Abstract
Context: Nutmeg [Myristica fragrans Houtt. (Myristicaceae)] has a long-standing reputation of psychoactivity. Anecdotal reports of nutmeg use as a cheap marijuana substitute, coupled to previous studies reporting a cannabimimetic-like action, suggest that nutmeg may interact with the endocannabinoid system.
Objective: The study evaluates nutmeg fractions for binding capacity with various CNS receptors and their potential interaction with the endocannabinoid system.
Materials and methods: Dichloromethane (DF) and ethyl acetate (EF) fractions were prepared from the methanol extract of powdered whole nutmeg. The HPLC-profiled fractions were assayed by the NIMH Psychoactive Drug Screening Program (PDSP) in a panel of CNS targets at a 10 μg/mL concentration. The fractions were also screened for fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) inhibition, initially at a concentration of 500 μg/mL, then by concentration-dependent inhibition studies.
Results: None of the tested fractions showed significant binding to CNS receptors included in the PDSP panel. However, both fractions exerted significant inhibition of the FAAH and MAGL enzymes. The DF fraction inhibited FAAH and MAGL enzymes at IC50 values of 21.06 ± 3.16 and 15.34 ± 1.61 μg/mL, respectively. Similarly, the EF fraction demonstrated FAAH and MAGL inhibition with IC50 values of 15.42 ± 3.09 and 11.37 ± 6.15 μg/mL, respectively.
Discussion and conclusion: The study provides the first piece of evidence that nutmeg interacts with the endocannabinoid system via inhibition of the endocannabinoid catabolizing enzymes. This mechanism provides insight into reported cannabis-like action as well as expands the potential therapeutic utility of nutmeg.
Keywords:
Introduction
Nutmeg, the commonly used kitchen spice, is the dried kernel of the seeds of Myristica fragrans Houtt (Myristicaceae), an evergreen tree indigenous to the Molucca (East Indies) and Caribbean (West Indies) islands. In addition to its common use as a spice, alternative medicine literature reports several medicinal uses of nutmeg as antidiarrheal, stimulant, bitter stomachic and aphrodisiac (Evans Citation1996; Nadkarni Citation1998; Tajuddin et al. Citation2005). Further preclinical studies have also attributed antimicrobial, antihelmintic, anti-inflammatory, as well as cardio- and hepatoprotective properties to nutmeg extracts (Ozaki et al. Citation1989; Takikawa et al. Citation2002; Morita et al. Citation2003; Kareem et al. Citation2009; López et al. Citation2015). Nutmeg kernel is rich in essential oils, fixed oils, lipids, starch and proteins (Khan & Abourashed Citation2010). Lignans/neolignans and diaryl alkanes also constitute major groups of bioactive secondary metabolites in terms of chemical diversity and number of compounds isolated from nutmeg (Cuong et al. Citation2014; Cao et al. Citation2015).
As early as the 12th century, nutmeg has been used and known for its central nervous system activity. Available literature has recognized a myriad of nervous system activities of nutmeg and its major constituents. Earlier anecdotes report psychoactive and hallucinogenic properties of nutmeg (Truitt et al. Citation1961). These reports were the basis of Shulgin’s hypothesis that attributed nutmeg’s psychoactivity to metabolic conversion of its main constituent, myristicin, to amphetamine-like metabolites (Shulgin Citation1966). So far, the hypothesis has not been experimentally supported. Inconsistent animal findings and lack of detection of the amphetamine-like metabolites in biological fluids of nutmeg abusers led to reevaluation of the validity of the hypothesis (Braun Citation1973; Beyer et al. Citation2006). Further experimental data have ascribed several additional nervous system effects to nutmeg. Hayfaa et al. (Citation2013) reported analgesic activity of alkaloids extracted from nutmeg in acetic acid-induced writhing animal model. This is in support of earlier reports of the analgesic activity of the n-hexane nutmeg extract (Sonavane et al. Citation2001; Grover et al. Citation2002).
Neurobehavioral effects exerted by nutmeg have been documented in various animal models, with numerous activities reported. Sonavane et al. (Citation2002) reported an anxiogenic activity exerted by the n-hexane extract of nutmeg as well by trimyristin. Additionally, an anxiogenic effect has been experimentally demonstrated by myristicin, another major nutmeg constituent (Leiter et al. Citation2011). On the other hand, Ayurvedic literature reports the use of aqueous nutmeg extract as an anxiolytic agent (Sharma Citation2001). Such claim has not been substantiated by experimental dependent anxiolytic activity of aqueous nutmeg extract in the open field test experimental model. Similar to reported results for its effect on anxiety, conflicting data have been documented for nutmeg (and its components) effect on depression. Dhingra and Sharma (Citation2006) and Moinuddin et al. (Citation2012) reported antidepressant activity of nutmeg extracts in both models of behavioral despair as well in reserpine reversal test paradigms, respectively. The studies also suggested the involvement of adrenergic, serotonergic and dopaminergic systems in the observed antidepressant effect, since it was inhibited by α1 and dopaminergic receptor antagonists as well as a serotonin synthesis inhibitor. On the other hand, tryimyristin exerted a depressant behavior when tested in behavioral despair animal models and potentiated hypothermia induced by reserpine. The observed effects were blocked by pre-administration of a serotonin 5-HT2A receptor antagonist studies (Kasture & Gujar Citation2005).
It is evident that various neurological activities have been reported for nutmeg. Currently, the use of nutmeg seems to be rapidly expanding from its traditional spice use to a low-cost, recreational drug use. Unfortunately, the widespread nutmeg use resulted in reported cases of psychotropic and rare fatal effects following high nutmeg intake (Servan et al. Citation1998; Sangalli & Chiang Citation2000; Stein et al. Citation2001; Demetriades et al. Citation2005; Forrester Citation2005; Ehrenpreis et al. Citation2014). Thus, the need for detailed pharmacological evaluation of the neurological effects of nutmeg and proper understanding of the mechanism of action of its constituents is ever increasing. A previous study in our laboratory evaluated the neurobehavioral effects of nutmeg in the four-point tetrad assay as compared to common drugs of abuse, Δ9-tetrahydrocannabinol (Δ9-THC), morphine, and amphetamine. The results of the study showed that nutmeg extracts have various activities in the assay, depending on the nature of the extract, as well as the route of administration. The study demonstrated that the dichloromethane nutmeg extract, when injected i.p., exerted some cannabimimetic activity in the tetrad assay (El-Alfy et al. Citation2009). Despite the numerous nervous system activities reported for nutmeg, the mechanism underlying these effects remains unclear.
Reviewing the literature reveals a few studies that examined the mechanism of neurological activities attributed to nutmeg. The CNS stimulant/hallucinogenic effects of nutmeg have been attributed to the metabolic conversion of myristicin to amphetamine-like compounds (Shulgin Citation1966). In addition to recent reports that disqualify Shulgin’s hypothesis, myristicin did not show any significant effect in the tetrad assay (El-Alfy et al. Citation2009). An earlier study has also associated myrsiticin with a monoamine oxidase (MAO) inhibition activity (Truitt et al. Citation1963). Recent studies reported an acetylcholinesterase inhibitory action for n-hexane nutmeg extract (Dhingra et al. Citation2006) and three pure compounds isolated from the ethyacetate extract (Cuong et al. Citation2014). As obvious, mechanistic studies are highly needed to better understand the full spectrum of nutmeg’s neurological activities and to provide a safe and effective medicinal use of this common natural product. In an effort to understand the mechanism of nutmeg action, this study aimed at evaluating the receptor binding capacity of specific nutmeg fractions, as well as their potential interaction with the endocannabinoid system in an effort to explain the previously reported partial cannabimimetic action.
Materials and methods
Preparation of nutmeg fractions
Whole nutmeg kernels were purchased from Mond Trading, Toronto, ON, Canada. A certificate of authenticity was provided by the supplier. Whole kernels were pulverized to a homogenous powder in a coffee grinder. All solvents used for extraction and fractionation were of reagent grade (Fisher Scientific, Waltham, MA). Powdered whole nutmeg (100 g) was ultrasonicated in methanol for 1 h, left to soak at room temperature overnight, filtered and the filtrate stored in a freezer overnight then refiltered on cold to remove waxy material. The filtrate was concentrated at 45 °C under vacuum to prepare the defatted total extract (DTE, 14.9 g). TE (1 g) was placed on a silica bed (10 g) in a glass column and successively eluted with four solvents of increasing polarity in the following order: n-hexane, dichloromethane, ethyl acetate and methanol (100 mL each). Each solvent was dried at 45 °C under vacuum to yield four separate fractions of the total extract: hexane fraction (HF, 0.05 g), dichloromethane fraction (DF, 0.59 g), ethyl acetate fraction (EF, 0.26 g) and residual methanol fraction (MF, 0.05 g).
HPLC profiling of extracts
An in-house HPLC method was used for fingerprinting nutmeg extract and fractions. It utilized an LC-2010 system (Shimadzu, Japan) equipped with an autoinjector, UV detector and a reversed-phase column (HyPurity®, C18, 3μ, 150 × 4.6 mm, Thermo Scientific, Waltham, MA). Gradient elution of acetonitrile (ACN) in 0.1% aqueous formic acid was performed as follows: 40% ACN, 2 min; 40–80%, 22 min; 100% ACN, 2 min; 40% ACN, 4 min. Flow rate, 1 mL/min; detection, 270 nm; injection volume, 10 μL. HPLC system control, data acquisition and management was performed by LCSolution® (Shimadzu) running under MS Windows Vista.
Pharmacological evaluation of extracts
Receptor binding assays
HPLC-profiled nutmeg extracts were assayed by the NIMH Psychoactive Drug Screening Program (PDSP) which provides screening services of pharmacological activity in the panel of cloned human and rodent receptors, channels and transporters (Besnard et al. Citation2012). Primary assays were performed at 10 μg/mL concentration. Four replicate experiments were conducted for each fraction at every receptor subtype tested. Data represent the mean percent inhibition, where significant was considered >50%.
FAAH inhibition assay
The FAAH inhibitor screening assay kit was purchased from Cayman Chemicals and was used for evaluation of nutmeg fractions’ enzyme inhibition activity. The assay procedure was performed according to the manufacturer’s instructions. All samples were dissolved in DMSO. The compound CAY10345 (Cayman Chemicals) was used as a positive control in concentrations of 0.3125–40 nM. The assay buffer (125 mM Tri-HCl with 1 mM EDTA, pH 9.0) was used to dilute the FAAH human recombinant enzyme and arachidonoyl amide, which was then used as the FAAH substrate at a concentration of 400 mM. Samples were mixed for 30 s and incubated at 37 °C for 30 min. The fluorescent byproduct 7-amino-4-methylcoumarin (AMC), released by the FAAH enzyme was detected and quantified at an excitation/emission wavelengths of 360 and 460 nm, respectively.
MAGL inhibition assay
The MAGL inhibitor screening assay kit was purchased from Cayman Chemicals and was used for evaluation of nutmeg fractions’ enzyme inhibition activity. All samples were dissolved in DMSO. The compound JZL 184 (Cayman Chemicals) was used as the positive control at concentrations of 0.0.125–80 nM. Manufacturer’s protocol was followed to perform the assay. Human recombinant MAGL enzyme was diluted with assay buffer (10 mM Tris-HCl with 1 mM EDTA, pH 7.2) and the substrate used was a 4.25 mM concentration of an ethanol solution of 4-nitrophenylacetate. The plate was mixed for 30 s and incubated at room temperature for 30 min. The microplate was read at an absorption wavelength of 410 nm in order to detect the byproduct 4-nitrophenol.
Data analysis
Percent inhibition was calculated for each compound using the formula [(100% Activity − Sample Activity)/100% Activity] × 100. All data is presented as mean percent inhibition ± standard error mean (SEM), unless otherwise noted. GraphPad Prism (Version 5.03, La Jolla, CA) was used to analyze the non-linear curve fit concentration inhibition curves and to determine the IC50 values of the active nutmeg fractions. All data represent the mean of three replicate experiments.
Results
HPLC profiling of nutmeg extract and fractions
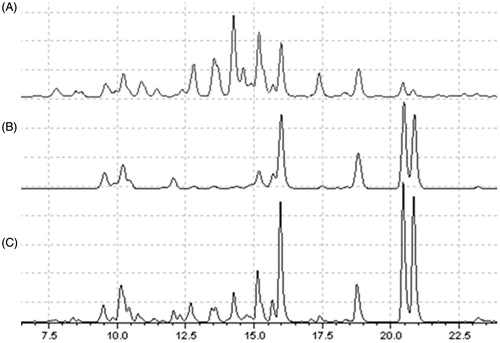
HPLC fingerprints obtained for DF () and EF () were not identical. When considered collectively, however, they contain all the major constituents present in DTE ().
Receptor binding assays
The dichloromethane (DF) and ethyl acetate (EF) fractions were both evaluated for binding to various CNS receptors. In primary screening assays at a concentration of 10 μg/mL, neither fractions showed significant binding (>50%) to 5HT receptors (5HT1-7, including subtypes), α- and β-adrenergic various subtypes, muscarinic M1-M5 receptors, benzodiazepine brain binding site, dopaminergic receptors (D1-D5), histaminergic H1 and H2 receptors, or cannabinoid receptors CB1 and CB2.
FAAH inhibition by nutmeg extracts
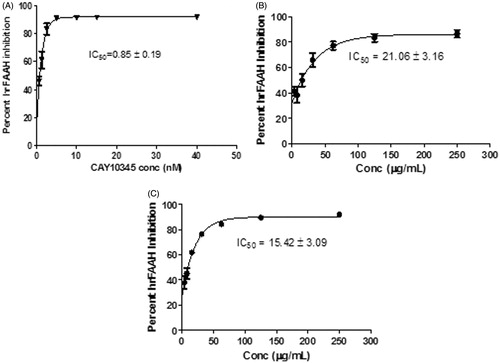
As shown in , the positive control CAY10345 strongly inhibited FAAH enzyme with an IC50 value of 0.85 ± 0.19 nM concentration. The dichloromethane fraction (DF) exhibited a concentration-dependent inhibition of the enzyme, with an IC50 value of 21.06 ± 3.16 μg/mL well concentration. Similarly, the ethyl acetate fraction (EF) exerted a concentration-dependent inhibition of FAAH enzyme, at an IC50 value of 15.42 ± 3.09 μg/mL. The degree of inhibition was not significantly different between the two nutmeg fractions.
MAGL inhibition by nutmeg extracts
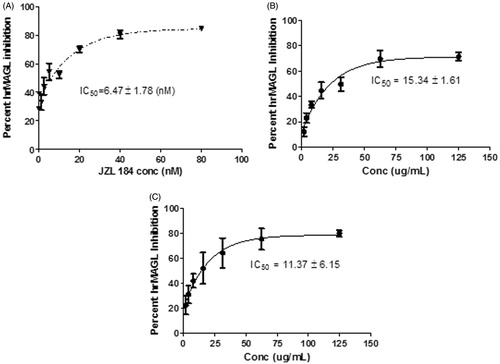
depicts the concentration inhibition curves for the positive control, JZL 184 and the two nutmeg fractions. The compound JZL 184 strongly inhibited FAAH enzyme with an IC50 value of 6.47 ± 1.78 nM concentration. The dichloromethane nutmeg fraction (DF) exhibited a concentration-dependent inhibition of the enzyme, with an IC50 value of 15.34 ± 1.61 μg/mL well concentration. Similarly, the ethyl acetate fraction (EF) exerted a concentration-dependent inhibition of FAAH enzyme, at an IC50 value of 11.37 ± 6.15 μg/mL well concentration. The degree of inhibition was not significantly different between the two nutmeg fractions.
Discussion
Our earlier work with nutmeg extracts prepared using different solvents showed that they displayed a common fingerprint with varying levels of UV-active marker compounds. In this study, we followed a classical phytochemical approach in preparing nutmeg samples. This approach is based on successive extraction of a defatted total methanol extract of nutmeg using solvents of increasing polarity. Of the four fractions prepared, the least and most polar ones (HF and MF, respectively) provided lowest yields and none of the characteristic constituents of the nutmeg HPLC fingerprint. The mid-polarity fractions (DF and EF) had the highest yields and clearly displayed the major constituents of the HPLC fingerprint (). Thus, DF and EF were the main focus of our in vitro evaluation. Despite earlier reports of prominent neurobehavioral effects exerted by nutmeg in experimental animal models (Sonavane et al. Citation2001, Citation2002; Grover et al. Citation2002; Leiter et al. Citation2011; Hayfaa et al. Citation2013) as well as human anecdotal reports (Truitt et al. Citation1961; Sharma Citation2001), receptor binding evaluation revealed no significant binding to common CNS receptors involved in the reported nutmeg activities. Previous data collected in our laboratory showed cannabimimetic effects of nutmeg extracts in the mouse tetrad assay (El-Alfy et al. Citation2009). However, no significant binding to the cannabinoid receptors CB1 or CB2 was observed in this study. In an attempt to understand the mechanism of action of nutmeg and the potential role of endocannabinoid system in its neurological effects, the current study focused on evaluating the interaction between the DF and EF nutmeg fractions and the endocannabinoid degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). The results of the study show that both DF and EF nutmeg fractions possess significant inhibitory effects on both enzymes. The effect proved to be concentration-dependent, and the IC50 of both fractions were determined. To our knowledge, this is the first study that reports an effect of nutmeg on the endocannabinoid system.
The endocannabinoid system is a complex neuromodulatory network involved in numerous physiological functions such as appetite, pain, reward, motor control, memory and cognition. The primary receptors component of the system are the cannabinoid 1 receptor (CB1), predominantly located in the central nervous system, and cannabinoid 2 receptor (CB2), located in the periphery. Numerous endogenous ligands such as anandamide (AEA), 2-arachidonoylglycerol (2-AG), palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) have been identified. These endogenous cannabinoids target the CB1 and CB2 receptors, acting as retrograde messengers. They bind presynaptically to modulate various neurotransmitters such as serotonin (5-HT), norepinephrine (NE), γ-aminobutyric acid (GABA) and glutamate. Neuronal reuptake is followed by enzymatic degradation. Serine hydrolases, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), degrade AEA and 2-AG, respectively, modulating their concentrations (Blankman & Cravatt Citation2013). Due to the diverse physiological roles of the endocannabinoid system, modulation of this system has emerged as a potential pharmacologic target for the treatment of various conditions as anxiety, depression, schizophrenia, drug-dependence, obesity, cancer, and pain. However, selective ligand targeting of CB1 and CB2 receptors has been associated with problematic adverse effects (Mechoulam & Parker Citation2003; Christensen et al. Citation2007). Accordingly, indirect modulation of the endocannabinoid system via the use of FAAH and/or MAGL inhibitors has gained recent attention. Reversible FAAH or MAGL inhibition has proven to be beneficial for analgesia (Sagar et al. Citation2009; Clapper et al. Citation2010) and anxiety (Scherma et al. Citation2008a, Citation2008b) with minimal adverse outcomes. This study demonstrated that the dichloromethane and ethyl acetate nutmeg fractions exhibit concentration-dependent inhibition of FAAH and MAGL enzymes. Such activities might explain the mechanism of neurological activities exerted by nutmeg, as well as shed some light on potential therapeutic applications of the evaluated fractions.
The inhibition of FAAH and MAGL enzymes provides insight into the mechanism of cannabimimetic effects attributed to nutmeg. Several reports have revealed that M. fragrans extracts exert cannabis-like effects. In vivo administration of M. fragrans extracts displayed anxiolysis at doses of 10 mg/kg without impairing locomotion (Sonavane et al. Citation2001, Citation2002). However, anxiogenic properties were seen with higher doses ranging from 30 to 100 mg/kg (Sonavane et al. Citation2001, Citation2002; Leiter et al. Citation2011). These effects are similar to the bimodal activity described with cannabis (Moreira & Wotjak Citation2010). Conversely, Nagaraju et al. (Citation2013) reported that a 7-day pretreatment with nutmeg extracts provided a protective effect on anxiety at doses of 25 and 50 mg/kg. Of note, tolerance was not seen with nutmeg extracts, which provides an added benefit for long term use. Another comparable cannabimimetic effect of interest is sedation and pain amelioration (Sonavane et al. Citation2001, Citation2002). Murine studies of nutmeg extracts injected orally or intraperitoneally demonstrated antinociception at effective doses of 300 mg/kg (El-Alfy et al. Citation2009) and 1 g/kg (Hayfaa et al. Citation2013). The effect of nutmeg on locomotor activity also resembled that of Δ9-THC. Dose dependent inhibition of locomotion was observed with the inhalation of nutmeg essential oils. Due to the apparent CNS effects of nutmeg its bioavailability to the brain should be appropriately considered. Thus, preliminary evaluation of the permeability of 15 compounds isolated from nutmeg was recently conducted in an in vitro MDCK-pHaMDR cell model of the blood-brain barrier (Wu et al. Citation2016). Passive diffusion across cell monolayers correlated with compound lipophilicity. For some compounds, an efflux pump mechanism was also involved.
Conclusion
This study provides evidence that nutmeg targets the endocannabinoid system indirectly by inhibiting both FAAH and MAGL enzymes. Such mechanism sheds light on the cannabis-like effects previously reported for Myristica fragrans. Our current and future studies are focusing on the characterization of nutmeg compounds responsible for these activities and their therapeutic evaluation for the management of anxiety and substance use disorders.
Funding information
Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R24DA036410.
Acknowledgements
The authors acknowledge Nataliya Sidelnikova and Triejaye McDowell for their help during earlier phases of this project.
Disclosure statement
The authors report no declarations of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, et al. 2012. Automated design of ligands to polypharmacological profiles. Nature. 492:215–220.
- Beyer J, Ehlers D, Maurer HH. 2006. Abuse of nutmeg (Myristica fragrans Houtt.): studies on the metabolism and the toxicologic detection of its ingredients elemicin, myristicin, and safrole in rat and human urine using gas chromatography/mass spectrometry. Ther Drug Monit. 28:568–575.
- Blankman JL, Cravatt BF. 2013. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 65:849–871.
- Braun UKD. 1973. Evidence for the biogenic formation of amphetamine derivatives from components of nutmeg. Pharmacology. 9:312–316.
- Cao GY, Xu W, Yang XW, Gonzalez FJ, Li F. 2015. New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production. Food Chem. 173:231–237.
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. 2007. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 370:1706–1713.
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, et al. 2010. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 13:1265–1270.
- Cuong TD, Hung TM, Han HY, Roh HS, Seok JH, Lee JK, Jeong JY, Choi JS, Kim JA, Min BS. 2014. Potent acetylcholinesterase inhibitory compounds from Myristica fragrans. Nat Prod Commun. 9:499–502.
- Demetriades AK, Wallman PD, McGuiness A, Gavalas MC. 2005. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 22:223–225.
- Dhingra D, Parle M, Kulkarni SK. 2006. Comparative brain cholinesterase-inhibiting activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J Med Food. 9:281–283.
- Dhingra D, Sharma A. 2006. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 9:84–89.
- Ehrenpreis JE, DesLauriers C, Lank P, Armstrong PK, Leikin JB. 2014. Nutmeg poisonings: a retrospective review of 10 years experience from the Illinois poison center, 2001-2011. J Med Toxicol. 10:148–151.
- El-Alfy AT, Wilson L, ElSohly MA, Abourashed EA. 2009. Towards a better understanding of the psychopharmacology of nutmeg: activities in the mouse tetrad assay. J Ethnopharmacol. 126:280–286.
- Evans WC. 1996. Trease and Evans Pharmacognosy. 14th ed. Singapore: Harcourt Brace & Co. pp. 273.
- Forrester MB. 2005. Nutmeg intoxication in Texas, 1998–2004. Hum Exp Toxicol. 24:563–566.
- Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D. 2002. Pharmacological studies on Myristica fragrans–antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 24:675–680.
- Hayfaa AA, Sahar AM, Awatif MA. 2013. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. J Pain Res. 6:611–615.
- Kareem MA, Krushna GS, Hussain SA, Devi KL. 2009. Effect of aqueous extract of nutmeg on hyperglycemia, hyperlipidemia and cardiac histology associated with isoproterenol-induced myocardial infarction in rats. Tropical J Pharmaceut Res. 8:337–344.
- Kasture SB, Gujar KN. 2005. Depressant effect of trimyristin and its inhibition by some antidepressant in mice. In: ISHS Acta Horticulturae 675: III WOCMAP Congress on Medicinal and Aromatic Plants – Volume 1: Bioprospecting and Ethnopharmacology. DOI: 10.17660/ActaHortic.2005.675.21.
- Khan IA, Abourashed EA. 2010. Nutmeg (and Mace). In: Khan IA, Abourashed EA, editors. Leung's encyclopedia of common natural ingredients used in food, drugs, and cosmetics. Hoboken (NJ): Wiley. pp. 467–470.
- Leiter E, Hitchcock G, Godwin S, Johnson M, Sedgwick W, Jones W, McCall S, Ceremuga TE. 2011. Evaluation of the anxiolytic properties of myristicin, a component of nutmeg, in the male Sprague-Dawley rat. Aana J. 79:109–114.
- López V, Gerique J, Langa E, Berzosa C, Valero MS, Gómez-Rincón C. 2015. Antihelmintic effects of nutmeg (Myristica fragans) on Anisakis simplex L3 larvae obtained from Micromesistius potassou. Res Vet Sci. 100:148–155.
- Mechoulam R, Parker LA. 2003. The endocannabinoid system and the brain. Annu Rev Psychol. 64:21–47.
- Moinuddin G, Devi K, Kumar Khajuria D. 2012. Evaluation of the anti-depressant activity of Myristica fragrans (nutmeg) in male rats. Avicenna J Phytomed. 2:72–78.
- Moreira FA, Wotjak CT. 2010. Cannabinoids and anxiety. Curr Top Behav Neurosci. 2:429–425.
- Morita T, Jinno K, Kawagishi H, Arimoto Y, Suganuma H, Inakuma T, Sugiyama K. 2003. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J Agric Food Chem. 51:1560–1565.
- Nadkarni K. 1998. Indian Materia Medica. Mumbai (India): Bombay Popular Prakashan. p. 830.
- Nagaraju B, Sahar SH, Bolouri A, Neha KZ, Zahra A, Zothanmawia C, Surendranatha A. 2013. Anxiolytic effect of Myristica fragrans. Int J Phytother Res. 1:1–7.
- Ozaki Y, Soedigdo S, Wattimena YR, Suganda AG. 1989. Antiinflammatory effect of mace, aril of Myristica fragrans Houtt., and its active principles. Jpn J Pharmacol. 49:155–163.
- Sagar DR, Gaw AG, Okine BN, Woodhams SG, Wong A, Kendall DA, Chapman V. 2009. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 5:1–13.
- Sangalli BC, Chiang W. 2000. Toxicology of nutmeg abuse. J Toxicol Clin Toxicol. 38:671–678.
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, et al. 2008a. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 54:129–140.
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinova Z, Mikics E, Haller J, Medalie J, et al. 2008b. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 327:482–490.
- Servan J, Chochon F, Duclos H. 1998. Hallucinations after voluntary ingestion of nutmeg: an unrecognized drug abuse. Rev Neurol (Paris). 154:708.
- Sharma PV. 2001. Dravyaguna Vijnana (vegetable drugs). Vol. 1. Varanasi (India): Chaukambha Bharti Academy. pp. 151–160.
- Shulgin AT. 1966. Possible implication of myristicin as a psychotropic substance. Nature. 210:380–384.
- Sonavane GS, Sarveiya V, Kasture V, Kastre SB. 2001. Behavioral actions of Myristica fragrans seeds. Indian J Pharmacol. 33:417–424.
- Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB. 2002. Anxiogenic activity of Myristica fragrans seeds. Pharmacol Biochem Behav. 71:239–244.
- Stein U, Greyer H, Hentschel H. 2001. Nutmeg (myristicin) poisoning–report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 118:87–90.
- Tajuddin Ahmad S, Latif A, Qasmi IA Amin KM. 2005. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 5:16–23.
- Takikawa A, Abe K, Yamamoto M, Ishimaru S, Yasui M, Okubo Y, Yokoigawa K. 2002. Antimicrobial activity of nutmeg against Escherichia coli O157. J Biosci Bioeng. 94:315–320.
- Truitt EB, Duritz G, Ebersberger EM. 1963. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 112:647–650.
- Truitt EB, Callaway E, Braude MC, Krantz JC. 1961. The pharmacology of myristicin. A contribution to the psychopharmacology of nutmeg. J Neuropsychiatr. 2:205–210.
- Wu N, Xu W, Yang YF, Yang XB, Yang XW. 2016. The blood-brain barrier permeability of lignans and malabaricones from the seeds of Myristica fragrans in the MDCK-pHaMDR cell monolayer model. Molecules. 21:134–143.