oleifera leaves
Fresh M. oleifera leaves (20 g) were cleaned, washed, and ground to a fine paste with addition of distilled water. The slurry obtained was left overnight at 4°C. Afterwards, the contents were centrifuged at 10,000 rpm for 15 min to obtain a clear extract and volume made up to 50 ml with distilled water. To prepare the extract of heat-treated M. oleifera leaves, fresh leaves were heated at 100°C for 15 min and the extract of heat-treated leaves was prepared as mentioned above. The freshly prepared extracts were used for platelet aggregation studies.
Isolation of platelets
Venous blood was collected in 3.8% tri-sodium citrate (9:1 v/v) from healthy volunteers who had not taken any medication for the past 10 days. This citrated blood was centrifuged at 1,100 rpm for 20 min to obtain platelet rich plasma (PRP), which was used within 3 h after collection. The residual blood was again centrifuged at 2,500 rpm for 20 min to obtain the homologous platelet poor plasma (PPP). Platelet count was adjusted to 1.6 × 107 platelets per μl of PRP (CitationGerrad, 1982).
Platelet aggregation assay
The aggregation experiments were carried out turbidimetrically in a Dual Path Aggro-meter (Chronolog, Havertown, PA) (CitationSuneetha & Krishnakantha, 2005a). PRP of 450 μL was kept stirred at 1,200 rpm in a glass cuvette at 37°C, while aggregation was induced by agonists such as adenosine diphosphate (ADP) (61 μM), collagen (0.005% in 0.1 N acetic acid), and epinephrine (76 μM). Similarly, PRP was pre-incubated with the extracts at different concentrations for 1 min followed by induction of aggregation by agonists mentioned above. The change in turbidity was recorded at least for 4 min in each case with reference to PPP using an Amersham Pharmacia Biotech Recorder 111, in the presence and absence of extracts. The slopes were calculated for control and experimental samples and percentage inhibition of aggregation due to control was calculated. The concentration of plant extract (mg) at which platelet aggregation was inhibited by 50% (IC50) was obtained by interpolation from linear regression analysis.
Effect of incubation time on platelet aggregation
The effect of duration of incubation of extracts with platelets on platelet aggregation was determined at IC50 of each extract. PRP (450 μL) was pre-incubated with sample extract for different times (1, 3, 5, & 10 min) before induction of aggregation, and percentage inhibition of platelet aggregation was calculated.
Estimation of malonaldehyde (MA) in agonist challenged platelets
The amount of MA formed in agonist challenged platelets was determined according to the method of CitationMaguire and Csonka-Khalifah (1987). After aggregation, platelets (450 μL) were taken and mixed with 20 μL of 1% butylated hydroxytoluene (BHT) in ethanol and 100 μL of 100% trichloroacetic acid in 3 N HCl. The pellet was centrifuged at 10,000 rpm for 10 min to obtain 450 μL of supernatant to which 100 μL of TBA reagent (0.12 M TBA in 0.26 M Tris-HCl solution) was added. The chromophor generated was measured at 532 nm, and MA equivalents were calculated using the following equation:
Statistical analysis
Data were recorded as means ± standard deviation of triplicate measurements. Analyses of variance were performed by ANOVA test and significance differences between the means were determined by Duncan’s multiple-range test (p ≤ 0.05) (CitationSteel & Torrie, 1980).
Results and discussion
Effect of M. oleifera extracts on human platelet aggregation
Aqueous extract of raw M. oleifera leaves inhibited platelet aggregation induced by the agonists, collagen, ADP, and epinephrine in a dose-dependent manner (). In the concentration range of 0.2-1 mg, the extract inhibited aggregation by 25.93%-89.26% in the case of collagen, and 20.65%-65.39% in the case of ADP. This indicated that M. oleifera extract was a potent inhibitor of collagen-induced platelet aggregation. When epinephrine was used as the agonist, the extract showed a dose-dependent activity (12.54%-38.18%) in the concentration range of 0.2–0. 8 mg. Increasing the concentration of extract to 1 mg appeared to suppress epinephrine-induced aggregation by almost 100%. The IC50 values of M. oleifera extract on collagen- and ADP-induced aggregation were 0. 48 mg and 0. 70 mg (), respectively, indicating that 50% inhibition of aggregation can be achieved by a significantly lower concentration of extract, when aggregation was induced by collagen.
Figure 1. Effect of aqueous extract of Moringa oleifera leaves on human platelet aggregation induced by collagen, ADP and epinephrine. *Percentage inhibition of aggregation relative to control.
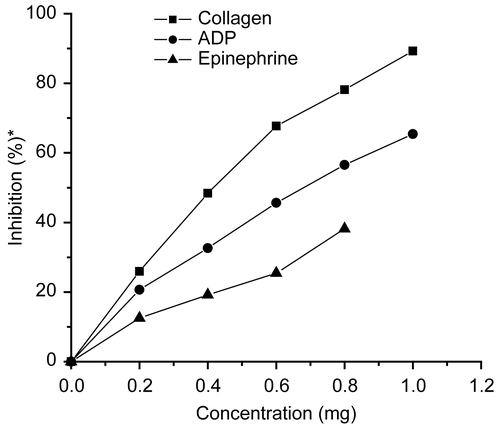
Table 1. IC50 (mg)* of aqueous extracts of Moringa oleifera leaves on agonist-induced platelet aggregation.
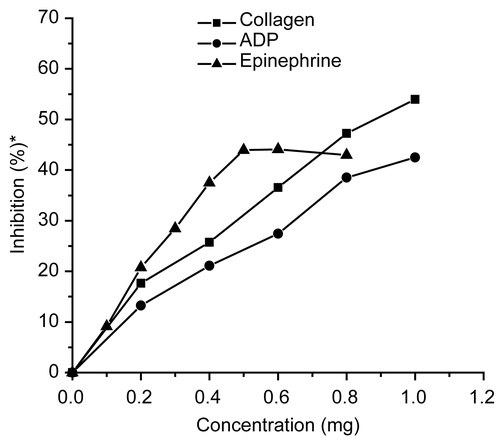
In general, plant foods are subjected to processing such as boiling, pressure-cooking, steaming, roasting, frying, etc., before consumption. Since in India, M. oleifera leaves are commonly used in cooked form (preparation of curry), it was reasonable to examine the effect of heat treatment on their inhibitory activity against platelet aggregation. The effect of extract obtained from heat-treated (100°C, 15 min) leaves on platelet aggregation is shown in . In the concentration range of 0.2- 1 mg, the inhibitory effect of extract against agonist-induced platelet aggregation was 17.64%-53.97% (in the case of collagen), and 13.25%-42.47% (in the case of ADP). The inhibitory effect of extract against epinephrine-induced aggregation reached its maximum (44.9%) with 0. 6 mg of extract, beyond which a decline in the activity of the extract was observed. From these results it is evident that 50% inhibition of ADP- and epinephrine-induced aggregation could not be achieved with the concentration range used in this study, i.e., there was poor inhibitory activity after heat processing of the sample. The IC50 value of extract against collagen-induced aggregation was 0. 87 mg, which was significantly (p ≤ 0.05) higher than that of raw leaf extract (0. 48 mg) (). This indicated that anti-aggregatory potential of M. oleifera leaves was reduced by heat processing, which could be attributed to the loss of active components responsible for activity of extract. However, the residual activity in the extract was about 54%, indicating that it can be still considered as a good inhibitor of platelet aggregation. It has been shown that about 86-91% of the active principle of spices was lost in turmeric, 13-17% in black pepper powder, and 4-19% in red pepper powder when boiled for 15 min (CitationSrinivasan et al., 1992). Similarly, a two–fold decrease in the consistency range was observed when garlic and onion were processed at high temperature (CitationAhmed, 2000).
Figure 2. Effect of aqueous extract of heat-treated (100°C, 15 min) Moringa oleifera leaves on human platelet aggregation induced by collagen, ADP, and epinephrine. *Percentage inhibition of aggregation relative to control.
The aqueous extracts of M. oleifera leaves (raw and heat-treated) were able to inhibit platelet aggregation induced by collagen, ADP and epinephrine. Reports indicate that activation of platelets with agonists brings about changes in the cyclo-skeletal structure, resulting in the loss of discoidal shape and pseudopodal projections (CitationMukherjee et al., 1990). It has been shown that ADP and epinephrine induce aggregation by reducing the intra-platelet c-AMP levels and inhibiting adenylate cyclase activity (CitationHawiger et al., 1980). Collagen-induced platelet aggregation has been reported to be associated with a burst of hydrogen peroxide (CitationPignatelli et al., 1998), a pro-oxidant which stimulates the arachidonic acid (AA) metabolism by contributing to the platelet production of thromboxane A2 (CitationPignatelli et al., 1999). Epinephrine-induced platelet aggregation is mediated through α2-adrenergic receptors that also decrease the PGE1 stimulated c-AMP levels (CitationFigures et al., 1986). The mode of action of these platelet agonists is to help increase the cytosolic levels of calcium either due to release from internal stores or through calcium reflux (CitationShah et al., 1999). A rise in the cytosolic levels of calcium accompanies platelet activation through stimulation of enzymes, which were otherwise not fully functional at low levels of calcium concentration present in resting platelets (CitationHeemsherk & Sage, 1994). Calcium antagonists usually may not bind to the specific receptor sites but may cause the thickening of membranes due to the insertion of these antagonists into the membrane bilayers, thus affecting the calcium mobilization (CitationBlache et al., 1987). The agonists, collagen, ADP, and epinephrine may also activate the surface glycoprotein receptors IIb–IIIa (GP IIb–IIIa) as well as interaction with the von Willebrand factor found in plasma (CitationScott et al., 1991; CitationBloekmans et al., 1995). Hence, the suppression of platelet aggregation indicated by M. oleifera extract in this study might be attributed to not only competition of extract components with the GP IIb – IIIa receptors along with the agonists, but also their interaction with membrane bilayers, causing the thickening of membranes and thus affecting calcium mobilization. It may also be likely that these extracts prevent reduction of intra-platelet c-AMP levels and adenylate cyclase stimulation caused by ADP and epinephrine. Inhibition of collagen-induced aggregation with the extract might be due to blocking the release of hydrogen peroxide, which will otherwise stimulate AA metabolism.
Effect of incubation time on inhibition of platelet aggregation
Human platelet aggregation is usually followed with the inhibitors being incubated for one min in the normal course. In this study, it was also of interest to determine the effect of longer exposure of platelets to inhibitors on the inhibition of platelet aggregation. The effect of incubation time on inhibition of platelet aggregation with the extracts (raw and heat-treated) is presented in . The maximum inhibition of collagen-induced platelet aggregation (69.84%) with the extract of raw sample was achieved due to incubation with PRP for 5 min. Further incubation (10 min) did not affect the activity of extract significantly (p ≤ 0.05). The inhibition of ADP-induced platelet aggregation significantly (p ≤ 0.05) increased with increase in duration of incubation. The extract incubated with platelets for 10 min, exhibited 83.78% anti-aggregatory activity on ADP. Epinephrine-induced platelet aggregation was highly affected by increasing the time of incubation. Maximum inhibition was seen at 3 min incubation for epinephrine-induced aggregation. When the extract of heat-treated leaves was incubated with PRP for 3 min, a 7% decline in the inhibition of platelet aggregation was noted.
Table 2. Effect of incubation time on inhibition of platelet aggregation with aqueous extracts of Moringa oleifera leaves at IC50.
The results showed that the efficacy of M. oleifera extract in inhibiting platelet aggregation was time-dependent. The increase in the inhibitory activity observed might be attributed to a better uptake or interactions of extract component(s) into the platelets or a better incorporation of active components into the platelet membrane bilayers during longer incubation. Prolonged incubation did not improve the anti- aggregatory activity of the extract of heat-treated M. oleifera leaves. The inhibitory activity of aqueous extracts of coriander, saffron and cardamom against platelet aggregation induced by ADP, collagen and epinephrine were found to be time-dependent at IC50 (CitationSuneetha & Krishnakantha, 2005a, Citation2005b, Citation2005c).
Estimation of malonaldehyde (MA) in agonist challenged platelets
Malonaldehyde is one of the end products of the cyclooxygenase pathway of AA metabolism (CitationRattan, 1988). It is believed that lipid peroxides (e.g., MA) along with thromboxane A2 (TXA2) increase the platelet sensitivity to agonists, thus leading to coronary heart diseases (CitationNeiva et al., 1999). The amount of MA released in agonist-challenged platelets decreased significantly (p ≤ 0.05) at IC50 of M. oleifera extract with different agonists (). The reduction in MA released during collagen-induced aggregation with the extract of raw leaves was higher than that of heat-treated leaves. The extract of raw leaves exhibited 34.37%, 29.68%, and 30% inhibitory activity toward MA formation during collagen-, ADP-, and epinephrine-mediated aggregation, respectively. The extract of heat-treated leaves exhibited 25.65% inhibitory activity against formation of MA in collagen-challenged platelets. The results indicate that the extract possessed component(s), which were able to inhibit the formation of release products through degradation of AA. It may also be likely that the cyclooxygenase pathway of AA metabolism was being affected, thus resulting in a decrease in platelet aggregation. Inhibition of cyclooxygenase pathway can lead to accumulation of AA, which in turn converts into 12-HPETE, a potent anti-aggregatory agent, through lipooxygenase pathway of AA metabolism (CitationRattan, 1988). Thus, the inhibition of platelet aggregation might be attributed to combined effects of reduced formation of MA and TXA2 and increased formation of 12-HPETE. It has been reported that ginger, omum, cloves, cumin, and turmeric increased the formation of platelet lipooxygenase products Citation(Srivastava, 1984, Citation1988, Citation1989; CitationSrivastava & Justesen, 1987). Onion has been shown to suppress both the cyclooxygenase and lipooxygenase pathways in platelets (CitationSrivastava, 1986).
Table 3. Effect of aqueous extracts of Moringa oleifera leaves on malonaldehayde (MA) formed in agonist challenged platelets at IC50.
Conclusions
The results of this investigation revealed that M. oleifera leaves have components that are capable of inhibiting human platelet aggregation in vitro. Their anti- aggregatory activity was dependent on the concentration of extract, duration of incubation with platelets, and type of inducer used. The extract appeared to suppress aggregation process mediated by agonists through the inhibition of cyclooxygenase pathway of AA metabolism. Further investigations are needed to isolate, identify and purify the active components, and to elucidate the exact biochemical interactions between the component(s) of the extracts and platelets, which may result in inhibition of platelet aggregation. Regular and increased consumption of M. oleifera leaves is recommended in order to exploit their nutritional and beneficial health effects such as their capacity as anti-platelet agents.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmed J (2000): Effect of temperature on rheological characteristics of garlic and onion pastes. J Food Sci Tech 37: 409–411.
- Blache D, Ciavatti M, Ojeda C (1987): The effect of calcium channel blockers on blood platelet function especially calcium uptake. Biochim Biophys Acta 923: 401–412.
- Bloekmans D, Peckmyn H, Vermylen J (1995): Platelet activation. Blood Rev 9: 143–156.
- Fahey JW (2005): Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J 1: 5–19.
- Figures WR, Scearce LM, Wachtfogel Y, Chen J, Colman RF, Colman RW (1986): Platelet ADP receptor and α2-adrenoreceptor interaction. J Biol Chem 261: 5981–5986.
- Freiberger CE, Vanderjagt DJ, Pastuszyn A, Glew RS, Mounkaila G, Millson M, Glew RH (1998): Nutrient content of the edible leaves of seven wild plants from Niger. Plant Foods Hum Nutr 53: 57–69.
- Gerrad JM (1982): Platelet aggregation and the influence of prostaglandins, in: Lands, WEM Smith, WL, eds. Prostaglandins and Arachidonate Metabolites ( Methods in Enzymology series, Vol. 86). London, Academic Press, pp. 642–643.
- Hawiger J, Parkinson S, Timmons S (1980): Prostacyclin inhibits mobilization of fibrinogen binding sites on human ADP- and thrombin-treated platelets. Nature 283: 195–197.
- Heemsherk JWM, Sage O (1994): Calcium signaling in platelets and other cells. Platelets 5: 295–316.
- Kroll MH, Schafer AI (1989): Biochemical mechanisms of platelet activation. Blood 74: 1181–1195.
- Maguire MH, Csonka-Khalifah L (1987): Vinca alkaloids inhibit conversion of arachidonic acid to thromboxane by human platelet microsoms: Comparison with other microtubule-active drugs. Biochim Biophys Acta 921: 426–436.
- Mathur BS (2005): Moringa oleifera: A potential life-saver. Available at: http://www.treesforlife.org/project/moringa/book/moringa_book_view.pdf. (Accessed 2005).
- Mukherjee G, Chatterjee GC, Banerjee D, Bhattacharya DK (1990): Differential effect of retinoic acid on ADP and collagen induced platelet aggregation. Indian J Exp Biol 28: 49–952.
- Nambiar VS, Seshadri S (2001): Bioavailability trials of β-carotene from fresh and dehydrated drumstick leaves (Moringa oleifera) in a rat model. Plant Foods Hum Nutr 56: 83–95.
- Neiva TJC, Morais L, Polack M, Simoes MD, D’Amico EA (1999): Effect of catechins on human platelet aggregation and lipid peroxidation. Phytother Res 13: 597–600.
- Pignatelli P, Pulcinelli FM, Lenti L, Gazzania PP, Violi F (1998): Hydrogen peroxide is involved in collagen induced platelet activation. Blood 91: 484–490.
- Pignatelli P, Pulcinelli FM, Lenti L, Gazzania PP, Violi F (1999): Vitamin E inhibits collagen induced platelet activation by blunting hydrogen peroxide. Aterioscler Thromb Vasc Biol 19: 2542–2547.
- Rattan ISS (1988): Science behind spices: Inhibition of platelet aggregation and prostaglandin synthesis. Bioassays 5: 161–162.
- Sastri BN (1962): The Wealth of India, Raw Materials. New Delhi, India, Council of Scientific and Industrial Research, pp. 425–429.
- Scott JP, Montgomery RR, Retzimer GS (1991): Dimeric ristocetin flocculates proteins, binds to platelets and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem 266: 8149–8155.
- Shah BH, Nawaz Z, Petrani SA, Roomi A, Mahmood H, Saeed SA, Gilani AH (1999): Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxan formation and Ca2+ signaling. Biochem Pharmacol 58: 1167–1172.
- Son DJ, Cho, MR, Jin YR, Kim Sy, Park YH, Lee SH, Akiba S, Sato, T, Yun YP (2004): Antiplatelet effect of green tea catechins: A possible mechanism through arachidonic acid pathway. Prostaglandins Leukot Essent Fatty Acids 71: 25–31.
- Srinivasan K, Sambiah K, Chandrasekhar N (1992): Loss of active principles of common spices during domestic cooking. Food Chem 43: 271–274.
- Srivastava KC (1984): Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system. Prostaglandins Leukot Med 13: 227–235.
- Srivastava KC (1986): Onion exerts antiaggregatory effects by altering arachidonic acid metabolism in platelets. Prostaglandins Leukot Med 24: 43–50.
- Srivastava KC (1988): Extract of a spice omum (Trachyspermum ammi) shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids 33: 1–6.
- Srivastava KC (1989): Extracts from two frequently consumed spices – cumin (Cuminum cyminum) and turmeric (Cucuma longa) - inhibit platelet aggregation and alter eicosanois biosynthesis in human blood platelets. Prostaglandins Leukot Essent Fatty Acids 37: 57–64.
- Srivastava KC, Justesen U (1987): Inhibition of platelet aggregation and reduced formation of thromboxane and lipoxygenase products in platelets with oil of cloves. Prostaglandins Leukot Med 29: 11–18.
- Steel RGD, Torrie JH (1980): Principles and Procedures of Statistics: A Biometrical Approach, 2nd edn. New York, NY, McGraw-Hill, p. 187.
- Subramoniam A, Ana MNS (1988): Influence of certain dietary plant constituents on platelet aggregation. J Food Safety 9: 201–214.
- Suneetha WJ, Krishnakantha TP (2005a): Inhibition of human platelet aggregation and membrane lipid peroxidation by food spice, saffron. Mol Cell Biochem 278: 59–63.
- Suneetha WJ, Krishnakantha TP (2005b): Antiplatelet activity of coriander and curry leaf spices. Pharm Biol 43: 230–233.
- Suneetha WJ, Krishnakantha TP (2005c): Cardamom extract as inhibitor of human platelet aggregation. Phytother Res 19: 437–440.
