Abstract
Context: Naringin is a natural flavanone glycoside that is found in the Chinese herbal medicines and citrus fruits. Studies have demonstrated that naringin possesses numerous biological and pharmacological properties, but few reviews of these studies have been performed.
Objective: The present review gathers the fragmented information available in the literature describing the extraction of naringin, its pharmacology and its controlled release formulations. Current research progress and the therapeutic potential of naringin are also discussed.
Methods: A literature survey for relevant information regarding the biological and pharmacological properties of naringin was conducted using Pubmed, Sciencedirect, MEDLINE, Springerlink and Google Scholar electronic databases from the year 2007–2015.
Results: Naringin modulates signalling pathways and interacts with signalling molecules and thus has a wide range of pharmacological activities, including anti-inflammatory, anti-cancer activities, as well as effects on bone regeneration, metabolic syndrome, oxidative stress, genetic damage and central nervous system (CNS) diseases. Information was gathered that showed the extraction of naringin can be improved using several modifications. There has been some progress in the development of controlled release formulations of naringin.
Conclusion: Naringin is a promising candidate for further in vivo studies and clinical use. More detailed studies regarding its mechanism of action are required.
Introduction
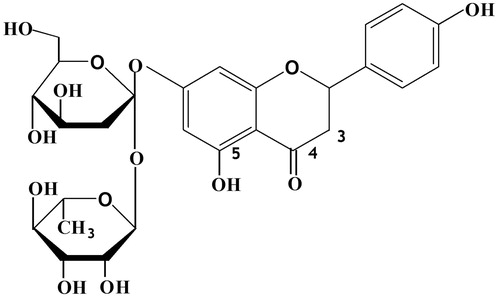
Naringin (), a flavanone glycoside that is formed from the flavanone naringenin and the disaccharide neohesperidose, is one of the main active components of Chinese herbal medicines, such as Drynaria fortunei (Kunze) J. Sm. (DF), Citrus aurantium L. (CA) and Citrus medica L. (CM) (Zhang et al. Citation2014; Yin et al. Citation2015b;). It is also present in citrus fruits (Wong et al. Citation2013) and imparts a bitter taste to citrus juices (Chtourou et al. Citation2015).
Flavonoids are an important group of secondary metabolites and a source of bioactive compounds in plants (Ghasemzadeh & Jaafar Citation2013). An extensive literature survey has revealed that naringin possesses antioxidant, anti-inflammatory, anti-apoptotic, anti-ulcer, anti-osteoporotic and anti-carcinogenic properties (Wang et al. Citation2013). However, there have been few reports, until recently, which describe naringin processing.
A relatively simple and high-yield method for the extraction and purification of naringin that is applicable to agricultural wastes such as citrus fruit peels, has recently been described (Kanokorn et al. Citation2009). Controlled release formulations of naringin could reduce the total dose of medication required (CitationKim & Tabata 2015), and these formulations should have high encapsulation efficiencies and stable drug release behaviours (Cordenonsi et al. Citation2015). Controlled release formulations of naringin have been shown to affect bone regeneration and to potentially promote bone healing (Chen et al. Citation2013).
The present review focuses on current studies describing the in vivo and in vitro effects of naringin, highlighting the potential value of this compound and the diversity of its pharmacological activities.
Extraction technology
Three steps are needed to isolate naringin from fruits: extraction, separation and purification (Wang et al. Citation2010). The naringin content in fruit depends on a number of factors the time of fruit collection, the part of the fruit used and if the peel is the source of naringin, the drying time (Zhao et al. Citation2015a). The naringin content of different fruits varies as follows: CA > Immature CA, Immature Ponciri Fructus > Citri Unshiu peel > Immature Citri Unshiu peel (Zhao et al. Citation2015a). A convection oven can be used to dry the peel more quickly than sun drying and to reduce the aerial exposure time and to prevent microbial activity, which could lead to the destruction of naringin and to the contamination with metabolites (Kanokorn et al. Citation2009). In previous studies, CM powder (0.5 g) was extracted with 50% methanol (25 mL) for 30 min using ultrasonication (Zhao et al. Citation2015b) and CA powder (100 g) was extracted by refluxing with methanol (1 L) for 2 h, which can help to retard or eliminate microbial infection (Zhang et al. Citation2014). The CA extract achieved a 25.8% naringin yield, and it was then redissolved in methanol to provide a crude drug solution with a final concentration of 0.1 g/mL (Zhang et al. Citation2014). Methanol extraction was followed by crystallization with water at 25 °C, with the addition of 14–15% (v/v) dichloromethane, and resulted in a fivefold higher yield than conventional hot water extraction. In this manner, ∼ 20 g of naringin (> 98% purity) was obtained from 1 kg of dry pomelo peel (Kanokorn et al. Citation2009). After being concentrated, the structure of naringin was confirmed using ultraviolet-visible spectroscopy (UV-VIS), Fourier transform-infrared spectroscopy (FTIR), 1H NMR spectroscopy, mass spectrometry and elemental analysis (Pereira et al. Citation2007).
Naringin, until recently, has been shown to exert potential therapeutic actions by modulating various protein and enzyme expressions as summarized in .
Table 1. The expression and activities of proteins and enzymes modulated by naringin.
Effects on bone regeneration
In vitro
Naringin has been shown to significantly affect osteogenic differentiation and cell proliferation by improving signalling pathway activity (Zhang et al. Citation2009; Yin et al. Citation2015a, Citation2015b). The effect of naringin on UMR-106 and MG-63 osteosarcoma cells is dose-dependent over the concentration range of 1–100 μg/mL (Chen et al. Citation2013; Bharti et al. Citation2014).
In UMR-106 cells, naringin has been demonstrated to augment osteoblastic activity via inhibition of hepatic 3-hydroxy-3-methyl CoA (HMG-CoA) reductase (Bharti et al. Citation2014). Naringin mimicked the effects of oestrogen by stimulating cell proliferation as well as modulating alkaline phosphatase (ALP) activity and OPG/RANKL mRNA expression (Wong et al. Citation2013). Naringin (10 nM) also stimulates osteoprotegerin (OPG) mRNA expression and suppresses the expression of the receptor activator for nuclear factor κ-B ligand (RANKL) mRNA, resulting in an increase in the OPG:RANKL ratio. These findings suggest that naringin may exert oestrogen-like effects by promoting osteoblastic functions and suppressing osteoclastogenesis (Pang et al. Citation2010).
In bone marrow stromal cells (BMSCs), there is a delay of 5–7 days between the start of naringin treatment and the burst of ALP expression, suggesting that naringin has a greater influence on osteogenic differentiation than on cell proliferation (Li et al. Citation2013b). Naringin not only promotes the secretion of bone morphogenetic proteins (BMPs), but also enhances the proliferation and osteogenic differentiation of BMSCs (Zhang et al. Citation2009). However, naringin concentrations >200 μg/mL are toxic and may lead to cell death (Zhang et al. Citation2009).
In the osteoclast precursor cell line RAW 264.7, the most significant effects of naringin (10 μg/mL) were the decrease in calcium release, suppression of tartrate-resistant acid phosphatase activity (Li et al. Citation2014b) and improvement in osteocalcin expression (Li et al. Citation2013b). Naringin restrained RANKL-induced activation of nuclear factor kappa B (NF-κB) by suppressing the degradation of the RANKL-mediated inhibitor α of NF-κB. Naringin also controlled RANKL-induced phosphorylation of extracellular regulated protein kinases (ERK) and perturbed osteoclast formation by inhibiting RANKL-mediated NF-κB and ERK signalling (Ang et al. Citation2011).
In MC3T3-E1 cells, naringin increased the expression of osteopontin and OPG and enhanced osteocalcin secretion (Yoon et al. Citation2012). Naringin raised osteoblast proliferation by increasing the BMP-2 expression (Yin et al. Citation2015a). The increase in BMP-2 expression and heightened osteogenic responses were mediated by the phosphoinositide 3-kinase, Akt, c-Fos/c-Jun and AP-1-dependent signalling pathways (Wu et al. Citation2008).
Naringin has been shown to markedly enhance MG-63 cell proliferation, differentiation and nodule formation in a concentration-dependent manner (1–100 μg/mL). The most effective concentrations were 1 and 10 μg/mL; at these levels naringin significantly improved osteoblastic cell differentiation by 50% (Chen et al. Citation2013).
Thus, naringin promotes cell proliferation and bone differentiation by increasing the OPG:RANKL ratio, enhancing BMP and osteocalcin expression, and decreasing HMG-CoA, NF-κB and ERK expression.
In vivo
In both the intact and gonadectomized animals, naringin has a significant effect on bone repair. It has been shown to increase bone mineral density (BMD) and bone strength as well as to inhibit urinary calcium excretion (Wei et al. Citation2007; Wong et al. Citation2013). In ovariectomized (OVX) mice, naringin suppressed OVX-induced enhancement in urinary calcium excretion as well as losses in bone mass and bone strength, and it improved the bone quality in the distal femur, proximal tibia and lumbar spine. Naringin and the DF flavonoid fraction might mimic oestrogen and suppress osteoclastogenesis by modulating OPG and RANKL expression in osteoblastic cells (Wong et al. Citation2013). In young retinoic acid-induced osteoporosis rats, naringin has been shown to significantly increase plasma insulin-like growth factor 1 levels, femoral BMD and strength, BMD of the fifth lumbar spine and bone calcium concentrations of the femur and lumbar spine (Wei et al. Citation2007). In OVX rats, naringin (200 mg/kg by oral gavages) has been demonstrated lead to the upregulation of BMD, bone volume and trabecular thickness and maximum load (Li et al. Citation2014a). In gonad-intact aged male rats, naringin has statin-like effects on reducing lipids and stimulating BMPs, as well as on the bone-fat mass relationship. However, it only affects the metaphyseal density, and the diaphyseal region is not responsive to naringin supplementation (Habauzit et al. Citation2011).
The efficacy of naringin has been demonstrated in other research models. In mice with polymethylmethacrylate (PMMA)-induced osteoclastogenesis, treatment with naringin by oral gavage (300 mg/kg daily) for 30 days or by local injection has been shown to ameliorate the PMMA-induced inflammatory tissue response and subsequent bone resorption and to significantly alleviate periprosthetic bone resorption (Li et al. Citation2014b). In rats with lipopolysaccharide (LPS)-induced alveolar bone resorption, treatment with naringin (400 mg/kg) by gastric perfusion for 10, 20 or 30 days improved the regeneration of alveolar bone, but its effect in vivo was less pronounced than that observed in an in vitro study (Chen et al. Citation2011b). In the ten-week-old male Sprague-Dawley rats, naringin improved the regeneration of alveolar bone, with the greatest effect apparent on day 30 (Chen et al. Citation2011b).
Treatment with naringin for 10–30 days can enhance the bone regeneration, BMD and bone strength in animal models, but the effects of the dose and route of administration, as well as the mechanism of action and side effects, remain unclear.
Anti-inflammatory effects
Inflammation is a part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells or irritants (Ferrero-Miliani et al. Citation2007). Although inflammation is a normal response to tissue injury, if uncontrolled, it can lead to chronic autoimmune diseases, and anti-inflammatory compounds may be needed to control the inflammatory response (Benavente-García & Castillo Citation2008). Plants rich in flavanones, such as hesperidin, naringin and neohesperidin, have been traditionally used for their anti-inflammatory properties (Manthey et al. Citation2001; Benavente-García & Castillo Citation2008).
Naringin did not inhibit cell proliferation but did restrain the production of regulated upon activation normal T-cell expressed and secreted (RANTES) via inhibition of the nuclear translocation of NF-κB in a human epidermal keratinocytes cell line (HaCaT cells) (Habauzit et al. Citation2011). Tumour necrosis factor alpha (TNF-α)/interferon-γ (IFN-γ) have been shown to modulate RANTES at 24 and 48 h. An increased amount of RANTES is released from HaCaT cells stimulated by TNF-α/IFN-γ at 48 h compared with that at 24 h (Wang et al. Citation2011).
Naringin has been shown to be effective in reducing the expression of signalling factors associated with the inflammatory response, e.g., interleukin-6 (IL-6), interleukin-8 (IL-8), inducible nitric oxide synthase (iNOS), nuclear factor erythroid 2-related factor 2 (Nrf2) and TNF-α, in animal models of inflammation. In the 20-month-old male Wistar rats, treatment with naringin potentially stopped an improvement in serum IL-6 during aging-related inflammation (Habauzit et al. Citation2011). In addition, naringin inhibited LPS-induced iNOS expression and NO production in macrophages (Liu et al. Citation2011). In a guinea pig model of chronic bronchitis, naringin reduced the concentrations of IL-8 and leukotriene B4 in bronchoalveolar lavage fluid (BALF) and decreased myeloperoxidase activity in both the BALF and lung tissue. It also increased the superoxide dismutase (SOD) activity in lung tissue and enhanced the lipoxin A4 level in BALF (Luo et al. Citation2012). In rats with 3-nitropropionic acid (3-NP)-induced experimental Huntington’s disease, naringin may reduce 3-NP-induced inflammation through the modulation of Nrf2-driven ARE gene expression and the reduction of TNF-α, COX-2 and iNOS expression. Nrf2-mediated upregulation of gene expression confers the protective effects of naringin in 3-NP-induced inflammation by reducing the deleterious production of pro-inflammatory mediators (Gopinath & Sudhandiran Citation2012).
Naringin has been shown to form a 1:1 complex with Cu (II), in which the Cu (II) ion is coordinated via positions 4 and 5 of naringin. The naringin-Cu (II) complex showed higher anti-inflammatory activity than free naringin without reducing cell viability (Pereira et al. Citation2007).
Painopowder is an ancient Chinese medicine that contains naringin, paeoniflorin, neohesperidin and platycodin-D. In a model of acute inflammation, the maximum anti-inflammatory effect was observed with the four-ingredient combination but, among the four ingredients, naringin was shown to have a dominant contribution to the effect (Chen et al. Citation2011a).
Anti-cancer effects
Suppressing, blocking and transforming agents have been used in attempts to control cancer. Suppressing agents prevent the formation of new cancer from procarcinogens, blocking agents stop carcinogenic compounds from reaching critical initiation sites and transforming agents facilitate the metabolism of carcinogenic components into less toxic materials or prevent their biological actions (Benavente-García & Castillo Citation2008). Naringin can act both as a suppressing and blocking agent.
Naringin has been shown to inhibit cell proliferation and to promote cell apoptosis in tumour cells, including triple-negative breast cancer (TNBC) cells, human cervical cancer (SiHa) cells and bladder cancer cells. In TNBC cells, the pro-apoptotic activity of naringin results from G1-phase cell cycle arrest. Suppression of the growth of breast cancer cells by naringin is mediated by inhibition of the β-catenin pathway, leading to a significantly increased p21 level and decreased cell survival (Li et al. Citation2013a). Naringin acts by a similar mechanism in SiHa cells, which exhibit apoptotic cell death, internucleosomal DNA fragmentation, morphological changes and a decline in the mitochondrial transmembrane potential through both death-receptor and mitochondrial pathways. Treatment with naringin also enhance the expression of caspases, p53, Bax and Fas death receptor as well as its adaptor protein, Fas-associated death domain protein. Naringin decreases SiHa cell proliferation in a dose-dependent manner in the 0–2000 μM concentration range (Ramesh & Alshatwi Citation2013). In chondrosarcoma, naringin is not an anti-tumour agent and does not possess pro-apoptotic activity; instead, it acts as an anti-migration agent that reduces the migration and invasion of chondrosarcoma cells. It downregulates vascular cell adhesion molecule-1, which is associated with metastasis, by increasing miR-126 expression (Tan et al. Citation2014). In the DU145 prostate cancer cell line, the 5-methyl-20-deoxycytidine level is significantly decreased after naringin treatment, indicating that naringin is a DNA hypomethylating agent that has the potential to modulate gene expression. In the 20–50 μM concentration range, naringin causes a decrease in DNA methyltransferase activity (Lewinska et al. Citation2015). Another study showed that naringin inhibits the proliferation of 5637 bladder cancer cells and induces G1 arrest by upregulation of p21 (Kim et al. Citation2008).
Numerous fundamental studies have shown that naringin inhibits cell proliferation and induces apoptosis in a majority of tumour cells and that it also plays a role in cell motility by reducing the migration and invasion of some tumour cells.
Effects on metabolic syndrome
In mice fed a high fat diet (HFD) for 20 weeks, naringin has been shown to significantly ameliorate mitochondrial dysfunction by restoring the mitochondrial matrix metalloproteinase activity and reactive oxygen species (ROS) and ATP levels in mitochondria (Wang et al. Citation2015). In addition, long-term administration of naringin to mice fed a HFD has been reported to reduce the risk of metabolic syndrome via upregulation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) and its related signalling pathway (Pu et al. Citation2012). The activation of AMPK upregulates peroxisome proliferator-activated receptor-α (PPARα) gene expression, downregulates fatty acid synthase and significantly decreases PPARγ expression (Pu et al. Citation2012).
In low-density lipoprotein (LDL) receptor knockout mice, naringin (0.02 g/100 g bw) has been shown to significantly reduce HMG-CoA reductase activity and to increase production of NO metabolites in the urine. In thoracic aortic ring preparations, naringin improved endothelial function via acetylcholine-mediated NO production (Alam et al. Citation2014). In rats fed a HFD, naringin improved metabolic syndrome to a similar extent as rutin or quercetin. Naringin enhanced NO production, improved endothelial function and decreased the cerebral thrombotic tendency (Alam et al. Citation2013). In diabetic rodents, administration of naringin resulted in the downregulation of key gluconeogenic enzymes including glucose-6-phosphatase and phosphoenolpyruvate carboxykinase (Pu et al. Citation2012). In rats fed high-fat and high-cholesterol diet and in rabbits fed high-cholesterol diet, naringin has been demonstrated to ameliorate hypercholesterolemia and atherosclerosis, inhibit HMG-CoA reductase, and decrease plasma cholesterol, LDL, triglycerides, and hepatic lipid levels without altering the high-density lipoprotein cholesterol level (Baskaran et al. Citation2015).
Thus, naringin reduces metabolic syndrome by upregulating AMPK and downregulating the expression of key gluconeogenic enzymes. It also reduces HMG-CoA reductase activity and increases the production of NO metabolites.
Effects on oxidative stress
Phenolic phytochemicals are thought to promote health partly via antioxidant activity and free radical scavenging effects (Johnson & Loo Citation2000). Disturbances in the normal redox state of cells can lead to toxicity through the production of ROS and free radicals, which damage all of the components of the cell (Li et al. Citation2015).
Naringin has been shown to have dose-dependent radical scavenging activity against 1,1-diphenyl-2-picryl-hydrazyl and tetraethylammonium chloride radicals (Rajadurai & Prince Citation2007). At concentrations of 5–2000 μM, naringin showed antioxidant activity and reduced the frequency of DNA damage by H2O2 in Chinese hamster fibroblast (V79) cells (Bacanli et al. Citation2015). In L6 myoblast cells subjected to oxidative stress, pretreatment with naringin (3 or 24 h) reversed the decrease in glutathione (GSH) and increases in both intracellular free radicals and glucose uptake. Naringin (100 μM) also led to a reduction (∼40%) in protein glycation (Dhanya et al. Citation2015). In the rat glomerular mesangial cell line HBZY-1, naringin (10 μM) obviously activated the Nrf2 signalling pathway and enhanced the expression and activity of its downstream target, HO-1 (Chen et al. Citation2015).
Treatment with naringin (25, 50 or 100 mg/kg) significantly and dose dependently resisted all of the biochemical and molecular alterations caused by cisplatin in aged rats (Chtourou et al. Citation2015). In pentylenetetrazole-induced seizure rats, naringin reduces oxidative stress by binding free radicals and regulating the GSH level. Pretreatment with naringin for several hours has been shown to significantly attenuate pentylenetetrazole-induced elevations in the brain malondialdehyde and TNF-α level and to conserve GSH (Golechha et al. Citation2014). In diabetic rats, naringin-reduced diabetes-induced lipid peroxidation and ROS accumulation in sperm and curbed the reduction in the GSH:oxidized GSH ratio (Bakheet & Attia Citation2011). In HFD/STZ-induced diabetic rats with hyperglycemia-induced oxidative damage, naringin enhanced the activity of the antioxidant defence system and thereby conferred protection by decreasing the activities of hepatic SOD, GSH reductase, GSH peroxidase and catalase (Mahmoud et al., Citation2012). In D-galactose-treated mice, chronic administration of naringin for a period of 6 weeks attenuated oxidative damage via decreases in lipid peroxidation and the nitrite concentration and restoration of the reduced GSH level as well as SOD, catalase and GSH-S-transferase activities (Kumar et al. Citation2010).
Effects on genetic damage
Some reagents and medicines (e.g., H2O2 and lomefloxacin) cause DNA damage that, if unresolved, can lead to genetic mutations and/or genomic instability (Lewinska et al. Citation2015).
In V79 cells, naringin has antioxidant properties and confers protection against H2O2-induced chromosome breakage and loss and DNA damage. Naringin may avoid H2O2-induced oxidative damage not only by decreasing DNA damage but also by increasing the DNA repair capacity (Bacanli et al. Citation2015).
Pretreatment with naringin has been shown to attenuate lomefloxacin-induced genomic instability in mice. A dose-dependent effect was observed at concentrations of 5–50 mg/kg and naringin significantly reduced cell proliferation, chromosomal aberrations and micronucleus formation in bone marrow and increased mitotic activity (Attia Citation2008). In male Wistar rats, naringin has been shown to modulate the production and expression of oxidative mediators and to reduce DNA damage to relieve the symptoms of inflammatory bowel disease (Kumar et al. Citation2014). In diabetic rats, administration of naringin for 4 weeks significantly decreased the rate of DNA strand breaks. The anti-genotoxic effect of naringin is mediated by the inhibition of hyperglycemia-induced generation of free radicals (Bakheet & Attia Citation2011).
Naringin exhibits anti-genotoxic activities and reduces DNA damage by modulating the expression of oxidative mediators and generation of free radicals.
Effects on central nervous system (CNS) diseases
Naringin has beneficial effects on many CNS diseases, including Alzheimer’s disease, Parkinson’s disease and epilepsy (Saaby & Jager Citation2011).
In rats treated intracerebroventricularly with streptozotocin (ICV-STZ rats), naringin has been shown to result in significant increases of the malondialdehyde and nitrite levels and marked reductions in the GSH level and enzymatic activities of SOD and catalase in the brain. Naringin also results in the suppression of acetylcholinesterase activity and TNF-α levels in the brains of ICV-STZ rats (Sachdeva et al. Citation2014). In a rat model of Parkinson’s disease, intraperitoneal injection of naringin protects the nigrostriatal dopaminergic (DA) projection by increasing glial cell line-derived neurotrophic factor expression and decreasing TNF-α expression in DA neurons and microglia (Jung & Kim Citation2014). In kainic acid (KA)-treated mice, naringin treatment significantly reduced seizure. Moreover, it protected hippocampal CA1 neurons in the KA-treated hippocampus, ameliorated KA-induced autophagic stress, decreased microtubule-associated protein light chain 3 (LC3) expression and attenuated an improvement in the TNF-α level (Jeong et al. Citation2015). In mice that were fed a HFD for 20 weeks, oral administration of naringin significantly improved learning and memory, as evidenced by a 52.5% improvement in the recognition index, 1.05-fold increase in the crossing-target number and amelioration of mitochondrial dysfunction (Wang et al. Citation2015). In a mouse model of Alzheimer’s disease, naringin (100 mg/kg/day) was shown to raise Thr286 phosphorylation by 47% compared with untreated APPswe/PS1dE9 mice, indicating that naringin enhances calmodulin-dependent protein kinase II (CaMKII) autophosphorylation and function (Wang et al. Citation2013).
Pharmacokinetics and toxicology
Naringin was quickly absorbed into serum and the first concentration peak at 15 min and another at 3 h after oral administration of a naringin monomer; 480 min after dosing, it could not be detected (Li et al. Citation2013d). The plasma concentration–time curves show that the AUC 0–24 h value for oridonin following oral administration was approximately three times larger than that of naringin, while the dose of naringin was nearly four times larger than that of oridonin, suggesting that the absorption of oridonin in rats is much higher than naringin (Jin et al. Citation2015). When the lower limit of quantification of naringin was 2.10 ng/mL, the main pharmacokinetic parameters in both male and female rats had no significant difference (Lu et al. Citation2015). Naringin (15 mg/kg) was shown to inhibit the function of P-gp and significantly increase the intestinal absorption of CDS by 3.2 times using in-situ single-pass intestinal perfusion technique (Surampalli et al. Citation2015). After naringin administration via duodenal cannula (600 and 1000 mg/kg), the average Cmax of naringin in portal plasma occurred at 18.8 ± 3.8 min (determined the concentration reached at tmax in portal plasma), while the absorption ratios of naringin in portal plasma and lymph fluid were approximately 95.9 and 4.1, respectively. This shows naringin could be primarily absorbed by portal blood rather than by mesenteric lymph fluid and could be eliminated through bile excretion with only a small amount entering systemic circulation after hepatic metabolism (Tsai & Tsai Citation2012).
Membrane toxicity studies, naringin did not cause any serious membrane damage or toxicity to the intestinal mucosa at the concentration (15 mg/kg, w/w) used; however, it negligibly enhanced the release of protein from the intestinal membrane (Surampalli et al. Citation2015). The acute and sub-chronic toxicology of naringin was shown to be practically nontoxic for SD rats in oral acute toxicity study and the no-observed-adverse-effect-level (NOAEL) of naringin in rats was greater than 1250 mg/kg/day when administered orally for 13 consecutive weeks (Li et al., Citation2013c) or 6 consecutive months (Li et al. 2014). There was no intestinal membrane damage observed in the presence of naringin by measuring the release of protein and ALP (Surampalli et al. Citation2015).
Controlled release formulations
Controlled release formulations maintain drug levels over a long time period to maximize therapeutic efficiency, increase the duration of action and reduce the total dose needed for efficacy (CitationKim & Tabata 2015).
GGT composite is a porous biodegradable composite that is obtained by combining genipin, cross-linked gelatin and tricalcium phosphate. In a previous study, naringin-GGT (GGTN) composites were prepared by mixing GGT composite with predetermined concentrations of naringin. The abilities of GGT and GGTN to repair bone defects were then evaluated. Radiographic analysis revealed greater new bone in-growth with the GGTN composite group compared with the GGT composite group at the same implantation time point (Chen et al. Citation2013). Naringin has also been incorporated into electrospun nanoscaffolds containing poly (ɛ-caprolactone) (PCL) and poly (ethylene glycol)-block-poly (ɛ-caprolactone) (PEG-b-PCL). PEG-b-PCL significantly improved the hydrophilic properties, drug release behaviour and material degradation rate of the scaffolds (Ji et al. Citation2014). Naringin has been reported to exhibit 80.90% association with Eudragit® E, a controlled release material formed from synthetic pharmaceutical excipients. After 42 days at 60 °C, naringin had degraded by ∼12%, and this level of degradation was maintained until day 82. Only a small amount of the drug was lost in the aqueous phase, resulting in a high encapsulation efficiency (Cordenonsi et al. Citation2015).
Conclusions
Naringin shows promise for treating a range of diseases because of its wide availability, low cost, variety of pharmacological actions and long history of use. Naringin, or combination therapy with naringin and other drugs, may be useful for treating diseases in which oxidative stress plays a role, such as Alzheimer’s disease and diabetes. The largest obstacle to the use of naringin as a novel therapy is the lack of in vivo data. The majority of published studies have only described in vitro experiments, and there are few detailed analyses of the pharmacokinetics of naringin. A 1:1 complex of naringin and Cu (II) shows higher antioxidant activity, anti-inflammatory activity and cytotoxicity towards tumour cells compared to naringin itself (Pereira et al. Citation2007). Exploration of the biological activities of complexes containing naringin and essential trace elements, such as Ca, Fe, and Zn, may provide a new direction for naringin research. The outcome of such research may provide convincing support for the future clinical use of naringin.
Disclosure statement
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the National Scientific Foundation of China under the National Scientific Grant [grant number 81160134]; by the Yunnan Provincial Science and Technology Department under the Grant Talent Reserve of Young Academic and Technical Leaders of Yunnan Province [grant number 2012HB027]; and by the Yunnan Provincial Bureau of Health under the Grant Medical Academic Leaders of Yunnan Province [grant number D-201232].
References
- Alam MA, Kauter K, Brown L. 2013. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients. 5:637–650.
- Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. 2014. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 5:404–417.
- Ang ES, Yang X, Chen H, Liu Q, Zheng MH, Xu J. 2011. Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-κB and ERK activation . FEBS Lett. 585:2755–2762.
- Attia SM. 2008. Abatement by naringin of lomefloxacin-induced genomic instability in mice. Mutagenesis. 23:515–521.
- Bacanli M, Basaran AA, Basaran N. 2015. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem Toxicol. 81:160–170.
- Bakheet SA, Attia SM. 2011. Evaluation of chromosomal instability in diabetic rats treated with naringin. Oxid Med Cell Longev. 2011:365292.
- Baskaran G, Salvamani S, Ahmad SA, Shaharuddin NA, Pattiram PD, Shukor MY. 2015. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug Des Devel Ther. 9:509–517.
- Benavente-García O, Castillo J. 2008. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 56:6185–6205.
- Bharti S, Rani N, Krishnamurthy B, Arya DS. 2014. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 80:437–451.
- Chen JC, Li LJ, Wen SM, He YC, Liu HX, Zheng QS. 2011a. Quantitative analysis and simulation of anti-inflammatory effects from the active components of Paino Powder () in rats. Chin J Integr Med. [Epub ahead of print]. doi:10.1007/s11655-011-0882-0.
- Chen F, Zhang N, Ma X, Huang T, Shao Y, Wu C, Wang Q. 2015. Naringin alleviates diabetic kidney disease through inhibiting oxidative stress and inflammatory reaction. PLoS One. 10:e0143868.
- Chen KY, Lin KC, Chen YS, Yao CH. 2013. A novel porous gelatin composite containing naringin for bone repair. Evid Based Complement Alternat Med. 2013:283941. doi:10.1155/2013/283941.
- Chen LL, Lei LH, Ding PH, Tang Q, Wu YM. 2011b. Osteogenic effect of Drynariae rhizoma extracts and naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch Oral Biol. 56:1655–1662.
- Chtourou Y, Gargouri B, Kebieche M, Fetoui H. 2015. Naringin abrogates cisplatin-induced cognitive deficits and cholinergic dysfunction through the down-regulation of AChE expression and iNOS signaling pathways in hippocampus of aged rats. J Mol Neurosci. 56:349–362.
- Cordenonsi LM, Bromberger NG, Raffin RP, Scherman EE. 2015. Simultaneous separation and sensitive detection of naringin and naringenin in nanoparticles by chromatographic method indicating stability and photodegradation kinetics. Biomed Chromatogr. 30:155–162.
- Dhanya R, Arun KB, Nisha VM, Syama HP, Nisha P, Santhosh Kumar TR, Jayamurthy P. 2015. Preconditioning L6 muscle cells with naringin ameliorates oxidative stress and increases glucose uptake. PLoS One. 10:e0132429.
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. 2007. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
- Ghasemzadeh A, Jaafar HZ. 2013. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement Altern Med. 13:341.
- Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, Arya DS. 2014. Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: possible mechanisms of neuroprotection. Epilepsy Behav. 41:98–102.
- Gopinath K, Sudhandiran G. 2012. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience. 227:134–143.
- Habauzit V, Sacco SM, Gil-Izquierdo A, Trzeciakiewicz A, Morand C, Barron D, Pinaud S, Offord E, Horcajada MN. 2011. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone. 49:1108–1116.
- Jeong KH, Jung UJ, Kim SR. 2015. Naringin attenuates autophagic stress and neuroinflammation in kainic acid-treated hippocampus in vivo. Evid Based Complement Alternat Med. 2015:354326. doi:10.1155/2015/354326.
- Ji Y, Wang L, Watts DC, Qiu H, You T, Deng F, Wu X. 2014. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent Mater. 30:1263–1273.
- Jin Y, Tian T, Ma Y, Xu H, Du Y. 2015. Simultaneous determination of ginsenoside Rb1, naringin, ginsenoside Rb2 and oridonin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Weifuchun tablet. J Chromatogr B Analyt Technol Biomed Life Sci. 1000:112–119.
- Johnson MK, Loo G. 2000. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat Res. 459:211–218.
- Jung UJ, Kim SR. 2014. Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regen Res. 9:1514–1517.
- Kanokorn S, Surachai P, Supason W. 2009. An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. Int J Food Sci Tech. 44:1737–1742.
- Kim DI, Lee SJ, Lee SB, Park K, Kim WJ, Moon SK. 2008. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis. 29:1701–1709.
- Kim YH, Tabata Y. 2015. Dual-controlled release system of drugs for bone regeneration. Adv Drug Deliv Rev. 94:28–40.
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. 2014. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 28:132–145.
- Kumar A, Prakash A, Dogra S. 2010. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol. 48:626–632.
- Lewinska A, Siwak J, Rzeszutek I, Wnuk M. 2015. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol in Vitro. 29:417–425.
- Li F, Sun X, Ma J, Ma X, Zhao B, Zhang Y, Tian P, Li Y, Han Z. 2014a. Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem Biophys Res Commun. 452:629–635.
- Li H, Yang B, Huang J, Xiang T, Yin X, Wan J, Luo F, Zhang L, Li H, Ren G. 2013a. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting beta-catenin signaling pathway. Toxicol Lett. 220:219–228.
- Li N, Jiang Y, Wooley PH, Xu Z, Yang SY. 2013b. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J Orthop Sci. 18:478–485.
- Li N, Xu Z, Wooley PH, Zhang J, Yang SY.. 2014b. Therapeutic potentials of naringin on polymethylmethacrylate induced osteoclastogenesis and osteolysis, in vitro and in vivo assessments. Drug Des Devel Ther. 8:1–11.
- Li P, Wang S, Guan X, Cen X, Hu C, Peng W, Wang Y, Su W. 2014c. Six months chronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem Toxicol. 66:65–75.
- Li P, Wang S, Guan X, Liu B, Wang Y, Xu K, Peng W, Su W, Zhang K. 2013c. Acute and 13 weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem Toxicol. 60:1–9.
- Li SG, Ding YS, Niu Q, Xu SZ, Pang LJ, Ma RL, Jing MX, Feng GL, Liu JM, Guo SX. 2015. Grape seed proanthocyanidin extract alleviates arsenic-induced oxidative reproductive toxicity in male mice. Biomed Environ Sci. 28:272–280.
- Li SQ, Dong S, Su ZH, Zhang HW, Peng JB, Yu CY, Zou ZM. 2013d. Comparative pharmacokinetics of naringin in rat after oral administration of chaihu-shu-gan-san aqueous extract and naringin alone. Metabolites. 3:867–880.
- Liu Y, Wu H, Nie YC, Chen JL, Su WW, Li PB. 2011. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int Immunopharmacol. 11:1606–1612.
- Lu Y, Li N, Deng Y, Zhao L, Guo X, Li F, Xiong Z. 2015. Simultaneous determination of icariin, naringin and osthole in rat plasma by UPLC-MS/MS and its application for pharmacokinetic study after oral administration of Gushudan capsules. J Chromatogr B Analyt Technol Biomed Life Sci. 993–994:75–80.
- Luo YL, Zhang CC, Li PB, Nie YC, Wu H, Shen JG, Su WW. 2012. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int Immunopharmacol. 13:301–307.
- Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. 2012. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 26:483–490.
- Manthey JA, Guthrie N, Grhmann K. 2001. Biological properties of Citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 8:135–153.
- Pang WY, Wang XL, Mok SK, Lai WP, Chow HK, Leung PC, Yao XS, Wong MS. 2010. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br J Pharmacol. 159:1693–1703.
- Pereira RM, Andrades NE, Paulino N, Sawaya AC, Eberlin MN, Marcucci MC, Favero GM, Novak EM, Bydlowski SP. 2007. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules. 12:1352–1366.
- Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY, Zhou NJ, Xie J, Jiang H. 2012. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys. 518:61–70.
- Rajadurai M, Prince PS. 2007. Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: an in vivo and in vitro study. Toxicology. 232:216–225.
- Ramesh E, Alshatwi AA. 2013. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem Toxicol. 51:97–105.
- Saaby L, Jager AK. 2011. Flavonoids and the CNS. Molecules. 16:1471–1485.
- Sachdeva AK, Kuhad A, Chopra K. 2014. Naringin ameliorates memory deficits in experimental paradigm of Alzheimer's disease by attenuating mitochondrial dysfunction. Pharmacol Biochem Behav. 127:101–110.
- Surampalli G, Nanjwade B, Patil P. 2015. Corroboration of naringin effects on the intestinal absorption and pharmacokinetic behavior of candesartan cilexetil solid dispersions using in-situ rat models. Drug Dev Ind Pharm. 41:1057–1065.
- Tan TW, Chou YE, Yang WH, Hsu CJ, Fong YC, Tang CH. 2014. Naringin suppress chondrosarcoma migration through inhibition vascular adhesion molecule-1 expression by modulating miR-126. Int Immunopharmacol. 22:107–114.
- Tsai Y, Tsai T. 2012. Mesenteric lymphatic absorption and the pharmacokinetics of naringin and naringenin in the rat. J Agric Food Chem. 60:12435–12442.
- Wang C, Pan Y, Fan G, Chai Y, Wu Y. 2010. Application of an efficient strategy based on MAE, HPLC-DAD-MS/MS and HSCCC for the rapid extraction, identification, separation and purification of flavonoids from Fructus aurantii immaturus. Biomed Chromatogr. 24:235–244.
- Wang D, Yan J, Chen J, Wu W, Zhu X, Wang Y. 2015. Naringin improves neuronal insulin signaling, brain mitochondrial function, and cognitive function in High-Fat Diet-induced obese mice. Cell Mol Neurobiol. 35:1061–1071.
- Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, Zhang LF. 2013. Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer's disease. Int J Mol Sci. 14:5576–5586.
- Wang SS, Liu L, Zhu L, Yang Y-X. 2011. Inhibition of TNF-alpha/IFN-gamma induced RANTES expression in HaCaT cell by naringin. Pharm Biol. 49:810–814.
- Wei M, Yang Z, Li P, Zhang Y, Sse WC. 2007. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med. 35:663–667.
- Wong KC, Pang WY, Wang XL, Mok SK, Lai WP, Chow HK, Leung PC, Yao XS, Wong MS. 2013. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br J Nutr. 110:475–485.
- Wu JB, Fong YC, Tsai HY, Chen YF, Tsuzuki M, Tang CH. 2008. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol. 588:333–341.
- Yin FM, Xiao LB, Zhang Y. 2015a. Research progress on Drynaria fortunei naringin on inflammation and bone activity. Zhongguo Gu Shang. 28:182–186.
- Yin L, Cheng W, Qin Z, Yu H, Yu Z, Zhong M, Sun K, Zhang W. 2015b. Effects of naringin on proliferation and osteogenic differentiation of human periodontal ligament stem cells in vitro and In Vivo. Stem Cells Int. 2015:758706.
- Yoon HY, Cho YS, Jin Q, Kim HG, Woo ER, Chung YS. 2012. Effects of ethyl acetate extract of Poncirus trifoliata fruit for glucocorticoid-induced osteoporosis. Biomol Ther (Seoul). 20:89–95.
- Zhang J, Gao W, Liu Z, Zhang Z, Liu C. 2014. Systematic analysis of main constituents in rat biological samples after oral administration of the methanol extract of Fructus aurantii by HPLC-ESI-MS/MS. Iran J Pharm Res. 13:493–503.
- Zhang P, Dai KR, Yan SG, Yan WQ, Zhang C, Chen DQ, Xu B, Xu ZW. 2009. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 607:1–5.
- Zhao BT, Kim EJ, Son KH, Son JK, Min BS, Woo MH. 2015a. Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Arch Pharm Res. 38:1512–1520.
- Zhao P, Duan L, Guo L, Dou LL, Dong X, Zhou P, Li P, Liu EH. 2015b. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 173:54–60.