Abstract
Cardiovascular complications of liver cirrhosis include cardiac dysfunction and abnormalities in the central-, splanchnic,- and peripheral circulation. Vasodilatation prevails, but vascular beds with various degrees of reduced and increased haemodynamic resistance are the results of massive activation of powerful homeostatic, regulatory systems. Cirrhotic cardiomyopathy implies systolic and diastolic dysfunction and electrophysiological abnormalities, an entity that is different from alcoholic heart muscle disease. Being often clinical latent, cirrhotic cardiomyopathy can be unmasked by physical and pharmacological strain. Cardiac failure is an important cause of mortality after liver transplantation and stressful procedures as insertions of transjugular intrahepatic portal systemic shunt (TIPS), peritoneal venous shunting, and other types of surgery. Improvement of liver function has been shown to reverse the cardiovascular complications. The clinical significance is an important topic for future research. At present, no specific treatment can be recommended, and the cardiac failure in cirrhosis should be treated as in non-cirrhotic patients with sodium restriction, diuretics, and beta-adrenergic blocking agents. Special care should be taken with the use of ACE-inhibitors and angiotensin antagonist in these patients.
Patients with cirrhosis often present with complications to portal hypertension and reduced liver function (e.g. bleeding from oesophageal varices, hepatic encephalopathy) Citation[1]. Other complications are related to low serum albumin (fluid retention and pleura effusion) and abnormal protein metabolism Citation[2], spontaneous bacterial peritonitis Citation[3], high circulating levels of ammonia Citation[4], hormone disturbances Citation[5], and electrolyte abnormalities (low serum sodium and magnesium) Citation[6]. All these abnormalities may together with anaemia Citation[7] have influence on cardiac function and haemodynamics in these patients. However, a substantial fraction of cirrhotic patients may also have complications relating more specifically to alterations in their systemic cardiovascular system. The hyperdynamic systemic circulation in cirrhosis was already described by Kowalski and Abelmann in 1953 Citation[8], and Schrier and co-workers Citation[9] in 1988 proposed arterial vasodilatation as a main cause of fluid retention in cirrhosis. The use of new investigative techniques has shown several lines of impaired cardiac contractility and vascular hyporeactivity in patients with cirrhosis mostly of reversible nature. This review will focus on recent advances in the pathophysiology of abnormal vascular tonus, volume distribution, and cardiac function in order to understand the cardiovascular dysfunction and related complications in advanced cirrhosis.
The systemic circulation in cirrhosis
The circulatory changes in patients with cirrhosis are summarised in . Cardiac output is raised as a consequence of increased stroke volume and heart rate Citation[10]. The mean arterial blood pressure is low normal or low, owing to a substantial reduction in the overall systemic vascular resistance Citation[11]. However, the vascular tonus is very different in the various vascular segments Citation[12]. Thus, substantial vasodilatation prevails in the splanchnic area and in parts of the skin, muscles, and lungs, whereas vasoconstriction is typically present in the kidneys Citation[13], Citation[14].
Table I. Circulatory changes in different vascular beds in cirrhosis.
The circulating medium is enlarged with increased blood and plasma volumes Citation[7], Citation[14], which are abnormally distributed with a low central and arterial blood volume and enlarged non-central blood volumes, including splanchnic volume Citation14–16. Total vascular, as well as arterial, compliance (i.e. rise in intravascular volume relative to rise in transmural blood pressure) is increased in patients with cirrhosis with the degree of decompensation Citation[17], Citation[18]. The altered static and dynamic characteristics of the wall of large arteries are closely associated with the circulatory and homoeostatic derangement Citation[19]. Arterial compliance is a property describing arterial wall function. It is important for the propagation and magnitude of the arterial pulse wave. Several lines of evidence indicate that the size of arterial compliance is important to the strain of the left ventricle Citation20–23. Increased arterial stiffness may predispose to heart failure even with normal ejection fraction Citation[22]. Conversely, reduced arterial stiffness, as reflected by increased or normal arterial compliance, may protect against the development of manifest heart failure Citation[21], Citation[23]. Arterial and venous compliance are important with respect to location of the intravascular volume, and reduced arterial filling in cirrhosis is a central feature in the activation of powerful neuroendocrine compensatory systems, such as the sympathetic nervous system, the renin-angiotensin-aldosterone system, and the neuropituitary release of vasopressin Citation[12], Citation[17], Citation[24]. In addition, patients with advanced cirrhosis have elevated circulating endothelins Citation[10]. The concept of central volume reduction and reduced arterial filling has been extensively investigated in cirrhosis Citation[9], Citation[11], Citation14–16. Direct measurements may be hampered by problems with evaluation in relation to body size Citation[14]. However, by a well-designed standardisation these problems may be overcome, see . From a clinical point of view elevated plasma renin activity is often taken as an index of arterial underfilling in cirrhosis Citation[25], Citation[26].
Table II. Evidence of reduced central and arterial filling in patients with cirrhosis Citation[19], Citation[25].
Whereas the arterial blood pressure is low during the daytime, it is normal at night, and the cirrhotic patients are more hyperdynamic in the supine position Citation[27]. Patients with advanced cirrhosis respond abnormally to volume loading and their baroreflex sensitivity is reduced Citation[12], Citation[28]. Changed vascular wall stress is important to the hyperdynamic circulation in cirrhosis Citation[29].
Pathophysiology of vasodilatation
Arteriolar vasodilatation in cirrhosis is brought about by a combination of increased concentration of circulating vasodilators and reduced sensitivity to vasoconstrictors. Overproduction of circulating vasodilators of intestinal or systemic origin, vasodilators that escape degradation in the diseased liver or bypass the liver through portosystemic collaterals, reduced resistance to vasoconstrictors and increased sensitivity to vasodilators has been suggested Citation[10], Citation[30]. Important vasodilators are illustrated in . Nitric oxide, calcitonin gene-related peptide, and endocannabinoids should especially be mentioned, but it should be stressed that the mechanisms behind the vasodilatation in cirrhosis are complex and not completely understood, although it has been the topic of intensive research for more than 25 years Citation[10], Citation[29], Citation[30], see . Hydrogen sulphide is a gaseous transmitter with potent vasodilating properties, which has recently been found to be implicated in vasodilatation in cirrhosis Citation[31]. New experimental data suggest that defective rho-kinase signalling may also contribute to the reduced vascular contractility in cirrhosis Citation[32]. According to the arterial vasodilatation hypothesis, splanchnic arteriolar vasodilatation leads to reduction of the systemic vascular resistance, central arterial underfilling with effective hypovolaemia, activation of vasoconstrictor systems (the sympathetic nervous system, the renin-angiotensin-aldosterone system, vasopressin, endothelins, and neuropeptide Y), and hence development of a hyperkinetic circulatory state Citation[33], Citation[34]. The predominantly splanchnic vasodilation in cirrhosis precedes the increase in cardiac output and heart rate, and it has recently been shown experimentally that mild increases in portal pressure upregulate nitric oxide synthase (eNOS) Citation[35]. With the progression of the disease, the splanchnic vasodilatation becomes more pronounced and the hyperdynamic circulation may no longer be sufficient to correct the effective hypovolaemia Citation[36], Citation[37]. The splanchnic circulation is less sensitive to the effects of angiotensin II, noradrenaline and vasopressin because of the surplus of vasodilators, which may play a role in the development of the vascular hyporesponsiveness to vasoconstrictors Citation[38]. The arterial blood pressure is mainly maintained by vasoconstriction in the renal, cerebral, and hepatic vascular beds where there seems to be diminished release of nitric oxide from endothelial cells Citation[29], Citation[39]. Blockade of nitric oxide formation in animal models and cirrhotic patients significantly increases arterial blood pressure and decreases plasma volume, sodium retention, and forearm blood flow Citation[40]. Taken together, there is a growing body of evidence that systemic nitric oxide production is increased and precedes the development of the hyperdynamic circulation in cirrhosis, thereby playing a major role in the arteriolar and splanchnic vasodilation and vascular hyporeactivity Citation[30]. In addition, vascular endothelial growth factor (VEGF) seems to stimulate angiogenesis and the development of portosystemic collaterals, and blockade of the VEGF receptor-2 has been shown experimentally to inhibit this process and revert portal hypertension and the hyperdynamic circulation Citation[35], Citation[41]. In addition, recent studies have suggested that the haemoxygenase-carbon monoxide pathway mediates hyporeactivity to phenylephrine in splanchnic vessels Citation[42]. Calcitonin gene-related peptide and adrenomedullin are powerful vasodilating peptides, which are both elevated in cirrhosis, especially in those patients with ascites and the hepatorenal syndrome correlating with markers of central hypovolaemia Citation[10]. Thus, an excess of vasodilators, combined with an inadequate haemodynamic response to vasoconstrictors, may explain both the vasodilatory state and the vascular hyporeactivity in the hyperdynamic syndrome of cirrhosis. Special cares should be taken with the use of drugs with vasodilating effects, like nitrates, ACE-inhibitors, angiotensin antagonists, and alpha-adrenergic blockers.
Figure 1. Vasodilatators and vasoconstrictors in the pathogenesis of a hyperdynamic systemic circulation in patients with cirrhosis.
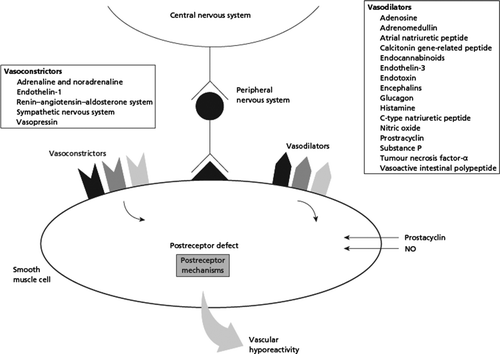
Figure 2. Cardiovascular hyporeactivity in cirrhosis may originate in the central nervous system, the autonomic nervous system, from local mediators, or within the heart muscle cell. The balance between vasodilatators and vasocontrictors is different in different vascular beds. Abbreviations: CGRP: Calcitonin gene-related peptide; ANP: atrial natriuretic peptide; CNP: C-type natriuretic peptide, TNFα: tumor necrosis factor-alpha; ET: endothelin; SNS: systemic nervous system.
Abnormal cardiovascular regulation in cirrhosis
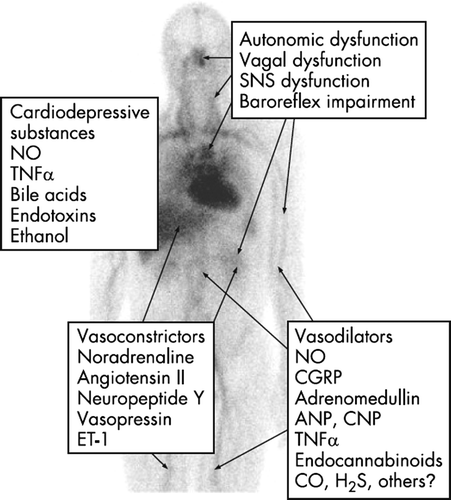
Autonomic dysfunction is often present in patients with cirrhosis and is associated with liver dysfunction and poorer survival Citation[43]. There are defects in the sympathetic nervous system and vagal impairment relating to the heart and splanchnic system Citation[44]. Abnormal cardiovascular response to vasoconstrictors has been known for some years in patients with cirrhosis. The vagal dysfunction may be related to angiotensin II. The massive activation of the sympathetic nervous system is brought about by stimulation of volume and baroreceptors, signalling reduced effective volaemia Citation[10], Citation[12], Citation[15]. The level of the arterial blood pressure is kept low in cirrhosis as a result of a primary vasodilation and secondary counterregulatory forces. The degree of arterial hypotension is related to the severity of cirrhosis, signs of decompensation, and survival Citation[45]. The sympathetic nervous system, the renin-angiotensin-aldosterone system, circulating vasopressin, and endothelin-1 are all important vasoconstrictors involved in the maintenance of the arterial blood pressure in these patients. A flat blood pressure/heart rate slope registered over 24 hours indicates that low baroreceptor sensitivity also contributes to the dysregulation of arterial blood pressure in cirrhosis Citation[27], Citation[28].
Cardiac dysfunction in cirrhosis
A cardiac dysfunction unrelated to alcoholic heart muscle disease was described 13 years ago Citation[46], and volume expansion in cirrhosis was clearly followed by an inadequate rise in cardiac output Citation[15]. The cardiac dysfunction in cirrhosis has been termed cirrhotic cardiomyopathy, a condition with systolic, diastolic, and electromechanic dysfunction, often latent owing to reduced afterload, but becoming manifest during various types of stress, see , Citation[47], Citation[48]. Most patients with cirrhosis have a normal or even elevated ejection fraction Citation[49]. Signs of early cardiac decompensation may be sparse because the cardiac afterload is reduced by peripheral vasodilatation and increased arterial compliance in these patients Citation[10], Citation[12], Citation[19]. Physiologic or pharmacologic stress may provoke manifest clinical signs of decompensation and perfusion abnormalities Citation[50]. Although cirrhotic cardiomyopathy can present with variable signs, all patients have four common features: 1) increased cardiac output; 2) attenuated systolic contraction and diastolic relaxation; 3) electrophysiologic abnormalities with repolarization changes; and 4) a reduced inotropic and chronotropic response to beta-adrenergic stimulation.
Table III. Diagnostic and supportive criteria for cirrhotic cardiomyopathy.
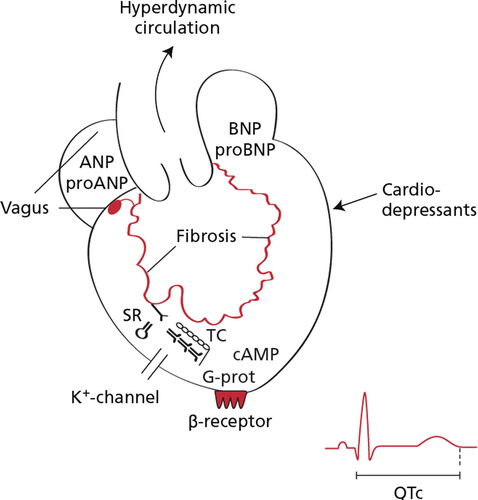
The cardiac output is controlled by venous return, heart rate, and myocardial contractility, which are all controlled by the autonomic nervous system and may be abnormal in patients with cirrhosis Citation[49]. The pathophysiology behind the cirrhotic cardiomyopathy is still under intensive study. Defects in the cardiomyocytic plasma membrane, abnormal beta-adrenergic signal pathway, negative inotropic effects of nitric oxide, carbon monoxide, cannabinoids, bile salts, and endotoxins have been implicated Citation[20], Citation[21], see .
Figure 3. Potential pathophysiological mechanism involved in the development of cirrhotic cardiomyopathy. Abbreviations: cAMP: cyclic adenosine monophosphate; pro-ANP: N-terminal propeptide of atrial natriuretic peptide; pro-BNP: pro-brain natriuretic peptide: N-terminal propeptide of brain natriuretic peptide, QTc: frequency adjusted Q-T interval; SR: sacroplasmic reticulum; TC: cardiac troponin C; G-prot: signal protein of G-type.
Systolic dysfunction
At rest, the systolic function of the left ventricle appears to be normal in most patients with cirrhosis Citation[46], Citation[49], Citation[50]. The mechanics of systolic contraction are commonly disturbed, as evidenced by examination of the systolic time interval Citation[19]. The length of the systole may be constant, but the left ventricular ejection period takes up a larger part of the time interval. This in turn shortens the pre-ejection period. Systolic dysfunction typically becomes evident during exercise, as ejection fraction does not increase as expected Citation[50]. An increase in left ventricular filling pressures does not adequately increase ejection fraction. As cirrhosis progresses, systolic function may be insufficient to meet tissue oxygen demands, leading to tissue hypoxaemia (Møller et al., unpublished). Latent systolic dysfunction may become manifest during such stressful events as normalisation of arterial blood pressure with terlipressin infusion, implantation of a peritoneovenous shunt, insertion of a transjugular intrahepatic portosystemic shunt (TIPS) with increased venous return, stress related to liver transplantation, etc. Citation[48], Citation50–53. On the other hand, the reversible and functional nature of the cirrhotic cardiomyopathy has been illustrated by improved systolic function in patients surviving orthotopic liver transplantation Citation[52], Citation[54].
Recent studies from our laboratory show that contractile dysfunction in the cirrhotic heart occurs in all parts of the left ventricle, and myocardial perfusion abnormalities are rare. The response to a pressor stimulation is reduced with enlargement of both systolic and diastolic left ventricular volumes Citation[53].
Diastolic dysfunction
Early changes in the heart of patients with cirrhosis include myocardial hypertrophy, interstitial and cellular oedema, and signs of myocyte injury Citation46–48. This causes global thickening of the left ventricle. Overall, these effects are more pronounced in patients with fluid retention. As wall thickness increases, diastolic dysfunction becomes manifest. Impaired diastolic relaxation prolongs the isovolumetric relaxation and produces a steep pressure-volume curve where the ventricular pressure is greater than normal for any given end-diastolic volume. The left atrium dilates in response to the higher "resistance" to left ventricular filling. When patients are exposed to acute changes in the circulation with increased filing pressure, they are at risk of developing congestive heart failure Citation[15], Citation[51], Citation[52]. This accounts for the high incidence of congestive heart failure after procedures such as TIPS, peritoneovenous shunt, and liver transplantation Citation[52], Citation[55]. Increased pulmonary pressure may develop during liver transplantation, and echocardiographic evaluation or right heart catheterisation should be performed before liver transplantation. Diastolic dysfunction, as described above seems to be a rather consistent feature of cirrhotic cardiomyopathy.
Electromechanical abnormalities
There is a large body of evidence of electrophysiological abnormalities in cirrhosis, primarily relating to prolonged repolarisation and compromised electromechanical coupling Citation[56], Citation[57]. The Q-T interval is prolonged in a substantial number of patients with cirrhosis Citation[44], Citation[56]. This is in line with a myocyte abnormality rather than regional abnormalities within the wall of the left ventricle Citation[44]. The prolonged repolarisation time as reflected by the prolonged Q-T interval may lead to ventricular tachyarrhythmias and sudden death. However, clinical evidence is sparse, but a significant number of cirrhotic patients die suddenly from cardiovascular collapse unrelated to bleeding and encephalopathy. The prolongation of the Q-T interval is partly reversible after improvement of liver function, liver transplantation and treatment with beta-adrenergic blockers Citation58–60. Chronotropic incompetence is marked in patients with cirrhosis, and failure to adapt the heart rate to pathophysiologic demand has important consequences in these patients. Because contractility often is compromised, heart rate becomes the major determinant of cardiac output in cirrhosis Citation[15].
Brain type natriuretic peptide (BNP) and pro-BNP are increased in patients with cirrhosis, not owing to reduced hepatic degradation but to increased cardiac generation Citation[61]. It has recently been shown that the raised cardiac output in advanced cirrhosis may fall, especially if the hepatorenal syndrome develops Citation[26], and recently Krag and co-workers showed that the reduction in cardiac output is related to poor survival of these patients Citation[62]. However, it should be stressed that the effect of cirrhosis on cardiac function and haemodynamics is mostly functional, since it may be reversible after liver transplantation. The renal dysfunction can also be normalized after transplantation, but may be difficult to evaluate, since cyclosporine and similar drugs may induce irreversible damage.
Conclusion
Cardiovascular dysfunction in cirrhosis is important for organ failure, fluid homoeostasis, and kidney dysfunction. It results from combined humoral, nervous, and haemodynamic changes. The cardiovascular complications in cirrhosis and cirrhotic cardiomyopathy are part of a multiorgan syndrome that affects survival. At present, no specific treatment can be recommended. Caution should be exercised with respect to such stressful procedures as TIPS, peritoneal venous shunting, large volume paracentesis, and surgery. Liver transplantation has been shown to reverse cardiovascular dysfunction in patients with cirrhosis, but on the other hand patients with cirrhotic cardiomyopathy are at greater risk during and after liver transplantation.
Further research should be directed towards a better understanding of the complex mechanisms underlying the cardiovascular dysfunction in liver disease, as this may be the key to benefits of potential treatment.
References
- Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: A nationwide population-based cohort study. Hepatology. 2008; 48: 214–20
- Henriksen JH, Siemssen O, Krintel JJ, Malchow-Møller A, Bendtsen F, Ring-Larsen H. Dynamics of albumin in plasma and ascitic fluid in patients with cirrhosis. J Hepatol. 2001; 34: 53–60
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999; 341: 403–9
- Keiding S, Sørensen M, Bender D, Munk OL, Ott P, Vilstrup H. Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology. 2006; 43: 42–50
- La Villa G, Gentilini P. Hemodynamic alterations in liver cirrhosis. Mol Aspects Med. 2008; 29: 112–8
- Cárdenas A, Ginès P. Predicting mortality in cirrhosis. Serum sodium helps. N Engl J Med. 2008; 359: 1060–2
- Henriksen JH, Bendtsen F, Møller S. Normal red cell cardiac output in the hyperkinetic syndrome of alcoholic cirrhosis. J Hepatol. 2001; 34: 782–3
- Kowalski HJ, Abelmann WH. The cardiac output in Laennec's cirrhosis. J Clin Invest. 1953; 32: 1025–33
- Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988; 8: 1151–7
- Møller S, Henriksen JH. The systemic circulation in cirrhosis. Ascites and renal dysfunction in liver disease, P Gines, V Arroyo, J Rodes, RW Schrier. Blackwell, Malden 2005; 139–155
- Møller, S, Henriksen, JH, Bendtsen, F. Pathogenetic background for treatment of ascites and hepatorenal syndrome. Hepatol Int. 2008;2:416–28.
- Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008; 57: 268–78
- Caraceni P, Dazzani F, Salizzoni E, Domenicali M, Zambruni A, Trevisani F, et al. Muscle circulation contributes to hyperdynamic circulatory syndrome in advanced cirrhosis. Hepatology. 2008; 48: 559–66
- Møller S, Henriksen JH, Bendtsen F. Central and noncentral blood volumes in cirrhosis: Relationship to anthropometrics and gender. Am J Physiol Gastrointest Liver Physiol. 2003; 284: G970–9
- Møller S, Bendtsen F, Henriksen JH. Effect of volume expansion on systemic hemodynamics and central and arterial blood volume in cirrhosis. Gastroenterology. 1995; 109: 1917–25
- Colombato LA, Albillos A, Groszmann RJ. The role of central blood volume in the development of sodium retention in portal hypertensive rats. Gastroenterology. 1996; 110: 193–8
- Henriksen JH, Møller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to low adrenaline and elevated circulating calcitonin gene-related peptide but not to activated vasoconstrictor systems. Gut. 2001; 49: 112–8
- Andreu V, Perello A, Moitinho E, Escorseli A, Garcia-Pagán JC, Bosch J, et al. Total effective vascular compliance in patients with cirrhosis. Effects of propranolol. J Hepatol. 2002; 36: 356–61
- Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Møller S. Arterial compliance in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2001; 280: G584–94
- Wang YX, Fitch RM. Vascular stiffness: Measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004; 2: 379–84
- Balmain S, Padmanabhan N, Ferrell WR, Morton JJ, McMurray JJ. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur J Heart Fail. 2007; 9: 865–71
- Shapiro BP, Lam CS, Patel JB, Mohammed SF, Kruger M, Meyer DM, et al. Acute and chronic ventricular-arterial coupling in systole and diastole: Insights from an elderly hypertensive model. Hypertension. 2007; 50: 503–11
- Hansen TW, Jeppesen P, Torp-Pedersen C. Prognostic significance of aortic pulse-wave velocity. Vascular hemodynamics. Bioengineering and clinical perspectives, PJ Yim. Hoboken, New Jersey: Wiley-Blackwell. 2008; 85–94
- Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. JRAAS. 2004; 5: 102–8
- Møller S, Bendtsen F, Henriksen JH. Determinants of the renin-angiotensin-aldosterone system in cirrhosis with special emphasis on the central blood volume. Scand J Gastroenterol. 2006; 41: 4518
- Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007; 46: 935–46
- Møller S, Wiinberg N, Henriksen J H. Noninvasive 24-hour ambulatory arterial blood pressure monitoring in cirrhosis. Hepatology. 1995; 22: 88–95
- Møller S, Iversen JS, Henriksen JH, Bendtsen F. Reduced baroreceptor reflex sensitivity in alcoholic cirrhosis. Relations to haemodynamic and humoral systems. Am J Physiol. 2007; 292: H2966–72
- Wiest R, Groszmann R J. The paradox of nitric oxide in cirrhsois and portal hypertension: Too much, not enough. Hepatology. 2002; 35: 478–91
- Iwakiri Y, Groszmann R J. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology. 2006; 43: 121–31
- Ebrahimkhani MR, Mani AR, Moore K. Hydrogen sulphide and the hyperdynamic circulation in cirrhosis: A hypothesis. Gut. 2005; 54: 1668–71
- Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, et al. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006; 130: 838–54
- Schrier R W. Water and sodium retention in edematous disorders: Role of vasopressin and aldosterone. Am J Med. 2006; 119: 1917–25
- Wiest R, Jurzik L, Herold T, Straub RH, Schölmerich J. Role of NPY for vasoregulation in the splanchnic circulation during portal hypertension. Peptides. 2007; 28: 396–404
- Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann R J. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006; 290: G980–7
- Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007; 46: 935–46
- Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis. A consensus workshop of the International Ascites Club. Gut. 2007; 56: 1310–8
- Helmy A, Newby DE, Jalan R, Hayes PC, Webb D J. Enhanced vasodilatation to endothelin antagonism in patients with compensated cirrhosis and the role of nitric oxide. Gut. 2003; 52: 410–5
- Langer DA, Shah V H. Nitric oxide and portal hypertension: Interface of vasoreactivity and angiogenesis. J Hepatol. 2006; 44: 209–16
- Ferguson JW, Dover AR, Chia S, Cruden NL, Hayes PC, Newby D E. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut. 2005; 55: 542–6
- Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodés J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005; 43: 98–103
- Bolognesi M, Sacerdoti D, Di Pascoli M, Angeli P, Quarta S, Sticca A, et al. Haeme oxygenase mediates hyporeactivity to phenylephrine in the mesenteric vessels of cirrhotic rats with ascites. Gut. 2005; 54: 1630–6
- Hendrickse MT, Triger D R. Vagal dysfunction and impaired urinary sodium and water excretion in cirrhosis. Am J Gastroenterol. 1994; 89: 750–7
- Hansen S, Møller S, Bendtsen F, Jensen G, Henriksen J H. Diurnal variation and dispersion in Q-T interval in cirrhosis. Relation to haemodynamic changes. J Hepatol. 2007; 47: 373–80
- Møller S, Henriksen J H. Cardiovascular dysfunction in cirrhosis. Patophysiological evidence of a cirrhotic cardiomyopathy. Scand J Gastroenterol. 2001; 36: 785–94
- Ma Z, Lee S S. Cirrhotic cardiomyopathy: Getting to the heart of the matter. Hepatology. 1996; 24: 451–9
- Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, et al. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007; 56: 869–75
- Møller S, Henriksen J H. Cirrhotic cardiomyopathy: A pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002; 87: 9–15
- Møller S, Søndergaard L, Møgelvang J, Henriksen O, Henriksen J H. Decreased right heart blood volume determined by magnetic resonance imaging: Evidence of central underfilling in cirrhosis. Hepatology. 1995; 22: 472–8
- Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, et al. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995; 22: 326–32
- Pacher P, Batkai S, Kunos G. Cirrhotic cardiomyopathy:an endocannabinoid connection?. Br J Pharmacol. 2005; 146: 313–4
- Myers RP, Lee S S. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000; 6: 44–52
- Krag A, Bendtsen F, Henriksen JH, Møller S. Cardiac effects of terlipressin in cirrhosis. Unmasking a cirrhotic cardiomyopathy. J Hepatol. 2007; 46: 96
- Mohamed R, Forsey PR, Davies MK, Neuberger J M. Effect of liver transplantation on Q-T interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996; 23: 1128–34
- Liu H, Gaskari S, Lee S S. Cardiac and vascular changes in cirrhosis: Pathogenic mechanisms. World J Gastroenterol. 2006; 12: 837–42
- Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Møller S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002; 36: 513–20
- Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006; 44: 994–1002
- Henriksen JH, Bendtsen F, Hansen EF, Møller S. Acute non-selective -adrenergic blockade reduces prolonged frequency-adjusted Q–T interval (QTc) in patients with cirrhosis. J Hepatol. 2004; 40: 239–46
- Zambruni A, Trevisani F, Di Micoli A, Savelli F, Berzigotti A, Bracci E, et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008; 48: 415–21
- Bernardi M, Calandra S, Colantoni S, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepotology. 1998; 27: 28–34
- Henriksen JH, Gøtze JP, Fuglsang S, Christensen E, Bendtsen F, Møller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003; 52: 1511–7
- Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac index predicts survival and renal failure in patients with ascites. Evidence of a heart-kidney axis in cirrhosis. J Hepatol. 2008; 48(Suppl 2)s118