Abstract
There are conflicting narratives over what drives demand for add-ons. We undertook an online survey of IVF patients to determine whether patients perceive that use of IVF add-ons is driven by patients or practitioners. People who underwent IVF in the UK in the previous five years were recruited via social media Survey questions focussed on the roles of clinician offer and patient request, including who first suggested use of add-ons in IVF consultations, where patients first heard about them, and which information sources they trusted. From a total of 261 responses, 224 met the inclusion criteria. Overall, 67% of respondents had used one or more IVF add-ons, most commonly: time-lapse imaging (27%), EmbryoGlue (27%), and endometrial scratching (26%). Overall, 81% of the add-ons used were offered to participants by clinicians (compared to 19% requested by themselves). Half (54%) reported being offered add-ons during consultations, compared to 24% who initiated discussion about add-ons. Higher proportions of private patients reported being offered (90%), requesting (47%) and using (74%) add-ons than those with NHS funding (74%, 29%, 52%, respectively). The main limitations of this study are the small sample size, recruitment via a convenience sample, and the self-reported data capture which is subject to recall bias.
Introduction
An estimated one in seven couples in the UK struggle to conceive and may be defined as subfertile (NHS, Citation2017). These couples may consider fertility treatments, such as in-vitro fertilisation (IVF). In 2019, 53,000 patients received over 69,000 cycles of IVF treatment in the UK (HFEA, Citation2021). Success rates vary substantially by age and gamete source; however, on average live birth rates are approximately 20-25% per cycle started (Chambers et al., Citation2021). The resulting uncertainty and sense of powerlessness are an integral feature of the treatment journey, which many describe as an ‘emotional rollercoaster’ (HFEA, Citation2018).
An increasing number of additional IVF treatments options (add-ons) have become available. Add-ons may be described as interventions, including drugs, devices, surgical procedures and diagnostic tests, which are not essential for treatment. Add-ons usually claim to increase the chance of success and are often provided at an extra cost to the patient (Harper et al., Citation2017; Lensen et al., Citation2021). However, for many IVF add-ons, there is no conclusive evidence that they increase live birth rates, and some have been demonstrated to be of no benefit, or even harmful to the overall probability of having a baby (Armstrong et al., Citation2019; Cornelisse et al., Citation2020; Harper et al., Citation2017; Kamath et al., Citation2019; Lensen et al., Citation2019). The Human Fertilisation and Embryology Authority’s ‘traffic light’ system for add-ons rates all of them as either amber (where the evidence is conflicting) or red (where there is no evidence of effectiveness from high-quality trials) (HFEA, Citation2022). Despite this, use of IVF add-ons is believed to be widespread. In the UK, add-ons are offered by most fertility centres and were reported to be used by 74% of people undergoing IVF (HFEA, Citation2018).
There are conflicting narratives over what drives demand for add-ons; some suggest patients predominantly request add-ons, possibly after learning about these online or from peers (Ben Rafael, Citation2020; Perrotta & Hamper, Citation2021; Wilkinson et al., Citation2019). Clinicians may oblige patients who request add-ons, either in order to reduce psychological distress in patients desperate to use the add-on (Sarwari et al., Citation2021), or for fear they may otherwise move to other providers. Alternatively, patients may feel compelled to use add-ons despite a weak evidence base, fearing that missing out on these extras may not give them the best chance of success. Even when aware of the limited evidence base, patients may reasonably conclude that if a consultant offers them an add-on, it is listed on the clinic’s website or price list, or a clinician agrees to using an add-on following a patient request ‘it must have some chance of working’ (Brody, Citation1997; Wilkinson et al., Citation2019). Despite ample discussion and speculation about the drivers for add-on use (Iacoponi et al., Citation2022; Lensen et al., Citation2019; Wilkinson et al., Citation2019), there is currently little real life data available. Understanding why these unproven procedures are used in practice can inform efforts to improve evidence-based care and decision-making. The aim of this survey is to understand the drivers behind the availability and use of add-ons from the perspective of the UK IVF patient.
Materials and methods
Eligibility and recruitment
An online survey was conducted in IVF patients. Eligible participants were men or women who have had IVF (including ICSI) treatment in the last five years and who live or sought fertility treatment in the UK. We primarily recruited by posting on Twitter and by providing organisations and influencers with drafts for blogs and social media posts (Figure S1). Potential participants were directed to a landing page to read more information about the study before beginning the survey. Participants were not offered any reward for completing the survey. The survey was hosted in Qualtrics and was open from 3 June to 31 July 2019. To reduce bias in this self-selecting sample, recruitment materials only mentioned IVF, and not add-ons themselves.
Survey design
The survey captured data on participant demographics (e.g. gender, age, stage of treatment, number of IVF cycles, funding type) (Appendix Questionnaire, Supplementary file). Participants were then provided with a description of add-ons, before reporting on whether they were offered or requested 12 individual add-ons by use of a matrix table. These 12 add-ons were those listed as ‘treatment add-ons’ on the HFEA website at the time of this study (). Information about specific add-ons used was captured by a free-text field, and provided an opportunity for participants to name other add-ons they had used (in addition to the 12 listed). Additionally, the survey captured where participants first remember hearing about add-ons and how, if at all, these were discussed in the consultation, and which information sources were considered authoritative. The survey did not distinguish between single and (possible) multiple instances (e.g. of add-on use or offers), so the figures presented refer to ‘ever-use’. This survey was undertaken prior to a randomised survey which investigated how IVF patients perceive and interpret information relating to IVF add-ons when delivered under different frameworks, which will be reported elsewhere. Utilisation of a core outcome set was not applicable.
This study embraced patient involvement throughout, two patients were involved in developing the initial idea, designing, and pilot-testing the questionnaire, and analysing the findings and drafting the manuscript.
Data analysis
Survey data was analysed using descriptive statistics in R and Excel (RStudio Team, Citation2020). We performed a subgroup analysis by funding type to provide insight into the difference in experience by NHS and private patients.
Ethical approval was obtained from the London School for Hygiene and Tropical Medicine Research Ethics Committee. (Reference: 16541, date of approval: 16 May 2019). Participants indicated their consent at the start of the survey by selecting ‘I consent, begin the study’.
Reflexivity statement
The research team has varying personal and professional experience with fertility services, and IVF add-ons specifically. We recognise the importance of existing opinions and perceptions in research and interpretation of findings. Although we included research team members with minimal previous IVF research (JCF, SC), we acknowledge the influence of our existing values and beliefs on the results reported here.
Results
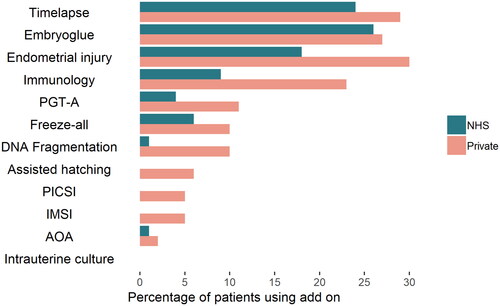
A total of 261 people consented to participate and 233 completed the survey beyond basic demographic details. Nine respondents either reported that they were ‘considering treatment’, or that they had not yet initiated IVF treatment or attended for a consultation, leaving a total of 224 eligible respondents. Participants appeared to be generally representative of people having IVF in the UK () (HFEA, Citation2021). Two-thirds of respondents (150, 67%) reported using one or more add-on; three-quarters of private patients (74%) and half of those who were publicly funded (52%) (). The most frequently used add-ons were time-lapse imaging (27%), EmbryoGlue (27%), and endometrial scratching (26%) (Table S1, Supplementary file). Use of add-ons was more prevalent for private patients: assisted hatching, PICSI, and IMSI use was reported exclusively by those undergoing private treatment (). A number of participants described (in free-text fields) that time-lapse imaging and EmbryoGlue were often included as part of their standard IVF treatment package.
Table 1. Baseline characteristics.
Table 2. Pattern of offering and requesting IVF add-ons.
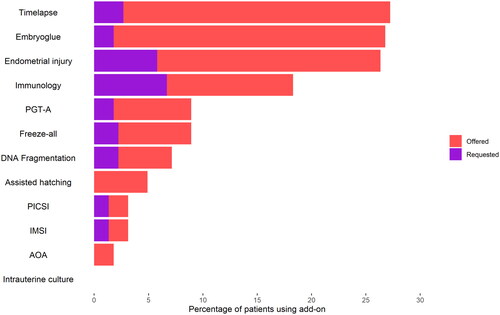
Overall, 19% of add-ons used were reported as being directly requested by the patient and 81% as being suggested to them by their clinic (Table S1). However, there was variation in the offer/request ratios for each add-on used. While time-lapse and EmbryoGlue were offered by treating clinicians 90% of the time, almost 40% of immunology treatments resulted due to patients asking for or expressing interest in them (Table S1, ). In consultations, participants reported that the clinician suggested add-ons as an option more often than patients bringing them up themselves (54% vs 24%) (Figure S2 Supplementary file). Clinician offers were significantly more common than patient requests for all add-ons (Figure S3, S4 Supplementary file). Private patients appear to both be offered and to request add-ons more than NHS patients, with time-lapse, EmbryoGlue and endometrial scratching being offered to almost half of all private patients (Figure S3, S4).
Patients reported that they were most often offered one or more add-ons without requesting any themselves (47%) (). While 44% of respondents who self-funded their treatment declared they were both offered and requested add-ons, this share is significantly lower for people with NHS funding (25%). Overall, 84% of patients were offered at least one add-on by their clinic while less than half (42%) requested any (47% of private patients vs. 29% of NHS patients). Use rates and the number of add-ons offered was also higher among patients who have undergone more IVF cycles (Table S2 Supplementary file). In the first three cycles, patients requested less than one add-on on average, but they were frequently offered a variety of optional extras. Among patients who have had at least five IVF cycles, over 90% had used at least one add-on across repeated cycles.
Overall, 45% of respondents first heard about add-ons from clinic sources, such as at their fertility clinic (33%) or an IVF clinic website (12%). Fewer patients (34%) had learned about add-ons from other sources such as online forums (23%), social media (6%) and news sites (5%) (Table S3 Supplementary file). Only 3% had not heard about add-ons before taking the survey. Of those privately funding their treatment, 37% first learned about optional extras at their fertility clinic, compared to 26% for NHS patients. Asked about which three sources they found most trustworthy, 66% participants said this was their fertility doctor, followed by the Human Fertilisation and Embryology Authority (HFEA) (64%) and scientific articles (48%) (Table S4 Supplementary file). A quarter of participants said they trusted online forums such as Mumsnet.
Discussion
This survey found that the majority of IVF patients have used treatment add-ons (67%), most commonly: time-lapse imaging (27%), EmbryoGlue (27%), and endometrial scratching (26%). Patients reported that IVF add-ons were offered to them by their fertility clinician or clinic much more commonly than requested by themselves. Over 80% of add-on use arose from a clinician’s offer rather than a patient’s request. Private and NHS patients appeared equally likely to bring up add-ons in IVF consultations and to request specific add-ons. However, private patients received more offers than NHS patients (90 vs 74%), and more private than NHS patients used add-ons (74 vs 52%).
This data suggests that IVF clinics and clinicians play a substantial role in the use of IVF add-ons. Almost half of the patients reported first hearing about add-ons from clinic-related sources (websites or in person at the clinic). Similarly, a survey of Australian IVF patients reported that add-on use was largely driven by clinicians, with 71% of add-ons used being suggested by the clinician rather than by the patient (Lensen et al., Citation2021). By comparison, online communities of fertility patients reported to play a smaller role in our study. In consultations specifically, discussions about add-ons were reported to be largely initiated by the IVF doctor, rather than the patient. Proactive patient requests for add-ons, on the other hand, accounted for less than a fifth of their overall use. Previous research exploring the clinician perspective has focussed on the notion of the ‘patient shopper’: that patients may see themselves as clients rather than patients, and often arrive at their consultation with a ‘shopping list’ of add-ons they would like to use (Armstrong in press; Iacoponi et al., Citation2022). However, the results of this survey suggest that patient request accounts for only a fifth of IVF add-on use.
Due to its design, this survey was not able to explore the motivation or reason behind clinician offers. It has been speculated that these reasons may be diverse, including the ability to offer patients options (and with this, some hope), especially in those patients with previous IVF failure (Perrotta & Geampana, Citation2020; Perrotta & Hamper, Citation2021; Sarwari et al., Citation2021). Clinicians may believe that certain add-ons benefit their patients, either by observing improvements in their personal practice or clinic data, or by drawing these conclusions from the available studies, many of which are poor quality.
Complex factors and considerations may underpin the demand for add-ons by patients, one of which may be unwarranted optimism regarding their benefit:risk ratio. IVF websites have been reported to make unsubstantiated claims about the benefit of IVF add-ons and other treatment options (Heneghan et al., Citation2016; Spencer et al., Citation2016), and present information that is often inaccurate and misleading (CMA, Citation2021; Lensen et al., Citation2021). In response to concerns about clinic advertising of add-ons, the UK’s Competition and Markets Authority has developed guidance on consumer law for UK IVF clinics (CMA, Citation2021). The HFEA, which regulates and licenses fertility clinics in the UK, developed a traffic-light system appraising add-ons for patients, which it has required clinics to signpost since 2019 (HFEA, Citation2018).
This survey has several limitations. Firstly, our recruitment method did not permit control over survey completion, which could lead to a biased sample, as more people with strong opinions about add-ons may have self-selected to participate. To minimise this, none of the advertising material mentioned add-ons. However, our findings about add-on use are consistent with the HFEA’s representative national patient survey (n = 1,017), which supports the generalisability of our sample (HFEA, Citation2018). We identified the same top three clinical add-ons with percentages for other add-ons all within a 2 percentage point range of our figures, except for reproductive immunology. For this, the HFEA found 3% of patients with NHS and 12% with private funding using it, compared to 8% and 22% respectively, reported here. While the HFEA questionnaire simply asks about ‘reproductive immunology treatments and tests’, we included examples for this umbrella term, which may have increased recall and therefore resulted in the higher figures reported. Secondly, as all the data is patient-reported, and does not include clinician or objective perspectives, it may not fully reflect actual clinical practice. Further, the survey did not capture information about the patient-clinician discussion that ensued following the offer of any add-on, which is key information to understanding the information patients are provided with and their decision-making process. The survey did not capture data per individual instance of add-on use or per IVF cycle. For instance, if a patient used IMSI on multiple occasions, we cannot distinguish this from a patient who used it once. Patients were requested to select whether their IVF treatment was NHS or privately funded, and we do not know how many may have undergone both NHS and privately funded IVF. As with any survey, there is opportunity for recall bias and misremembering, especially for patients who had treatment more than two years ago (8% of the sample). Thirdly, there is an ambiguity around which treatments are considered add-ons. Some argue that, for example, time-lapse imaging is not an add-on if it is offered free of charge, is part of a standard package, or is the only available incubator at the clinic. In this way, IVF add-ons such as time-lapse imaging and EmbryoGlue may have been used by IVF patients without an explicit offer per se, particularly at NHS clinics where IVF treatment packages are not usually permitted to be altered or to include extras. The finding that IVF add-ons are offered more to private patients may therefore be exaggerated.
To ensure participants had a common understanding, we provided a description of add-ons within the survey (HFEA, Citation2018). An indication that this was successful at creating shared understanding is that none of the respondents gave details on so-called ‘alternative’ add-ons like acupuncture. These are currently not featured on the HFEA add-ons page, although they are advertised on many IVF clinic websites and the national patient survey reported widespread use (HFEA, Citation2018; Lensen et al., Citation2021; Stein & Harper, Citation2021). We conducted subgroup analyses by whether participants underwent NHS funded or private IVF treatment. However, we did not capture whether patients had been treated at privately-owned or NHS-run clinics, which may accept both private and NHS funded patients. The survey was undertaken in 2019 and the results may not represent the current picture of IVF add-on offers, requests, or use.
The results of survey capture the patient perspective only, and little data is available from the clinician perspective. Further qualitative research with clinicians and patients is required to explore the attitudes, beliefs and psychosocial factors that may underpin drivers of both supply and demand. Understanding both these factors, as well as supply-side health economics, may be key to understanding IVF add-on use. This can then be used to deliver information aimed at reducing decisional uncertainty and regret and improving evidence-based treatment provision in IVF care. Such initiatives should undergo robust user and pilot-testing to ensure usability, correct interpretation, and user satisfaction, and to avoid unintended consequences. The HFEA traffic light system was developed to provide IVF patients with an independent source of evidence-based information for the benefits and risks of IVF add-ons. However, it remains unclear to what extent this website is accessed by patients and used to inform their decision-making, and how they might use and interpret the traffic light labels.
Conclusion
Two-thirds of UK IVF patients used add-ons during their IVF treatment, and use was more common among private than NHS-funded patients. Patients reported that clinician offers accounted for more than 80% of add-on use, and discussions about IVF add-ons were more commonly initiated by the clinician during consultations.
Author contributions
SC conceived the idea for the study, designed and carried out the survey, and prepared the first draft of the paper. JCF contributed to study objectives and survey design. JW performed the data analysis. SL contributed to the data analysis and preparation of the manuscript. All authors contributed to interpretation of the data, manuscript preparation, and approved the final version.
Supplemental Material
Download MS Word (680.7 KB)Acknowledgements
The authors thank the patients who provided feedback on the study design and all patients who completed the survey.
Disclosure statement
EJ and JCF declare no conflicts of interest. SC was employed via an agency contract by the HFEA whilst designing and conducting the study. JH was a member of the HFEA Scientific and Clinical Advances Advisory Committee (SCAAC) who developed the HFEA traffic lights system. SL, JW, JH, and KL declare research and personal (KL) interests in IVF add-ons, and have published extensively on IVF add-ons and their evidence base.
Data availability statement
The data is available upon reasonable request to the corresponding author.
Additional information
Funding
References
- Armstrong, S., Atkinson, M., MacKenzie, J., Pacey, A., & Farquhar, C. (2019). Add-ons in the laboratory: hopeful, but not always helpful. Fertility and Sterility, 112(6), 994–999. https://doi.org/10.1016/j.fertnstert.2019.10.031
- Ben Rafael, Z. (2020). Repeated implantation failure (RIF): an iatrogenic meaningless definition that generates unnecessary and costly use of add-on procedures. Human Reproduction (Oxford, England), 35(7), 1479–1483. https://doi.org/10.1093/humrep/deaa134
- Brody, H. (1997). Medical futility: a useful concept?. In M. B. Zucker, & H. D. Zucker (Eds.), Medical Futility: And the Evaluation of Life-Sustaining Interventions. (pp. 1–14). Cambridge University Press. https://doi.org/10.1017/CBO9780511530227
- Chambers, G. M., Dyer, S., Zegers-Hochschild, F., de Mouzon, J., Ishihara, O., Banker, M., Mansour, R., Kupka, M. S., & Adamson, G. D. (2021). International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology, 2014†. Human Reproduction (Oxford, England), 36(11), 2921–2934. https://doi.org/10.1093/humrep/deab198
- CMA. (2021). Guidance for fertility clinics on consumer law: Helping fertility clinics comply with their consumer law obligations. Competition & Markets Authority.
- Cornelisse, S., Zagers, M., Kostova, E., Fleischer, K., Wely, M., & Mastenbroek, S. (2020). Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. The Cochrane Database of Systematic Reviews, 9(9), CD005291. https://doi.org/10.1002/14651858.CD005291.pub3
- Harper, J., Jackson, E., Sermon, K., Aitken, R. J., Harbottle, S., Mocanu, E., Hardarson, T., Mathur, R., Viville, S., Vail, A., & Lundin, K. (2017). Adjuncts in the IVF laboratory: where is the evidence for 'add-on’ interventions? Human Reproduction (Oxford, England), 32(3), 485–491. https://doi.org/10.1093/humrep/dex004
- Heneghan, C., Spencer, E. A., Bobrovitz, N., Collins, D. R., Nunan, D., Plüddemann, A., Gbinigie, O. A., Onakpoya, I., O'Sullivan, J., Rollinson, A., Tompson, A., Goldacre, B., & Mahtani, K. R. (2016). Lack of evidence for interventions offered in UK fertility centres. BMJ (Clinical Research ed.), 355, i6295. https://doi.org/10.1136/bmj.i6295
- HFEA. (2018). Human Fertilisation and Embryology Authority. Treatment add-ons. https://www.hfea.gov.uk/treatments/explore-all-treatments/treatment-add-ons/
- HFEA. (2021). Fertility treatment 2019: trends and figures. https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures/).HFEA
- HFEA. (2022). Treatment add-ons with limited evidence. https://www.hfea.gov.uk/treatments/treatment-add-ons/
- Human Fertilisation and Embryology Authority. (2018). Pilot national fertility patient survey. https://www.hfea.gov.uk/media/2702/pilot-national-fertility-patient-survey-2018.pdf
- Iacoponi, O., van de Wiel, L., Wilkinson, J., & Harper, J. C. (2022). Passion, pressure and pragmatism: how fertility clinic medical directors view IVF add-ons. Reproductive Biomedicine Online, 45(1), 169–179. https://doi.org/10.1016/j.rbmo.2022.02.021
- Kamath, M. S., Mascarenhas, M., Franik, S., Liu, E., & Sunkara, S. K. (2019). Clinical adjuncts in in vitro fertilization: a growing list. Fertility and Sterility, 112(6), 978–986. https://doi.org/10.1016/j.fertnstert.2019.09.019
- Lensen, S., Chen, S., Goodman, L., Rombauts, L., Farquhar, C., & Hammarberg, K. (2021). IVF add-ons in Australia and New Zealand: A systematic assessment of IVF clinic websites. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 61(3), 430–438. https://doi.org/10.1111/ajo.13321
- Lensen, S., Hammarberg, K., Polyakov, A., Wilkinson, J., Whyte, S., Peate, M., & Hickey, M. (2021). How common is add-on use and how do patients decide whether to use them? A national survey of IVF patients. Human Reproduction (Oxford, England), 36(7), 1854–1861. https://doi.org/10.1093/humrep/deab098
- Lensen, S., Shreeve, N., Barnhart, K. T., Gibreel, A., Ng, E. H. Y., & Moffett, A. (2019). In vitro fertilization add-ons for the endometrium: it doesn’t add-up. Fertility and Sterility, 112(6), 987–993. https://doi.org/10.1016/j.fertnstert.2019.10.011
- NHS. (2017). Infertility. https://www.nhs.uk/conditions/infertility/
- Perrotta, M., & Geampana, A. (2020). The trouble with IVF and randomised control trials: Professional legitimation narratives on time-lapse imaging and evidence-informed care. Social Science & Medicine (1982), 258, 113115. https://doi.org/10.1016/j.socscimed.2020.113115
- Perrotta, M., & Hamper, J. (2021). The crafting of hope: Contextualising add-ons in the treatment trajectories of IVF patients. Social Science & Medicine (1982), 287, 114317. https://doi.org/10.1016/j.socscimed.2021.114317
- RStudio Team. (2020). RStudio: Integrated Development for R [computer software].
- Sarwari, M., Beilby, K., Hammarberg, K., Hickey, M., & Lensen, S. (2021). Endometrial scratching in Australia, New Zealand and the United Kingdom (UK): a follow-up survey. Human Fertility, 14, 1–6. https://doi.org/10.1080/14647273.2021.1995902
- Spencer, E. A., Mahtani, K. R., Goldacre, B., & Heneghan, C. (2016). Claims for fertility interventions: a systematic assessment of statements on UK fertility centre websites. BMJ Open, 6(11), e013940. https://doi.org/10.1136/bmjopen-2016-013940
- Stein, J., & Harper, J. C. (2021). Analysis of fertility clinic marketing of complementary therapy add-ons. Reproductive Biomedicine & Society Online, 13, 24–36. https://doi.org/10.1016/j.rbms.2021.04.001
- Wilkinson, J., Malpas, P., Hammarberg, K., Mahoney Tsigdinos, P., Lensen, S., Jackson, E., Harper, J., & Mol, B. W. (2019). Do à la carte menus serve infertility patients? The ethics and regulation of in vitro fertility add-ons. Fertility and Sterility, 112(6), 973–977. https://doi.org/10.1016/j.fertnstert.2019.09.028