Abstract
Objectives. The purinergic system has not been investigated in detail following ischemia/reperfusion (I/R) injury in the heart. In the present study, we focus on both release and response to extracellular adenosine triphosphate (ATP). Pannexin (Panx) channels have been shown to be involved in ATP release from myocytes and can activate P2X1 and P2Y2 receptors on the coronary artery. Design. We applied a well-characterized I/R model in rats, with 24 hours of reperfusion. Panx expression in the myocardial tissue was measured with quantitative polymerase chain reaction (qPCR) and flow cytometry. ATP release was detected in situ using luminescence and the vascular response to nucleotides determined in a wire myograph. Results. Here, we show that Panx expression is increased after experimental myocardial I/R, leading to an increase in extracellular ATP release, which could be inhibited by probenecid. Functional studies revealed that the P2Y2 receptor-dependent contraction is reduced in the coronary artery after I/R, which might be a response to the increased ATP levels. Conclusion. We, therefore, conclude that the regulation of the arterial purinergic system minimizes coronary contractions following ischemia.
Introduction
In recent years, Pannexin (Panx) single membrane channels have gained interest as a potential extracellular adenosine triphosphate (ATP) releasing pathway. During cardiac ischemia, several changes occur both in the myocytes and in the coronary vasculature. The role of possible ATP release and its metabolites has been studied in relation to cardiac protection, but the main focus has been on the cardiac myocytes with limited attention to the vasculature.
Previous studies have shown that ATP released during acute ischemia was mediated through Panx single membrane channels [Citation1]. ATP release through Panx has been shown to activate nearby sympathetic fibers [Citation1]. Interestingly, studies by Vessey and colleagues concluded that Panx was involved in pre- and post-conditioning by reducing the infarct from acute myocardial ischemia [Citation2,Citation3]. ATP is a potent vasoconstrictor, and changes in post-ischemic ATP release and vascular purinergic receptors have not been investigated. It is worth mentioning that experimentally induced cerebral ischemia in Panx knockout mice showed a reduced infarction, with potential involvement of neurons [Citation4]. Hence, changes in Panx and its implications on the microenvironment following ischemia/reperfusion (I/R) is not fully understood. In addition to expression and function of Panx, it is important to investigate other potential targets of the released ATP. Myocardial cells and inflammation has been broadly studied, but not the coronary artery smooth muscle cells, where extracellular ATP mainly activates the P2X1 and P2Y2 receptors [Citation5,Citation6]. For other vasoconstrictive agents, such as receptors for ET-1, receptor upregulation is an important part of I/R pathology [Citation7].
Materials and methods
Surgery
Detailed method can be found elsewhere [Citation7]. Left-sided thoracotomy was performed on Sprague Dawley rats (280 – 350 g) and a proline suture was placed around the LAD (left anterior descending) coronary artery and tightened for 30 min. 24 hours of reperfusion was achieved by loosening the ligature and allowing the animal to recover. All procedures performed were approved by the Danish animal inspectorate (Dyrefors⊘gstilsynet) approval number 2013-15-2934-00940, and therefore also according to the guidelines of Directive 2010/63/EU of the European Parliament.
RNA extraction and qPCR
RNA was isolated from myocardium from sham or I/R rats using the NucleoSpin® miRNA kit (Macherey-Nagel). cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time qPCR was performed on a CFX384 system (Bio-Rad) using the following TaqMan primer assays: Panx1 (Rn01447976_m1), Panx2 (Rn01308054_m1) and peptidylprolyl isomerase A (PPIA) (Rn00690933_m1).
Preparation of single cell suspensions and intracellular flow cytometry
The cardiomyocytes were isolated by enzymatic digestion (Liberase TM Research Grade, Roche) and sieved through 70 µm cell strainer to obtain a single cell suspension. Fixable Viability Dye eFluor 780 (FVD, eBioscience) was used to irreversibly label dead cells. The cell suspension was incubated with primary rabbit anti-Panx1 (1:100, ACC-234, Alomone) or rabbit isotope control IgG (1 mg/ml, Abcam, UK) overnight, followed by incubation with Alexa 488-conjugated donkey anti-rabbit IgG (1:100, Invitrogen) for 2 hours The suspension was analyzed on the BD FACSVerse machine (BD Biosciences, USA).
ATP release
Myocardial left ventricle tissue was enzyme-digested (Collagenase, Roche). The ATP release experiments were conducted similarly to Haanes et al. [Citation8] using the (ATP kit, SL 144–041, BioThema) and recorded on a NOVOstar Optima (BMG, Labtech). Experiments were conducted at 37 °C in the presence of 5% CO2. Sample protein concentrations were measured with DC™ Protein Assay kit (Bio-Rad) on a Tecan Infinite M200 Microplate Reader. Absorbance values below the limit of detection were set to the lowest bovine serum albumin concentration.
Myograph
A detailed method is described elsewhere [Citation9]. Two segments of the LAD distal to the ligation were isolated and mounted on a Mulvany–Halpern wire myograph (Danish Myo Technology, Denmark). The myographs were connected to a PowerLab Unit and responses were sampled in LabChart™ (ADInstruments, UK). Vascular smooth muscle contractile function and the contractile reproducibility were confirmed by stimulating the segments twice with 125 mmol/L K+. Contractile responses are expressed as a percentage of the K+ response, or as a percent of the precontraction.
Immunohistochemistry
Details on immunohistochemistry and specificity of the antibody can be found elsewhere [Citation10]. The samples were incubated with primary antibodies to the P2Y2 receptor (rabbit anti-P2Y2, 1:100, #APR-010, Alomone) overnight, followed by the secondary antibody (Alexa A488 donkey anti-rabbit 1:400)
Chemicals and statistics
All chemicals were from Sigma-Aldrich, except α,β-Methyleneadenosine 5′-triphosphate (αβ-metATP), 10Panx and probenecid (Tocris, UK) and Uridine-5'-(γ-thio)-triphosphate (UTPγS, Biolog, Germany). Data are expressed as mean ± SEM (standard error of the mean, n equals the number of animals). Statistical significance was tested using two-way analysis of variance (ANOVA) with Bonferroni post-hoc test or unpaired, two-tailed Student’s t-test where appropriate. Data were considered statistically significant only if p < .05.
Results
We initially embarked on analyzing the expression of Panx1 and Panx 2 in the tissue surrounding the left anterior descending (LAD) coronary artery in animals exposed to (I/R) or a sham operation. Our analysis showed that Panx1 was significantly upregulated in the myocytes after I/R () and Panx1 was also expressed to a larger extent than Panx2. The expression of Panx2 was unaffected by the I/R ().
Figure 1. Myocardial Panx mRNA changes and ATP release. (a) Relative expression of Panx1 to PPIA (peptidylprolyl isomerase A) was increased in I/R rats compared to sham (n = 3). (b) Relative expression of Panx2 to PPIA was unaltered following I/R compared to sham (n = 3). (c) Representative histograms demonstrating Panx1-positive events of viable cells suspension (n = 5). The green line indicates the IgG for Panx1. The bar graph summarizes the Panx1 data. (d) Extracellular ATP release corrected for sample protein in the sham (n = 5) and I/R (n = 6). The increase in ATP release was significantly different between sham and I/R and inhibited by probenecid. *: p < .05, unpaired, two-tailed Student’s T-test.
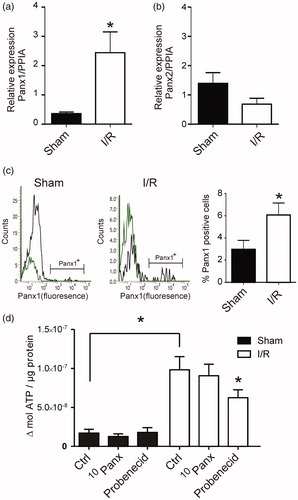
To determine Panx1 expression, a cardiomyocyte suspension was double stained with fixable viability dye e780 (FVD e780) and primary antibody Panx1. A negative signal for FVD e780 indicated viable cells. The viability of the cardiomyocytes from sham and I/R animals were not statistically significant (88.8% ± 3% vs 86% ± 2.5%, n = 5). FVD e780 negative events (viable single cells) were further sub-gated and Panx1 was detected based on their fluorescence (). The bar graph in shows summarized data of the cells positive for Panx1 expression, higher than the threshold. There was a significantly higher number of myocytes expressing Panx1 from I/R animals compared to sham animals (6.1% ± 1.1% vs 3% ± 0.8%, n = 5).
To evaluate if the increase in Panx1 expression correlated with a functional increase in extracellular ATP release we stimulated isolated myocytes with 40 mM K+. Hence, the myocytes were both depolarized (which induces contraction), and mechanically stimulated. There was an increase in the extracellular ATP release in myocytes from sham and I/R when stimulated. Importantly, the ATP release from the ventricle of I/R animals was significantly higher than that of the sham controls (). In addition, to ensure that the ATP release was linked to increased functionality of Panx1 we incubated the cells with 10 µM 10Panx inhibitory peptide or 500 µM probenecid before triggering ATP release. 10Panx did not have a significant effect, in contrast to probenecid which significantly reduced the ATP release.
Released extracellular ATP might activate purinergic receptors in vivo, located on the vascular smooth muscle cells. We therefore investigated the LAD in a myograph setup, following I/R. There were no differences in the contractions induced by specific stimulation of P2X1 receptors with αβ-metATP (). For the stimulation of P2Y2 receptors, UTPγS was applied on arteries depolarized using 30 mM K+ to prevent any P2Y2 dependent dilation from endothelial hyperpolarization [Citation11,Citation12]. Here we observed a reduced contraction to UTPγS in I/R compared to sham (p < 0.05, ). We postulated that P2Y2 receptor expression could be altered. Using immunohistochemistry, a representative picture from sham and I/R operated rats (n = 4) shows that P2Y2 receptor immunoreactivity is nearly absent in smooth muscle cells of I/R arteries (). The specificity of this antibody has been determined elsewhere [Citation10].
Figure 2. Rat distal coronary artery contractions and expression of P2Y2. (a) Contractions induced by αβmetATP in I/R and sham operated animals relative to 125 mM K+ (n = 6). (b) Arteries pre-contracted with 30 mM K+ to prevent endothelial dependent hyperpolarizing influence [Citation11], were stimulated with UTPγS, a specific P2Y2 ligand. There was a significantly decreased contraction in the I/R arteries (n = 6). (c) Representative immunohistochemistry on rat coronary arteries (n = 4), where a weaker staining (immunoreactivity) for P2Y2 can be observed after I/R. Scale bar is 25 µm in both pictures. In general, the expression of P2Y2 appears decreased in these arteries. Statistical significance was tested using two-way ANOVA with Bonferroni post-hoc test. *p < .05; **p < .01; ***p < .001.
![Figure 2. Rat distal coronary artery contractions and expression of P2Y2. (a) Contractions induced by αβmetATP in I/R and sham operated animals relative to 125 mM K+ (n = 6). (b) Arteries pre-contracted with 30 mM K+ to prevent endothelial dependent hyperpolarizing influence [Citation11], were stimulated with UTPγS, a specific P2Y2 ligand. There was a significantly decreased contraction in the I/R arteries (n = 6). (c) Representative immunohistochemistry on rat coronary arteries (n = 4), where a weaker staining (immunoreactivity) for P2Y2 can be observed after I/R. Scale bar is 25 µm in both pictures. In general, the expression of P2Y2 appears decreased in these arteries. Statistical significance was tested using two-way ANOVA with Bonferroni post-hoc test. *p < .05; **p < .01; ***p < .001.](/cms/asset/e88bcd79-52e5-4335-8a48-7cc5c17f4b6c/icdv_a_1552793_f0002_c.jpg)
Discussion
There are several studies showing that ATP and UTP have protective features following ischemic damage (reviewed by Burnstock and Pelleg [Citation13]). Nevertheless, the Panx1 knockout mice have smaller ischemic damage after middle cerebral occlusion [Citation4,Citation14], which appears as a paradox. One explanation could be differences in Panx location, for example, their role on autonomic nerves might have separate physiological functions from their expression in the innervated tissue. Therefore, we believe that in respect to purinergic signaling, there might be differences between cerebral and coronary ischemia, which deserves future attention.
We have in previous work focused on the upregulation of endothelin receptors, particularly Endothelin receptors type B, (ETB receptors) after coronary artery ischemia [Citation7]. In the ischemic heart, the levels of endohelin-1 (ET-1) mRNA was increased [Citation15] and the contractile response to ETB receptor stimulation was enhanced [Citation7,Citation16]. Altogether, these observed changes in the endothelin system, can contribute to a reduction in blood flow/supply and exacerbating the ischemic damage following reperfusion injury. In the present study, we focused on extracellular ATP, which causes arterial contractions mainly via P2X1 or P2Y2 receptors upon application to the vascular smooth muscle cells [Citation5,Citation6]. The contraction induced by an P2X1 agonist did not significantly change after I/R, in contrast to P2Y2, which induces a weaker contraction following I/R.
It has been shown that P2Y2 is cardioprotective since P2Y2 activation leads to reduced inflammation [Citation17]. The compensatory response observed in our study in the coronary arteries, with reduced contractility, suggests that UTP/ATP could have a pure cardioprotective effect since the increased extracellular ATP would not cause increased arterial contraction. Indeed, previous studies have concluded that Panx is involved in coronary pre- and post-conditioning [Citation2,Citation3].
In conclusion, Panx1 is upregulated following myocardial I/R injury, leading to increased probenecid sensitive ATP release. However, for the purinergic receptors investigated here, we do not see a functional receptor upregulation, as is seen for the endothelin system; in contrast, we observe reduced P2Y2-mediated contraction.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dong F, Yang XJ, Jiang TB, et al. Ischemia triggered ATP release through Pannexin-1 channel by myocardial cells activates sympathetic fibers. Microvasc Res. 2016;104:32–37.
- Vessey DA, Li L, Kelley M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am J Physiol Heart Circ Physiol. 2011;301:H881–H887.
- Vessey DA, Li L, Kelley M. Pannexin-I/P2X 7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre- and postconditioning. J Cardiovasc Pharmacol Ther. 2010;15:190–195.
- Cisneros-Mejorado A, Gottlieb M, Cavaliere F, et al. Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab. 2015;35:843–850.
- Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340.
- Welsh DG, Brayden JE. Mechanisms of coronary artery depolarization by uridine triphosphate. Am J Physiol Heart Circ Physiol. 2001;280:H2545–H2553.
- Skovsted GF, Kruse LS, Larsen R, et al. Heart ischaemia-reperfusion induces local up-regulation of vasoconstrictor endothelin ETB receptors in rat coronary arteries downstream of occlusion. Br J Pharmacol. 2014;171:2726–2738.
- Haanes KA, Kowal JM, Arpino G, et al. Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal. 2014;10:431–440.
- Kristiansen SB, Haanes KA, Sheykhzade M, et al. Endothelin receptor mediated Ca2+ signaling in coronary arteries after experimentally induced ischemia/reperfusion injury in rat. J Mol Cell Cardiol. 2017;111:1–9.
- Haanes KA, Spray S, Syberg S, et al. New insights on pyrimidine signalling within the arterial vasculature – different roles for P2Y2 and P2Y6 receptors in large and small coronary arteries of the mouse. J Mol Cell Cardiol. 2016;93:1–11.
- Kristiansen SB, Sheykhzade M, Edvinsson L, et al. Changes in vasodilation following myocardial ischemia/reperfusion in rats. Nitric Oxide. 2017;70:68–75.
- Kilpatrick EV, Cocks TM. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br J Pharmacol. 1994;112:557–565.
- Burnstock G, Pelleg A. Cardiac purinergic signalling in health and disease. Purinergic Signal. 2015;11:1–46.
- Bargiotas P, Krenz A, Hormuzdi SG, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA. 2011;108:20772–20777.
- Tonnessen T, Christensen G, Oie E, et al. Increased cardiac expression of endothelin-1 mRNA in ischemic heart failure in rats. Cardiovasc Res. 1997;33:601–610.
- Skovsted GF, Kruse LS, Berchtold LA, et al. Myocardial ischemia-reperfusion enhances transcriptional expression of endothelin-1 and vasoconstrictor ETB receptors via the protein kinase MEK-ERK1/2 signaling pathway in rat. PLoS One. 2017;12:e0174119
- Cohen R, Shainberg A, Hochhauser E, et al. UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem Pharmacol. 2011;82:1126–1133.
