ABSTRACT
The Chinese artichoke (Stachys affinis) is a vegetable that is also used as a medicinal plant. There are two major problems in growing Chinese artichoke. One is the re-infection of viruses when propagating virus-free seed tubers, and the other is too long a cultivation period before harvest. The application of rooted cuttings technology to tuber production could allow virus-free cuttings to be propagated annually and planted at any time of the year. We showed that 100% of the cuttings were rooted within 14 days when the electrical conductivity of the nutrient solution was less than 0.42 dS·m−1. There was no significant difference in yield or tuber size between tubers derived from rooted cuttings and those derived from seed tubers (the conventional method of cultivation). Although the yield of rooted cuttings planted in July, August and September decreased slightly with delayed planting time, tubers of marketable size were harvested even when planted in September, the shortest growing season. The stachyose content, polyphenol content and antioxidant activity of the tubers did not differ significantly with planting time. In conclusion, short-term cultivation of Chinese artichoke using rooted cuttings is feasible for commercial tuber production.
Introduction
The Chinese artichoke (Stachys affinis, synonym S. sieboldii), chorogi, is a member of the Lamiaceae family and is edible as a tuber with an enlarged stolon that grows underground (Lim, Citation2016). Native to northern China, it is widely used as a vegetable in East Asia (Mercier & Perennes, Citation1982) and has been cultivated in Europe since the 19th century (Lim, Citation2016). In Japan, red pickles made from harvested tubers and coloured red with perilla leaves are traditionally served during the New Year celebrations. It can also be used in soups and eaten raw in salads (Mercier & Perennes, Citation1982). Chinese artichoke is a nutritious vegetable that contains carbohydrates, starches, lipids and cellulose (Rana & Yadav, Citation2017). In China, Chinese artichoke is also used as a medicinal plant for the treatment of ischaemic brain injury, dementia and gastrointestinal disorders (Cho et al., Citation2014). In addition, Chinese artichoke has antitoxin activity, inhibits hyaluronidase activity, and improves ischaemic stroke and senile dementia (Harada et al., Citation2015; Takeda et al., Citation1985). Thus, Chinese artichoke is an important crop, especially in East Asia.
Foods and herbs containing oligosaccharides have been approved as functional foods for specific health uses in many countries. Non-digestible oligosaccharides such as raffinose and stachyose found in soybeans are called soybean oligosaccharides (Y. Hata et al., Citation1991). These oligosaccharides have been reported to promote the growth of bifidobacteria in the human gut and to protect the function of organs that eliminate harmful substances. They may also be used as a sugar substitute for diabetics, as they are not digested or absorbed by the body (Yin et al., Citation2006). Chinese artichoke tubers contain large amounts of stachyose, a type of oligosaccharide, which is said to make up about 63% of the dried weight of the tubers (Yazawa et al., Citation1979). Chinese artichoke may be useful as a functional food because it contains more than 10 times more stachyose than soybeans. As a prebiotic ingredient in functional foods, stachyose is expected to have intestinal regulatory effects (Manthey & Xu, Citation2010). It is also a nutritionally functional ingredient with hypoglycaemic effects (Takeda et al., Citation1985; Zhang et al., Citation2004). In addition, the phenylethanoid glycosides, acetonide and stachyoside C of stachyose have been reported to inhibit the lethality of potassium cyanide in mice (Yamahara et al., Citation1990). Acetonide is also thought to have anti-inflammatory properties (Hayashi et al., Citation1996). Chinese artichoke tubers are also useful as a tonic for anaemia due to their high iron content (Instituto Botanico Boreali-Occidentali Academiae Sinicae Edita, Citation1983). Previous phytochemical and pharmacological studies have isolated terpenes, flavonoids and phenolic compounds and evaluated their antibacterial, antioxidant and antitumour effects (Venditti et al., Citation2017).
Although Chinese artichoke is such a high-potential food, there are two major problems with its conventional cultivation. The first is the long cultivation period, and the second is the problem of virus infection of seed tubers. In Japan and other East Asian countries, Chinese artichoke is usually grown by planting tubers in early spring and harvesting them in winter, which takes six months (Yazawa et al., Citation1983). After planting, runners develop underground and the tuber begins to enlarge in response to low temperatures below 10°C (Yazawa et al., Citation1983). Such conventional cultivation methods, which require a long growing season, make it difficult to double-crop with other major crops. In addition, tuber-transmitted viruses such as Chinese artichoke mosaic virus (ChAMV), a potyvirus (Fuji et al., Citation2003), alfalfa mosaic virus (AMV), cucumber mosaic virus (CMV) and tobacco mosaic virus (TMV) are problematic in current Chinese artichoke cultivation (Kim et al., Citation2018). Virus-free plants produced thorough shoot-tip culture have been produced to address these problems (Yamamoto et al., Citation2004), but growing tubers from the previous year poses a risk because cuttings can be re-infected during outdoor cultivation (Yamamoto et al., Citation2007).
Establishing a cuttings-based production system is a practical way of tackling both the problems of long cultivation periods and virus infection simultaneously. In the case of dahlias, a bulbous plant, it is now possible to use shoot-tip cultures as mother plants for cutting propagation for cut flower production (Naka et al., Citation2007). For dahlias, the use of cuttings has allowed a free choice of growing seasons. If the yield and quality of tubers from rooted cuttings are comparable to those from seed bulbs, a production system using virus-free cuttings could be established and the growing season shortened. It is also desirable to maintain the yield and functionality even when the cultivation period is shortened, and to increase the percentage of usable cuttings. This study evaluated the usefulness of a new cultivation technique for Chinese artichoke using rooted cuttings from these perspectives.
Materials and methods
This study consisted of three experimental essays. The first two essays were carried out under controlled environmental conditions in the Kindai University, Nara, Japan. The third essay was conducted under field conditions in the Azono region of Himeji City, Hyogo Prefecture, Japan (34° 98’ 59” N, 134° 62’ 98” W).
First essay: propagation and rooting of cuttings
In this experiment, we used Chinese artichokes conserved at Kindai University, Japan. According to Hosokawa et al. (Citation2004), leaf primordia-free shoot apical meristem culture was used to produce plants in vitro. Young plants were grown on modified MS medium (Murashige & Skoog, Citation1962) containing 2% sucrose and acclimated after two months of tissue culture in 2020. The conditions used for in vitro plant growth were 20°C, 14 h day length and 63 µmol·m−2·s−1 (photosynthetic photon flux density; PPFD). The acclimatised and rooted plants, mother plants for the harvesting of cuttings, were transplanted into pots filled with 7 g of polyester medium (Neo Agri Earth, Nara, Japan) and grown hydroponically in 6.4 L containers placed in a closed room at 23°C, 16 hours day length, 182.9 µmol·m−2·s−1 light intensity using a white fluorescent lamp (FHF32EX-N-H, Panasonic, Tokyo, Japan). The nutrient solution was a mixture of 1.5 g·L−1 of OAT House No. 1 (OAT Agrio, Tokyo, Japan) and 1.0 g·L−1 of OAT House No. 2 (OAT Agrio) containing 18.6 mM nitrogen, 5.1 mM phosphorus, 8.6 mM potassium and was maintained at an electrical conductivity (EC) of 2.6 dS·m−1. An air pump (e-AIR 6000 WB GEX, Osaka, Japan) and an air stone (18φ30 cm, King Stone, Aichi, Japan) were used for aeration during hydroponic cultivation (Fig. S1A, B). For the production of rooted cuttings, a 50-hole plug tray was placed upside down in a tray containing 7 L of water or nutrient solution, and cuttings from the top of the stem to the third or fourth node of the mother plant were collected and inserted into the holes of the plug tray for rooting (Fig. S1C, D).
After propagating, the cuttings immediately taken from the mother plant were subjected to the following cultivation conditions: with and without aeration and nutrient solution concentrations. Two treatments – one with aeration (aerated treatment) and one without aeration (unaerated treatment) during the rooting period – were set up to determine the need for aeration for rooting. Fifty cuttings were used per treatment. In addition, to study the optimal nutrient solution concentration for rooting and stolon development of cuttings, tap water (referred to as × 0) and diluted Otsuka A solution (1.5 g·L−1 for OAT house No. 1 and 1.0 g·L−1 for OAT house No. 2) consisting of total nitrogen 260, ammonia nitrogen 23, nitrate nitrogen 233, P2O5 120, K2O 405, CaO 230, MgO 60, MnO 1.5, B2O2 1.5, Fe 1.35, Cu 0.03, Zn 0.09 and Mo 0.03 ppm, were used as culture solution at three concentration levels: ×1/10, ×1/4 and × 1/2. Their electrical conductivity values were 0.42, 0.84 and 1.44 dS·m−1, respectively. The number of rooted cuttings and the number of cuttings that developed stolons were counted every two days after the start of the culture. The nutrient solution was not changed during the experiments. The number of rooted and stolonised cuttings was counted every seven days after the start of the culture.
Second essay: tuber yield with plants derived from rooted cuttings or from tubers
On 28 May 2020, two plants were planted in a planter (22.5 cm wide × 64 cm long × 18.5 cm deep) with 30 cm between plants. Five planters were used per treatment (10 plants in total for each treatment) and tuber yields from rooted cuttings and tuber plants were compared. The medium was composed of vermiculite and peat moss in a ratio of 1:1 by volume with an appropriate amount of lime added, and 3 g of N:P:K = 8:8:8 granular compound fertiliser (Fertiliser 8-8-8, Ohmiya Green Service, Saitama, Japan) was applied to the base of each plant once a month during the growing season. Each pot was automatically watered with 1 L of tap water every 5 hours. The underground part of the plant was checked for stolon tip enlargement several times during the growing period. The harvest date was 20 December 2020. Fresh weight and number of tubers were assessed for each of the four size classes A-D according to the length of the tubers: size A for 4 cm or more, size B for 3 cm to 4 cm, size C for 2 cm to 3 cm and size D for 2 cm or less. Temperatures at a depth of 5 cm below the soil surface of the planter were monitored using a data logger (TR-52i, T&D, Tokyo, Japan).
Third essay: differences in yield depending on the planting date of rooted cuttings
Fertiliser was applied according to local cultural practices, with 3 g of N:P:K = 8:8:8 granular chemical fertiliser (Fertiliser 8-8-8, Ohmiya Green Service) applied to the base of each plant once a month during the growing season. Rooted cuttings were planted in a clay soil field on 5 July, 5 August and 8 September 2021 with a spacing of 50 cm between plants. Fifty plants were planted at each of the three planting dates. Fifteen plants growing to uniform size were randomly selected from the 50 plants in each treatment and harvested on 16 December 2021. Data logger sensors were buried 5 cm into the soil to monitor soil temperature. As described above, the total length of the tubers was classified into size classes A-D. In 2021, the fresh weight of tubers was measured for each size class.
We analysed the functional components of tubers planted in the clay soil field (the same field in Himeji city) on 4 July and 5 September 2022 to evaluate the stachyose content and other food functionalities of tubers from rooted cuttings compared to conventional cultivation. As a conventional cultivation method, tubers were planted in the same field in April. Three tubers per plant per size class (A-C) were harvested and used as a sample for analysis. Three biological replicates were used for each planting date. Stachyose, polyphenol content and antioxidant activity were quantified in the tubers. Stachyose content was measured using a capillary electrophoresis system (Agilent 7100, Santa Clara, California) and polyphenol content and antioxidant activity were measured using a spectrophotometer (Shimadzu, UV-1800, Kyoto, Japan).
For each sample, three tubers frozen in liquid nitrogen were crushed in a blender (CCG-U021-WJP, Vesync, USA) until a fine powder was obtained. The fine powders were placed in a 1.5 mL tube with four times the volume of distilled water (w/v) and then heated in a dry bath (AccuBlock, Labnet, New Jersey, USA) at 95°C for 1 min to inactivate the enzymatic activity as described by Ogiwara et al. (Citation1999) and Sato et al. (Citation2009). The extract was centrifuged at 21,200 × g for 1 min at room temperature and the supernatant was collected. The extract was filtered through a syringe filter (SLNY1345NB, Nippon Genetics, Tokyo, Japan) with a pore size of 0.45 μm and finally diluted 20-fold with distilled water. The electrophoresis conditions for capillary electrophoresis were −10 kV and 30°C for 34 min. The reference wavelength was 275.0 nm (10 nm bandwidth) and the detection wavelength was 350.0 nm (10 nm bandwidth). A solution of 0.5 mM cetyltrimethylammonium bromide (CTAB), 50 mM sodium borate and 20 mM 2,6-pyridine carboxylic acid were mixed and the pH was adjusted to 9.2 with sodium hydroxide (NaOH) (Anderson et al., Citation2003). A fused silica capillary tube with an inner diameter of 75 μm was used as the capillary tube, adjusted to a length of 80.5 cm, with an effective length of 72 cm. The capillary tube was washed with a mixture of water and methanol (1:1) (v/v) for 5 min, 0.1 M NaOH for 5 min and the electrophoresis solution for 5 min before injection of the sample to avoid contamination of the capillary tube by the sample and to improve the reproducibility of the separation. Analyte samples were injected at 50 mbar for 5 seconds. The calibration curve was constructed by adjusting stachyose n-hydrate (Fujifilm-Wako, Osaka, Japan) at 10,000, 5,000 and 2,500 mg·L−1 dissolved in distilled water (R2 = 0.99). Polyphenol measurements were performed according to the Folin-Ciocalteu method (Folin & Ciocalteu, Citation1927). Briefly, methanol was added to 10 times the weight (w/v) of a fine powder in a 1.5 mL tube and mixed well to prepare the sample for polyphenol measurement. The samples were then centrifuged at 21,200 × g for 5 min, and the collected supernatant was further diluted 10-fold with distilled water and mixed well. Then, 100 μL of the sample or 100 μL of the standard solution of gallic acid monohydrate (Nakarai Tesuque, Kyoto, Japan) was mixed with 500 μL of a 10% (v/v) phenol solution, followed by the addition of 400 μL of a 7.5% (w/v) sodium carbonate solution. The mixture was allowed to react for 1 hour at room temperature and the absorbance was measured spectrophotometrically at 765 nm. The polyphenols in the samples were expressed as gallic acid monohydrate. A calibration curve was established by diluting gallic acid monohydrate in five concentrations (R2 = 0.98).
Antioxidant activity was determined by the trolox equivalent antioxidant capacity (TEAC) method according to Re et al. (Citation1999). Briefly, 7 mM 2,2’-casino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) solution and 10 mM potassium peroxydisulphate solution were mixed at a ratio of 3:1 (v/v) and the reaction was carried out overnight in the dark. This solution was diluted 50-fold with phosphate-buffered saline (PBS) (pH 7.4) to give an ABTS working solution. A 100 mg sample was taken and PBS buffer was added to make a 10-fold dilution. The sample was centrifuged at 21,200 × g and the supernatant was collected. Finally, 10 μL of the sample supernatant was added to 1 mL of ABTS working solution. Absorbance was measured spectrophotometrically at a wavelength of 734 nm. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was diluted with ethanol and used for the calibration curve. The absorbance at 734 nm was measured spectrophotometrically to obtain a calibration curve (R2 = 0.98). The Steel-Dwass test was used to determine significant differences in the functionalities of tubers from different cultivation methods.
Statistical analysis
All statistical analyses were performed using R software version 4.1.0. Student’s t-test was used to statistically evaluate the weight and number of tubers harvested from rooted cuttings and tuber-derived plants in Second Essay. Tukey-Kramer test was performed for tuber weights and numbers between three planting dates in Third Essay. The relationship between the weight of each tuber and its length at three planting dates was also investigated and the slopes of the regression lines were compared to discuss the relationships between tuber weight and its length at different planting dates. The dry matter ratio of Chinese artichoke tubers was calculated as ((dry weight (D.W.)/fresh weight (F.W.)) × 100).
Results
First essay: propagation and rooting of cuttings
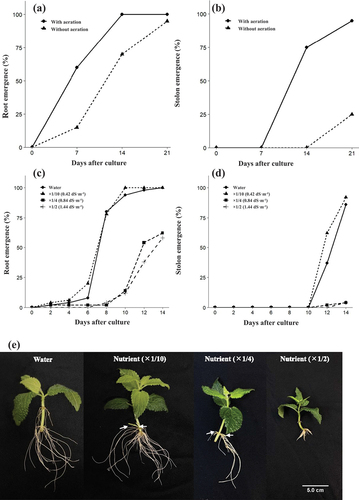
All cuttings with aeration treatment were rooted at 14 days (, Fig. S1D, E), while the rooting percentage without aeration treatment was 95% even at 21 days (). With aeration treatment, stolon development reached 75% on day 14 and almost 100% on day 21 after culturing the cuttings (). In contrast, without aeration treatment, no stolon was observed on the 14th day and the percentage of stolon development was only 25% even on the 21st day. Furthermore, when rooting and stolon development were examined according to the concentration of the nutrient solution, the rooting percentage on day 14 was 100% in the × 0 (water) and × 1/10 (0.42 dS·m−1) nutrient solution treatments, and the change in rooting percentage over time was almost identical in these two treatments (). Root percentage in the × 1/2 (1.44 dS·m−1) and × 1/4 (0.84 dS·m−1) nutrient solution treatments was 62% and 58%, respectively. Similarly, stolon elongation on day 14 was observed in more than 85% of the cuttings in the × 0 and × 1/10 nutrient solution treatments, but in only 4% of the cuttings in the × 1/2 and × 1/4 nutrient solution treatments (). The root development on day 14 of the nutrient solution concentration above 0.84 dS·m−1 was inferior to that of the low concentrations (water and 0.42 dS·m−1) ().
Figure 1. Rooting and stolon development after culture of Chinese artichoke cuttings. Time course of the percentage of (a) rooting and (b) developing stolons during 21 days on culturing cuttings with or without aeration (n = 50). Time course of the percentage of (c) rooting and (d) developing stolons during 14 days on cuttings cultured in different nutrient concentrations (n = 50). (e) Rooting of cuttings after 14 days of rooting under different nutrient concentrations. Arrows indicate elongating stolons.
Second essay: tuber yield with plants derived from rooted cuttings or from tubers
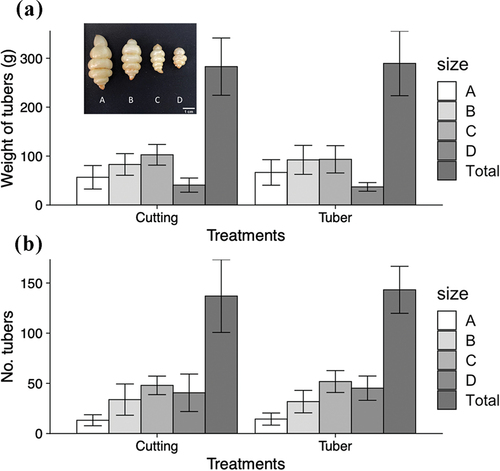
The maximum soil temperature in the planter dropped below 20°C on 17 October and below 15°C on 12 November (). It dropped further below 5°C on 27 November (). Mean soil temperatures were 13.3°C (17 October − 1 November), 10.3°C (2 November − 27 November) and 7.2°C (27 November − 18 December) (). Stolon enlargement was observed on 17 November 2021 (). The above-ground part was completely dried up by 20 December 2021, the date of harvest. There were no significant differences in total weight, total number of harvested tubers or number of harvested tubers per size class between plants derived from rooted cuttings and plants derived from tubers ().
Figure 2. Changes in soil temperature and timing of tuber formation in the planter crop. The figure shows the average daily soil temperature at 5 cm depth in the planter from 9 October to 28 December. The photographs show the tip of the stolon in the planter at the dates indicated by the arrows.
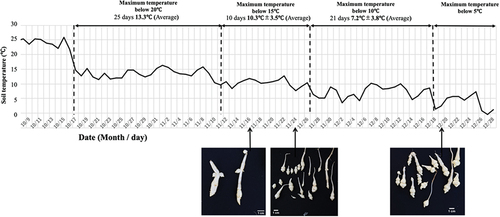
Figure 3. The yield of tubers by size class when grown from rooted cuttings and tuber plants. Five planters (10 plants) were used for each treatment. (a) Average tuber weight of each size class and all tubers harvested per planter. (b) Average number of tubers of each size class and all tubers harvested per planter. The inset shows the classification based on tuber length as: size a = greater than 4 cm, size b = greater than 3 cm but less than 4 cm, size c = greater than 2 cm but less than 3 cm and size d = less than 2 cm. No significant difference in weights and numbers at the 5% level by Student t-test between cuttings and tubers in each size class or all tubers harvested per planter. Bars are standard deviations.
Third essay: differences in yield depending on the planting date of rooted cuttings
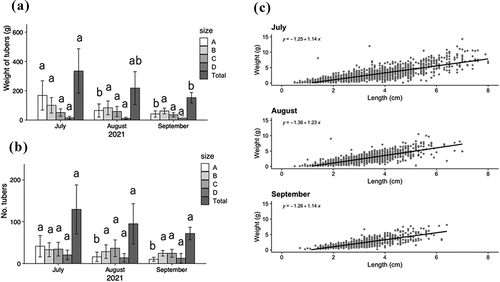
In both 2021 and 2022 in Himeji, soil temperature at 5 cm depth started to decrease in late September and reached the temperature of around 10°C in mid-November (Fig. S2), which is required for tuber enlargement as reported by Yazawa et al. (Citation1979). In the 2021 crop, the total tuber weight was highest in the July planting and decreased as the planting date was delayed (). The mean ± standard deviation of fresh weight per plant was 335.6 ± 147.0 g, 218.2 ± 108.3 g and 151.6 ± 33.8 g for the July, August and September plantings, respectively. Furthermore, the number of tubers also decreased with the delay in planting date (). The Tukey-Kramer analysis for the average yield per plant for the different planting dates showed that planting in August and September reduced the yield of A-size tubers (>4 cm) at the 5% level (). The slope of the regression line between fresh weight and harvested tuber length was almost the same regardless of planting date ().
Figure 4. Yield of tubers in each size class and all harvested tubers per plant (2021) when grown from rooted cuttings planted at different dates in the open field. (a) Average tuber weight of each size class and all harvested tubers per plant. (b) Average tuber number of each size class and all harvested tubers per plant. Statistical analysis by Tukey-Kramer at planting date was performed for yield of each size class or all harvested yield per plant. There is a significant difference at 5% for the different letters (n = 5). A-D indicate tuber size class. (c) Regression of length and weight of tubers harvested from rooted cuttings planted at each date. The regression equations are shown in the upper left of the figure.
No statistically significant differences in stachyose, polyphenol content and antioxidant activity were found among the three cultivation methods for all three size categories (). Stachyose content did not change significantly with tuber size (). Without significance, the polyphenol content decreased with delay in planting time in all three size classes (). Antioxidant activity tended to be lower in the September planting than in the July planting (). In conclusion, there were no significant differences in stachyose content, polyphenol content or antioxidant activity by size class, confirming that the difference in component content was detected by planting date.
Table 1. Nutritional analysis of tubers during different planting dates of rooted cuttings.
Discussion
Rooting and stolon elongation of Chinese artichoke were very easy (), indicating that commercial propagation of rooted cuttings is feasible (). In the case of cuttings, the nodes that produce the stolons that form the tuber must be secured at the time of cutting. Interestingly, the lateral buds of the lowest node of Chinese artichoke cuttings produced elongated stolons that grew downwards by gravity (). Since the stolons from the lowest node of the cuttings develop during the rooting of the cutting and go underground after planting in the field, there is no need to consider the planting depth of the rooted cuttings.
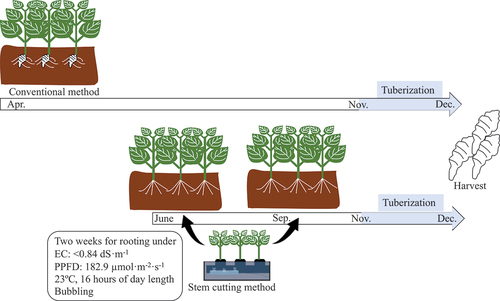
Figure 5. Conventional method of planting tubers (top panel) and a new method of planting rooted cuttings (bottom panel). In the conventional method, tubers are planted in April and cultivated until December, resulting in a long cultivation period. On the other hand, since rooted cuttings can be planted at any time of the year, the use of rooted cuttings makes it possible to shorten the cultivation period. In this study conducted in Japan, tuber growth is expected to start around 15 November when soil temperatures drop below 10°C.
Immersion of the cuttings in water was found to be effective for rooting and 100% of the cuttings were rooted within two weeks (). In an experiment with Forsythia suspensa grown on rockwool as a medium, the application of × 1/10 nutrient solution of the Otsuka A protocol showed promotion of rooting, and the growth of roots from cuttings in × 1 unit nutrient solution was inhibited compared to those grown from cuttings in tap water (N. Hata et al., Citation2009). According to Hyndman et al. (Citation1982), the addition of sucrose reduced rooting inhibition at high nutrient concentrations, and N. Hata et al. (Citation2009) explained this phenomenon by sucrose compensating for the lack of photosynthesis of the cuttings. Furthermore, root growth of Japanese knotweed was inhibited when the nutrient concentration was × 1/4 (0.8 dS·m−1 in EC) of Otsuka A nutrient solution (Mizushima, Citation2016). Therefore, nutrient supply at the time of cutting has been reported to inhibit rooting; however, in our experiment, nutrient supply below × 1/10 Otsuka A nutrient solution (0.42 dS·m−1 in EC) did not appear to interfere with Chinese artichoke rooting (). In short-rotation crops, growth recovery after planting is considered particularly important, but a 2-week rooting treatment in the absence of nutrients may weaken the plants and slow the resumption of growth (). Therefore, the production of rooted cuttings with a nutrient solution of about 0.42 dS·m−1 would be preferable.
Yazawa et al. (Citation1979) reported that soil temperature below 10°C was required for tuber enlargement in Chinese artichoke. In our planter experiment, temperatures dropped rapidly after mid-October, and tuber enlargement was observed one month after the average soil temperature dropped to 10°C, from which tubers rapidly enlarged (). Although the temperature in the planter was unstable and could not be said to reflect the actual soil temperature, the soil temperature in the open field also declined rapidly in October and dropped to 10°C from early November (Fig. S2), suggesting that early November was the onset of tuber enlargement in this study. Chinese artichokes derived from rooted cuttings showed no difference in total harvested weight or number of tubers of each size compared to plants derived from tubers (conventional method), suggesting that tuber production using rooted cuttings is feasible ().
Yields per area by planting time in 2021 were 1271.7 g·m−2 (18 plants in 4.75 m2, calculated with an average yield of 335.6 g per plant as shown in ) for July planting, 826.9 g·m−2 (18 plants in 4.75 m2, an average yield of 218.2 g per plant as shown in ) for August planting and 606.4 g·m−2 (18 plants in 4.75 m2, an average yield of 151.6 g per plant as shown in ) for September planting. The present results are in agreement with the reported yield of 1175.1 g·m−2 in Hokkaido, Japan (Akimoto & Ichikawa, Citation2014) and 300–600 g·m−2 in Honshu, Japan (Yazawa et al., Citation1979). Our yield results were not lower, even for the September planting, when comparing these yields. As the tuberisation state of Chinese artichoke starts below 10°C in the soil (Yazawa et al., Citation1979), it is difficult to choose the harvesting time. In 2021 and 2022, in the Azono region of Himeji City, Hyogo Prefecture, the soil temperature dropped below 10°C from mid to late October (Fig. S2). As a result, in a short-duration crop, such as a crop planted in September, the above-ground part had to grow to a sufficient size in about 1.5 months, from September to mid-October. shows that delaying the planting date reduces the yield by reducing the number and weight of A-size tubers, whereas tubers can still be harvested in such a short-period crop. In our opinion, the yield per unit area can be secured by increasing the planting density.
Tuber length is an important indicator for commercial processing and marketing. The slope of the regression line between fresh weight and total length of harvested tubers was almost the same regardless of planting time (). This suggests that the relationship between apparent shape (tuber length) and weight is the same when the growing season is shortened, which means that shortening the growing season does not affect apparent quality. The increase in the proportion of small tubers due to short-term cultivation () suggests that they may become easier to process as food.
The dry matter ratio of Chinese artichoke tubers was about 20.0% (data not shown), and the stachyose content per D.W. for size A calculated from was 498 mg·g−1 D.W. for tubers planted in April, 437 mg·g−1 D.W. in July, and 493 mg·g−1 D.W. in September. In another study on Chinese artichoke, Yin et al. (Citation2006) reported that tubers contained 236.0 mg·g−1 D.W. of stachyose. The reported stachyose content in soybean seeds is 5–6 mg·g−1 fresh weight (Ohtani et al., Citation2000), 8.4 mg·g−1 F.W., around 15 mg·g−1 F.W (Rhee et al., Citation2001), 30–50 mg·g−1 F.W (Nomura et al., Citation2013), while the content in Chinese artichoke tubers is much higher. Stachyose content per D.W. did not differ significantly with tuber size (), suggesting that stachyose accumulates at a constant rate with tuber enlargement and is not translocated all at once at the end of the enlargement stage. No significant differences in stachyose content were observed as a function of planting date and cultivation method (), leading to the conclusion that stachyose does not decrease with short-term cultivation using rooted cuttings. Although no statistical differences were found, a delay in planting time resulted in a decrease in polyphenol content (). The polyphenol content of the tubers did not seem to vary with tuber size, but rather with planting date (). The decrease in antioxidant activity was also observed with September planting. It was suggested that part of this decrease was due to a reduction in polyphenols.
Conclusion
In this study, the rooting of Chinese artichoke cuttings is shown to be easy. Unlike conventional tuber planting, the use of propagated cuttings diversifies the planting time. This study demonstrates the use of rooted cuttings for the production of tubers with commercial value in stachyose content even in a short cultivation period such as 3–4 months. By using rooted cuttings, it would be possible to use Chinese artichoke for double cropping.
Supplemental Material
Download MS Word (18.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14620316.2024.2304559.
Additional information
Funding
References
- Akimoto, M., & Ichikawa, N. (2014). Trial cultivation of Chinese artichoke (Stachys sieboldii miq.) in Tokachi region. Research Bulletin of Obihiro University, 35, 9–14.
- Anderson, K. E., Bjergegaard, C., Møller, P., Sørensen, J. C., & Sørensen, H. (2003). High-performance capillary electrophoresis with indirect UV detection for determination of r-galactosides in leguminosae and brassicaceae. Journal of Agricultural and Food Chemistry, 51(22), 6391–6397. https://doi.org/10.1021/jf030328m
- Cho, H. K., Kim, C. S., Woo, K. W., & Kang, R. L. (2014). A new triterpene saponin from the tubers of Stachys sieboldii. Bulletin of the Korean Chemical Society, 35(5), 1553–1555. https://doi.org/10.5012/bkcs.2014.35.5.1553
- Folin, O., & Ciocalteu, V. (1927). On tyrosine and tryptophan determinations in proteins. The Journal of Biological Chemistry, 73(2), 627–650. https://doi.org/10.1016/S0021-9258(18)84277-6
- Fuji, S., Yamamoto, H., Furuya, H., & Naito, H. (2003). Characterization of a new potyvirus isolated from Chinese artichoke in Japan. Archives of Virology, 148(11), 2249–2255. https://doi.org/10.1007/s00705-003-0169-7
- Harada, S., Tsujita, T., Ono, A., Miyagi, K., Mori, T., & Tokuyama, S. (2015). Stachys sieboldii (Labiatae, Chorogi) protects against learning and memory dysfunction associated with ischemic brain injury. Journal of Nutritional Science and Vitaminology, 61(2), 167–174. https://doi.org/10.3177/jnsv.61.167
- Hata, N., Okazawa, N., Morimoto, K., Ono, E., Satake, H., & Kobayashi, A. (2009). Effect of IBA treatment, fertigation, photoperiod, and light intensity on rooting of softwood cuttings of forsythia suspense. Journal of Society of High Technology in Agriculture, 21(1), 15–23. https://doi.org/10.2525/shita.21.15
- Hata, Y., Yamamoto, M., & Nakajima, K. (1991). Effects of soybean oligosaccharides on human digestive organs: Estimation of fifty percent effective dose and maximum non-effective dose based on diarrhea. Journal of Clinical Biochemistry and Nutrition, 10(2), 135–144. https://doi.org/10.3164/jcbn.10.135
- Hayashi, K., Nagamatsu, T., Ito, M., Yagita, H., & Suzuki, Y. (1996). Acteoside, a component of Stachys Sieboldii Miq, may be a promising antinephritic agent (3) effect of acteoside on expression of intercellular adhesion molecule-1 in experimental nephritic glomeruli in rats and cultured endothelial cells. The Japanese Journal of Pharmacology, 70(2), 157–168. https://doi.org/10.1254/jjp.70.157
- Hosokawa, M., Otake, A., Sugawara, Y., Hayashi, T., & Yazawa, S. (2004). Rescue of shoot apical meristems of chrysanthemum by culturing on root tips. Plant Cell Report, 22(7), 443–448. https://doi.org/10.1007/s00299-003-0719-1
- Hyndman, S. E., Hasegawa, P. M., & Bressan, R. A. (1982). The role of sucrose and nitrogen in adventitious root formation on cultured rose shoots. Plant Cell, Tissue and Organ Culture, 1(1), 229–238. https://doi.org/10.1007/BF02318919
- Instituto Botanico Boreali-Occidentali Academiae Sinicae Edita. (1983). Flora Tsinlingensis (Vol. 1). Science Press.
- Kim, E. K., You, J. W., Park, J. S., Min, D. J., Park, S. H., & Hong, J. S. (2018). Detection and identification of a mixed infection of three viruses in Chinese artichoke in Korea. Research in Plant Disease, 24(1), 81–85. https://doi.org/10.5423/RPD.2018.24.1.81
- Lim, T. K. (2016). Stachys affinis. In T. K. Lim (Ed.), Edible medicinal and non-medicinal plants: Volume 11 modified stems, roots, bulbs (pp. 41–46). Springer International Publishing.
- Manthey, F. A., & Xu, Y. (2010). Chapter 2: Glycobiology of foods: Food carbohydrates-occurrence, production, food uses, and healthful properties. In F. Yildiz (Ed.), Advances in food biochemistry (p. 29). CRC Press, Taylor & Francis.
- Mercier, J., & Perennes, M. (1982). Le crosne. Muséum Nationald’histoire Naturelle.
- Mizushima, S. (2016). Effects of nutrient solution concentration on rooting and growth of cuttings in Elatostema involucratum Franch. & Sav. cultured by hydroponics. Horticultural Research, 15(4), 371–376. https://doi.org/10.2503/hrj.15.371
- Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Naka, T., Maeda, S., & Sumikawa, Y. (2007). Tuberous root production of dahlia (dahlia ×cultorum) by cottage from apical meristem culture plants. Bulletin of the Nara Agricultural Experiment Station, 38, 23–30.
- Nomura, S., Furutani, N., Ohtani, K., Muramoto, Y., & Matsui, M. (2013). Characterization of ‘kyo-shiro-tamba’ (glycine max (L.) merr.), new yellow soybean cultivar derived from black soybean. Trace Nutrients Research, 30, 79–85. https://doi.org/10.51029/jtnrs.30.0_79
- Ogiwara, I., Ohtsuka, Y., Yoneda, Y., Sakurai, K., Hakoda, N., & Shimura, I. (1999). Extraction method by water followed by microwave heating for analyzing sugars in strawberry fruits. Journal of the Japanese Society for Horticultural Science, 68(5), 949–953. https://doi.org/10.2503/jjshs.68.949
- Ohtani, K., Rhee, O., & Minamide, T. (2000). The contents of stachyose in “murasakizukin”, one of Kyoto traditional vegetables. Trace Nutrients Research, 17, 103–107.
- Rana, M. K., & Yadav, N. (2017). Chapter 70: Chinese artichoke. In M. K. Rana (Ed.), Vegetable crop science (p. 8). CRC press, Taylor & Francis.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
- Rhee, O., Minamide, T., & Ohtani, K. (2001). The contents of stachyose in tanba black soybeans (tanbaguro). Trace Nutrients Research, 18, 123–127.
- Sato, K., Horie, H., & Kitajima, N. (2009). Analysis of sugar composition in strawberry using capillary electrophoresis. Bulletin of the Fukuoka Agricultural Research Center, 28, 56–60.
- Takeda, Y., Fujita, T., Satoh, T., & Kakegawa, H. (1985). On the glycosidic constituents of Stachys sieboldii miq. And their effects on hyaluronidase activity. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan, 105(10), 955–959. https://doi.org/10.1248/yakushi1947.105.10_955
- Venditti, A., Frezza, C., Celona, D., Bianco, A., Serafini, M., Cianfaglione, K., Fiorini, D., Ferraro, S., Maggi, F., Lizzi, A. R., & Celenza, G. (2017). Polar constituents, protection against reactive oxygen species, and nutritional value of Chinese artichoke (Stachys affinis Bunge). Food Chemistry, 221, 473–481. https://doi.org/10.1016/j.foodchem.2016.10.096
- Yamahara, J., Kitani, T., Kobayashi, H., & Kawahara, Y. (1990). Studies on Stachys sieboldii miq II. anti-anoxia action and the active constituents. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan, 110(12), 932–935. https://doi.org/10.1248/yakushi1947.110.12_932
- Yamamoto, H., Fuji, S., & Asari, Y. (2004). Production of virus-free Chinese artichoke through shoot tip culture. Journal of the Japanese Society for Horticultural Science, 73(1), 82–84. https://doi.org/10.2503/jjshs.73.82
- Yamamoto, H., Sato, G., & Fuji, S. (2007). Reinfection by viruses and growth of virus-free Chinese artichoke. Annual Report of the Society of Plant Protection of North Japan, 58, 69–70.
- Yazawa, S., Kanno, E., & Takashima, S. (1979). Studies on the growth habit of chorogi (Stachys sieboldii miq.). The Bulletin of Experimental Farm Faculty of Agriculture, Kyoto Prefectural University, 9, 24–28.
- Yazawa, S., Kanno, E., & Takashima, S. (1983). Dormancy of tuber of chorogi (Stachys sieboldii miq.). The Bulletin of Experimental Farm Faculty of Agriculture, Kyoto Prefectural University, 10, 21–25.
- Yin, J., Yang, G., Wang, S., & Chen, Y. (2006). Purification and determination of stachyose in Chinese artichoke (Stachys sieboldii miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta, 70(1), 208–212. https://doi.org/10.1016/j.talanta.2006.03.027
- Zhang, R. X., Jia, Z. P., Kong, L. Y., Ma, H. P., Ren, J., Li, M. X., & Ge, X. (2004). Stachyose extract from Rehmannia glutinosa Libosch. to lower plasma glucose in normal and diabetic rats by oral administration. Pharmazie, 59(7), 552–556.
