Abstract
Context: Agastache mexicana (Kunth) Lint & Epling (Lamiaceae) is a plant used in Mexican traditional medicine for the treatment of hypertension, anxiety and so on.
Objective: To determine the vasorelaxant effect and functional mode of action of dichloromethane-soluble extract from A. mexicana (DEAm) and isolate the constituents responsible for the pharmacological activity.
Materials and methods: Extracts were prepared from the aerial parts of A. mexicana (225.6 g) by successive maceration with hexane, dichloromethane and methanol (three times for 72 h at room temperature), respectively. DEAm (0.01–1000 μg/mL), fractions (at 174.27 μg/mL), acacetin and ursolic acid (UA) (0.5–500 μM) were evaluated to determine their vasorelaxant effect on ex vivo rat aorta ring model. In vivo UA antihypertensive action was determined on spontaneously hypertensive rats.
Results and discussion: DEAm induced a significant vasorelaxant effect in concentration-dependent and endothelium-independent manners (EC50 = 174.276 ± 5.98 μg/mL) by a calcium channel blockade and potassium channel opening. Bio-guided fractionation allowed to isolate acacetin (112 mg), UA (2.830 g), acacetin/oleanolic acid (OA) (M1) (155 mg) and acacetin/OA/UA (M2) (1.382 g) mixtures, which also showed significant vasodilation. UA significantly diminished diastolic (80 mmHg) and systolic blood pressure (120 mmHg), but heart rate was not modified.
Conclusion: DEAm produced significant vasorelaxant action by myogenic control cation. The presence of acacetin, OA and UA into the extract was substantial for the relaxant activity of DEAm. In vivo antihypertensive action of UA corroborates the use of A. mexicana as an antihypertensive agent on Mexican folk medicine.
Introduction
In Mexico, arterial hypertension has been increasing in its epidemiology due to personal habits of people (Arredondo & Zúñiga Citation2006; Rubinstein et al. Citation2009). Moreover, the treatment of hypertension is expensive, and patients do not adhere to therapy (Arredondo & Zúñiga 2006; Rubinstein et al. Citation2009). Furthermore, hypertension represents one of the main risk factors that provokes cardiovascular and cerebrovascular diseases, causing about 122,000 deaths in 2011. Medicinal plants have been used since ancient times as traditional therapeutics sources and currently, using this knowledge, for the discovery and development of new drugs. Thus, World Health Organization (WHO) recommends the use of medicinal plants to relieve various ailments, and the validation of therapeutic and toxicological effects for adequate quality and security of herbal medicinal products (Steinhoff Citation2004).
Agastache mexicana (Kunth) Lint & Epling (Lamiaceae) is a medicinal plant widely used in the Mexican traditional medicine for the treatment of anxiety, hypertension, and relief of heart disease, insomnia, and diabetes as well as to reduce stomach ache (Monroy-Ortiz & Castillo-España Citation2007). It has been previously reported that A. mexicana induced spasmolytic (Gónzalez-Trujano et al. Citation2012), antinociceptive and anti-inflammatory (González-Ramírez et al. Citation2012; Verano et al. Citation2013), anxiogenic (Molina-Hernández et al. Citation2000), depressant and anxiolytic (Estrada-Reyes et al. Citation2014; González-Trujano et al. Citation2015) and antioxidant effects (Ibarra-Alvarado et al. Citation2010). Moreover, we isolated tilianin from a methanol-soluble extract of A. mexicana, which showed a significant vasorelaxant action by NO/cGMP pathway and potassium channel opening in functional studies on aortic rat rings, and also showed a significant decrease in both systolic blood pressure (SBP) and diastolic blood pressure (DBP) on spontaneously hypertensive rats (SHR) in acute model (Hernández-Abreu et al. Citation2009). Other phytochemical studies allowed the identification of several secondary metabolites, such as acacetin, (2-acetyl)-7-O-glucosyl acacetin and so on (Estrada-Reyes et al. Citation2004). Thus, the aim of the current work was to determine the vasorelaxant effect and functional mode of action of dichloromethanic extract from A. mexicana (DEAm) and also conduct a bio-guided phytochemical analysis to identify the constituents responsible for the pharmacological activity.
Materials and methods
Chemicals and drugs
Carbamylcholine chloride (carbachol), noradrenaline bitartrate (NA), tetraethylammonium chloride (TEA) and nifedipine were purchased from Sigma-Aldrich Co. (St. Louis, MO). All other reagents were analytical grade from local sources. For ex vivo experiments, DEAm and acacetin were dissolved in DMSO (1% v/v) and other reagents dissolved in distilled water and sonicated just before use. Preliminary experiments showed that DMSO at 1% (v/v) had no effect on tension development of isolated aorta. For in vivo experiments, ursolic acid (UA) suspension was prepared in distilled water.
Plant material, extraction, isolation and structural elucidation of bioactive compounds
Aerial parts of A. mexicana were collected, at 4 months of age, in September 2012 in San Felipe Neri (19°02′21.4′′N 98°56′30.0′′W), Morelos, Mexico and identified by Dr. Patricia Castillo-España of the CEIB-UAEM. A voucher specimen (26336) was deposited at the HUMO-Herbarium of the ‘Centro de Investigación en Biodiversidad y Conservación (CIBYC-UAEM)’ from Morelos State University. Briefly, the aerial parts of dried plant material were ground into powder (225.6 g) and crude extracts were prepared by successive maceration with hexane, dichloromethane and methanol (three times for 72 h at room temperature), respectively. After filtration, extracts were concentrated in vacuo at 40 °C until dry. In order to isolate bioactive compounds from DEAm, the extract (40 g) was separated by column chromatography to yield 643 fractions (200 mL each), grouped into nine pools according to similar chromatographic profiles. The mixture of oleanolic acid (OA)/acacetin (M1) was obtained from pool 6, and UA, acacetin and the mix acacetin/UA/OA (M2) was obtained from pool 7 (see “Results” section). Structural elucidation of UA and acacetin was achieved by comparison of their physical and spectral data (IR, 1H NMR and 13C NMR) with those reported in the literature (Supporting Information, S1) (Bei-Bei et al. Citation2011; Topçu et al. Citation2011; Gónzalez-Trujano et al. Citation2012).
Animals
To determine the vasorelaxant activity and the mode of action of DEAm, acacetin, UA, M1 and M2, male Wistar rats (250–350 g) were used. For the evaluation of the antihypertensive effect of UA and captopril (positive control) male SHR (250–350 g) were used. All animal procedures were conducted in accordance with our Federal Regulations for Animal Experimentation and Care (SAGARPA, NOM-062-ZOO-1999, México) and approved by the Institutional Animal Care and Use Committee based on US National Institute of Health publication (No. 85-23, revised 1985). All experiments were carried out using six animals per group.
Preparation of aortic rings
Rats were sacrificed by exposure to ether and the thoracic aortas were removed and immersed in Krebs solution at room temperature. Aortic rings (3–5 mm) were obtained free of connective tissue and fat (in some rings the endothelium was removed), and suspended by platinum hooks under an optimal tension of 3 g in a Krebs solution (composition, mM: NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; EDTA, 0.026; glucose, 11.1, pH 7.4), maintained at 37 °C in an atmosphere of 95% O2 and 5% CO2. Isometric tension was measured and recorded using Grass-FT03 force transducers (Astromed®, West Warwick, RI) connected to a MP100 analyser (BIOPAC® Instruments, Santa Barbara, CA) (Estrada-Soto et al. Citation2010).
Effect of DEAm, fractions, acacetin, UA, M1 and M2 on the contraction induced by NA and KCl
The aortic rings with and without endothelium were pre-contracted with NA (0.1 μM) and KCl (80 mM). Once the plateau was attained, concentration–response curves (CRCs) of DEAm, acacetin, UA, M1 and M2 and positive controls were obtained by adding cumulative concentrations of the test samples to the bath (Estrada-Soto et al. Citation2010).
Determination of DEAm mode of action
For this purpose, three sets of experiments were designed (Estrada-Soto et al. Citation2010; Vergara-Galicia et al. Citation2010).
Effect of DEAm on the contraction induced by NA
Endothelium-denuded aortic rings were incubated with 112, 200 and 625 μg/mL of DEAm during 15 min, and then NA was cumulative added at different concentrations (0.1 nM to 10 μM). Finally, the contractile effect induced by NA was compared in the absence (control group) and presence of the extract.
Effect of DEAm on extracellular Ca2+-induced contraction activated by KCl
To determine whether the inhibition of extracellular Ca2+ influx was involved in DEAm-induced relaxation, the experiments were carried out in Ca2+-free Krebs solution. Endothelium-denuded aortic rings were washed with Ca2+-free solution (approximately 30 min) and then rinsed with Ca2+-free solution containing KCl (80 mM). The cumulative CRCs for CaCl2 (80 μM to 27 mM) were obtained in the absence of DEAm (control group) or after 30 min incubation with test sample (112, 200 and 625 μg/mL). Finally, the contractile effect induced by CaCl2 was compared in the absence (control group) and presence of DEAm.
Role of K+ channel in DEAm-induced relaxation
In order to know the role of K+ channels on DEAm-induced relaxation, endothelium-denuded arterial rings were pre-incubated with the nonselective K+ channel blocker TEA (5 mM), which was added 15 min before NA (0.1 μM), and then DEAm was added cumulatively (0.01 to 1000 μg/mL).
In vivo experiments
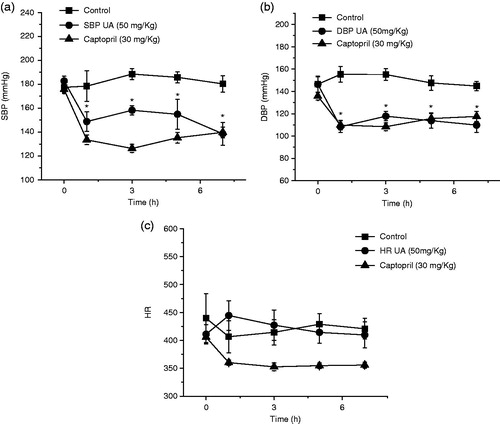
Antihypertensive activity study of UA was conducted in SHR rats. Animals were allotted into three groups (six animals each): control rats (CR, group 1), positive control (captopril 30 mg/kg) and treated rats (UA, group 3). Treated group received a single intragastric dose of UA (50 mg/kg). Measurements [blood pressure and heart rate (HR)] were recorded before and after the treatment of test compound at 0, 1, 3, 5 and 7 h a tail cuff method using a LE 5001 automatic blood pressure meter (PanLab™ Harvard Apparatus, Barcelona ES). Percent decrease in HR, SBP and DBP was calculated.
Data analysis
Data were expressed as means ± standard error of the mean (SEM). Statistical analysis was conducted using one-way ANOVA, followed by Tukey’s post hoc test. The p < 0.05 was considered to be significant. CRC were plotted and the obtained experimental data were adjusted by a nonlinear curves fitting program (ORIGIN 8.0, Northampton, MA). Maximal changes (expressed as a percentage of baseline values) in HR, SBP and DBP along the recording time were used to construct time–response curves.
Results and discussion
Novel therapeutic strategies focused on vascular smooth muscle relaxation hold great potential for the prevention and treatment of hypertension. Moreover, modern treatment of hypertension is mainly based on impede vascular smooth muscle contraction (Brunton et al. Citation2006). Medicinal plants are important element of Mexican folk medicine and many are considered as a part of the cultural heritage. In this context, several of them are used for the treatment of hypertension and constituted a wonderful source for the isolation of new leads for development of new antihypertensive drugs (Castillo-España et al. Citation2009). Thus, A. mexicana preparations are currently used in herbal Mexican medicine for the treatment of several diseases, including hypertension. Previous reports indicate that A. mexicana has not been widely studied from the pharmacological (antihypertensive) and chemical point of view, since only had been reported that methanol-soluble extract and tilianin, obtained from A. mexicana, induce vasorelaxant effect on aortic rings and antihypertensive activity (Hernández-Abreu et al. Citation2009). Therefore, we decided to examine the mode of vasodilator action of dichloromethane-soluble extract acquired from A. mexicana (DEAm), realize the bio-guided phytochemical study for the isolation of main vasorelaxant agents, among tilianin, and to determine the in vivo antihypertensive effect of UA on SHR rat model.
DEAm induced a concentration-dependent and endothelium-independent relaxant activity on NA 0.1 μM and KCl 80 mM pre-contracted aortic rings with maximal relaxant effect values (), suggesting that vasodilation is produced by the interference on a common pathway which several receptor agonists exert, such as the augment of free cytosolic Ca2+ levels. In this regards, in smooth muscle cells there are two kinds of Ca2+ channels, voltage-dependent Ca2+ channels (high KCl induced contraction due to membrane depolarization, leading to augmented Ca2+ entry through voltage-dependent channels) and receptor operated Ca2+ channels (contraction induced by NA in Ca2+-free medium is due to intracellular Ca2+ release, through sarcoplasmic reticulum Ca2+ channels activated by IP3). Therefore, agents acting directly on the vascular smooth muscle cell may alter tone by three mechanisms: altering intracellular Ca2+ concentrations ([Ca2+]i), varying the sensitivity of the contractile regulatory apparatus to [Ca2+]i or modulating the sensitivity to other vasoactive inputs (Huang & Ho Citation1996). So, DEAm was capable of inhibiting vasoconstriction induced by KCl and NA in endothelium-denuded aortic rings, and also inhibited the concentration–response contraction of NA in a nonparallel manner and depressed its maximal response (, and and , respectively), suggesting that extract might block both voltage-dependent and receptor operated Ca2+ channels. In addition, we found that 112, 200 and 625 μg/mL of the extract significantly inhibited CaCl2-induced contraction in a nonparallel manner and decreased its maximal responses compared with control (), giving evidence that DEAm possesses a Ca2+ entry blocking activity. Moreover, the opening of K+ channels in vascular smooth muscle cells induces hyperpolarization of membrane potential, which provides an important mechanism to dilate arteries (Estrada-Soto et al. Citation2010). In this context, the Ca2+-activated K+ channel blocker TEA (), significantly inhibited the vasodilator effect of DEAm. Therefore, both results suggest that opening K+ channels perhaps are indirect involved in the mechanism of action of DEAm by means of blocking of Ca2+ channels.
Figure 1. (a) Vasorelaxant effect of DEAm on the contraction induced by NA (0.1 μM) in endothelium-intact and -denuded aortic rings, (b) vasorelaxant effect of DEAm on the contraction induced by KCl (80 mM) on endothelium-denuded aortic rings. Results are presented as mean ± SEM, n = 6, *p < 0.05 compared with control.
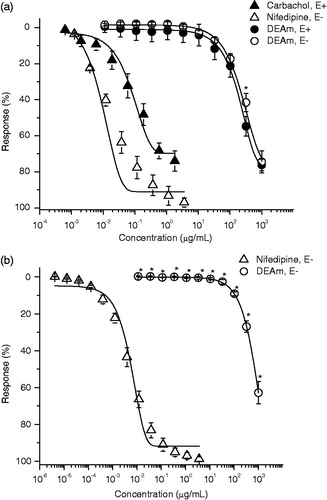
Figure 2. (a) Inhibitory effects of DEAm on the cumulative contraction induced by NA (0.1 nM to 10 μM) in endothelium-denuded aortic rings, (b) inhibitory effect of DEAm on the cumulative-contraction effect dependent on extracellular Ca2+ influx induced by KCl (80 mM) in Ca2+-free solution, and (c) effect of TEA (5 mM) on DEAm induced relaxation in endothelium-denuded aortic rings pre-contracted by NA (0.1 μM). Results are presented as mean ± SEM, n = 6, *p < 0.05 compared with control.
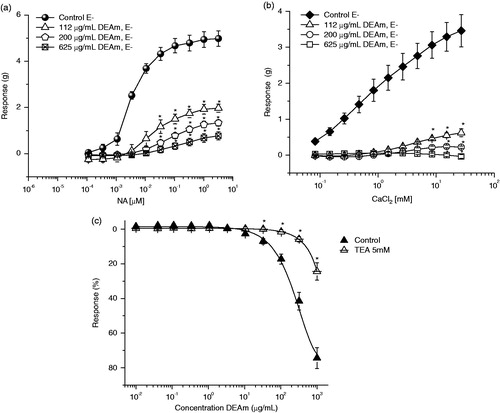
Table 1. Relaxatory effects induced by test samples on the contraction induced by NA (0.1 μM).
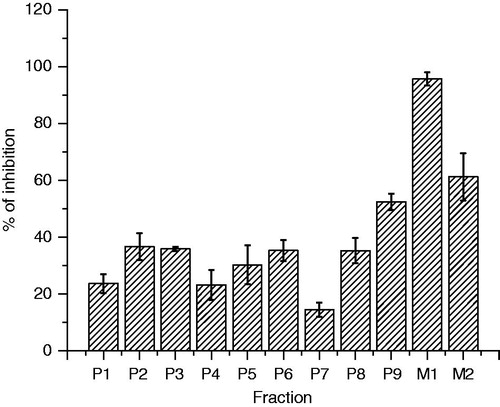
However, in order to isolate the responsible compounds of the vasorelaxant effect of DEAm, extract was subjected to a bio-guided fractionation by open column chromatography and was obtained 643 fractions (). From a primary fractionation, it was obtained nine pool fractions (P1–P9), which were evaluated using the EC50 calculated for DEAm (175 μg/mL) in rat aortic rings bioassay. P1–P9 vasorelaxant evaluations allowed us to establish that those with the best activity were P2, P3, P5, P6, P8 and P9 as shown in .
Figure 3. Bio-guided fractionation of A. mexicana by using column chromatography and the chemical structure of acacetin, UA, and OA.
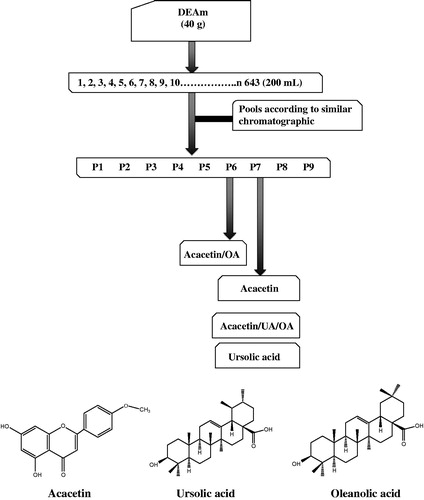
Figure 4. Percentage inhibition of primary fractions obtained DEAm phytochemical study. Results are presented as mean ± SEM, n = 6, *p < 0.05 compared with control.
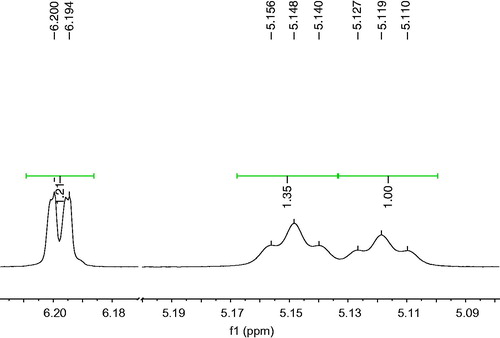
From active fraction P6 (36% of inhibition) a mixture of acacetin/OA (M1) was obtained, which was evaluated using the same ex vivo bioassay, showing a significant percentage of inhibition of 95.65% at 175 μg/mL (). P7 showed a lower percentage of inhibition; however, from this it was isolated acacetin, UA and the mixtures of acacetin/UA/OA (M2) by sequential precipitation. To establish the proportion of each compound in M1 and M2, it was analysed their 1H NMR spectra (). For mixture M1, it was compared the area under the curve (AUC) of the selective signals and their corresponding integrals at δ 6.12 ppm (H6 for acacetin) and δ 5.14 ppm (H12 for OA) from which were obtained the following proportion: acacetin (43%)/OA (57%). Moreover, for M2 analysis content, it was followed same procedure described above where signals at δ 6.12 ppm (H6 for acacetin), δ 5.11 ppm (H12 for UA) and δ 5.14 ppm (H12 for OA) were analysed to establish next proportion: acacetin (33.98%)/UA (37.92%)/OA (28.08%).
Figure 5. AUC of the selective signals and their corresponding integrals of the mixture of acacetin/UA/OA.
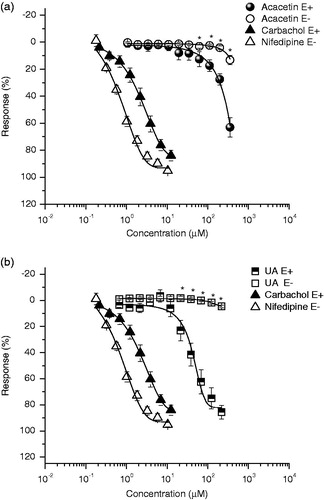
M1 and M2 showed 98.1% and 61.22% of inhibition of the contraction induced by NA (), respectively. Also, acacetin (Emax = 63.4% and EC50 = 210.84 μM) and UA (Emax = 86% and EC50 = 39.56 μM) induced a significant concentration-dependent and endothelium-dependent relaxant activity on the contraction induced by NA (0.1 μM) (, ). UA was the most potent agent evaluated, but less potent than positive controls used (carbachol and nifedipine). Our results are in agreement with those previously relaxant effect described for UA (Aguirre-Crespo et al. Citation2006; Rios et al. Citation2012), OA (Rodriguez-Rodriguez et al. Citation2004; Rios et al. Citation2012) and acacetin (Calderone et al. Citation2004), which induce their effect by an augment of NO production-derived endothelium. With these results, we partially explain the relaxant effect of DEAm, because all compounds isolated induced their effect in an endothelium-dependent manner. Further experiments are in progress in order to isolate bioactive compounds responsible for the nonendothelium-dependent relaxation.
Figure 6. (a) Vasorelaxant effect of acacetin on the contraction induced by NA (0.1 μM) in endothelium-denuded aortic rings and (b) vasorelaxant effect of UA on the contraction induced by NA (0.1 μM) in endothelium-denuded aortic rings. Results are presented as mean ± SEM, n = 6, *p < 0.05 compared with control.
Finally, UA was capably of evoking a significant decrease of SBP and DBP and did not modify HR (1 h after administration) on SHR rats (p < 0.05) (). However, UA was less potent than captopril (30 mg/kg) in reducing SBP and showed a similar effect in lowering DBP. To the best of our knowledge, there are no previous studies describing the antihypertensive effect of UA on SHR model; however, it was reported the antihypertensive effect of UA due its diuretic–natriuretic-saluretic activities on Dahl salt-sensitive rat model (Somova et al. Citation2003).
Conclusions
In conclusion, DEAm produced significant vasorelaxant action by myogenic control action (calcium channel blockade and potassium channel opening). The presence of acacetin, OA and UA into the extract was substantial for the relaxant activity of DEAm. In addition, in vivo antihypertensive action of UA corroborates the use of A. mexicana as an antihypertensive agent on Mexican folk medicine.
Funding information
This study was financed by a grant from ‘SEP-CONACyT (Proyecto de Ciencia Básica CB-2011-01 No.167044)’ and Faculty of Pharmacy Budgets (2014 and 2015).
Disclosure statement
The authors declare no conflict of interest.
References
- Aguirre-Crespo F, Vergara-Galicia J, Villalobos-Molina R, López-Guerrero JJ, Navarrete-Vázquez G, Estrada-Soto S. 2006. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 79:1062–1068.
- Arredondo A, Zúñiga A. 2006. Epidemiologic changes and economic burden of hypertension in Latin America: evidence from Mexico. Am J Hypertens. 19:553–559.
- Bei-Bei Z, Yuan D, Zhi-Xin L. 2011. Chemical constituents of Saussurea eopygmaea. Chin J Nat Med. 9:33–37.
- Brunton LL, Lazo JS, Parker KL. 2006. Goodman and Gilman’s. The pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill.
- Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, Breschi MC, Martinotti E. 2004. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 370:290–298.
- Castillo-España P, Cisneros-Estrada A, Garduño-Ramírez ML, Hernández-Abreu O, Ramírez R, Estrada-Soto S. 2009. Preliminary ethnopharmacological survey of plants used in Mexico for the treatment of hypertension. Pharmacog Rev. 3:41–65.
- Estrada-Reyes R, Aguirre Hernández E, García-Argáez A, Soto Hernández M, Linares E, Bye R, Heinze G, Martínez-Vázquez M. 2004. Comparative chemical composition of Agastache mexicana subsp. mexicana and A. mexicana subsp. xolocotziana. Biochem Syst Ecol. 32:685–694.
- Estrada-Reyes R, López-Rubalcava C, Ferreyra-Cruz OA, Dorantes-Barrón AM, Heinze G, Moreno Aguilar J, Martínez-Vázquez M. 2014. Central nervous system effects and chemical composition of two subspecies of Agastache mexicana; an ethnomedicine of Mexico. J Ethnopharmacol. 153:98–110.
- Estrada-Soto S, Rivera-Leyva JC, Ramírez-Espinosa JJ, Castillo-España P, Aguirre-Crespo F, Hernández-Abreu O. 2010. Vasorelaxant effect of Valeriana edulis ssp. procera (Valerianaceae) and its mode of action as calcium channel blocker. J Pharm Pharmacol. 62:1167–1174.
- González-Ramírez A, González-Trujano ME, Pellicer F, López-Muñoz FJ. 2012. Anti-nociceptive and anti-inflammatory activities of the Agastache mexicana extracts by using several experimental models in rodents. J Ethnopharmacol. 142:700–705.
- González-Trujano ME, Ponce-Muñoz H, Hidalgo-Figueroa S, Navarrete-Vázquez G, Estrada-Soto S. 2015. Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin. Asian Pac J Trop Med. 8:185–190.
- González-Trujano ME, Ventura-Martínez R, Chávez M, Díaz-Reval I, Pellicer F. 2012. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 78:793–796.
- Hernández-Abreu O, Castillo-España P, León-Rivera I, Ibarra-Barajas M, Villalobos-Molina R, González-Christen J, Vergara-Galicia J, Estrada-Soto S. 2009. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem Pharmacol. 78:54–61.
- Huang Y, Ho IH. 1996. Separate activation of intracellular Ca2+ release, voltage-dependent and receptor-operated Ca2+ channels in the rat aorta. Chin J Physiol. 39:1–8.
- Ibarra-Alvarado C, Rojas A, Mendoza S, Bah M, Gutiérrez DM, Hernández-Sandoval L, Martínez M. 2010. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm Biol. 48:732–739.
- Molina-Hernández M, Téllez-alcántara P, Martínez E. 2000. Agastache mexicana may produce anxiogenic-like actions in the male rat. Phytomedicine. 7:199–203.
- Monroy-Ortiz C, Castillo-España P. 2007. Medicinal plants used in the State of Morelos. 2nd ed. Morelos, Mexico: Centro de Investigaciones Biológicas, Universidad Autónoma del Estado de Morelos.
- Rios MY, López-Martínez S, López-Vallejo F, Medina-Franco JL, Villalobos-Molina R, Ibarra-Barajas M, Navarrete-Vázquez G, Hidalgo-Figueroa S, Hernández-Abreu O, Estrada-Soto S. 2012. Vasorelaxant activity of some structurally-related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: ex vivo and in silico studies. Fitoterapia. 83:1023–1029.
- Rodriguez-Rodriguez R, Herrera MD, Perona JS, Ruiz-Gutiérrez V. 2004. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br J Nutr. 92:635–642.
- Rubinstein A, Alcocer L, Chagas A. 2009. High blood pressure in Latin America: a call to action. Ther Adv Cardiovasc Dis. 3:259–285.
- Somova LO, Nadar A, Rammanan P, Shode FO. 2003. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 10:115–121.
- Steinhoff B. 2004. New documents for the assessment of herbal medicinal products. Phytomedicine. 11:467–468.
- Topçu G, Yapar G, Türkmen Z, Gören AC, Öksüz S, Schilling JK, Kingston DGI. 2011. Ovarian antiproliferative activity directed isolation of triterpenoids from fruits of Eucalyptus camaldulensis Dehnh. Phytochem Lett. 4:421–425.
- Verano J, González-Trujano ME, Déciga-Campos M, Ventura-Martínez R, Pellicer F. 2013. Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol Biochem Behav. 110:255–264.
- Vergara-Galicia J, Ortiz-Andrade R, Rivera-Leyva JC, Castillo-España P, Villalobos-Molina R, Ibarra-Barajas M, Gallardo-Ortiz I, Estrada-Soto S. 2010. Vasorelaxant and antihypertensive effects of methanolic extract from roots of Laelia anceps are mediated by calcium-channel antagonism. Fitoterapia. 81:350–357.