ABSTRACT
Introduction: The number of patients with end-stage kidney disease is increasing worldwide, creating an unprecedented organ shortage. The kidney is a highly complex structure performing many crucial functions. Dialysis replaces filtration but not all other kidney functions and transplant is limited by kidney availability. Numerous innovative ways are being explored to obtain new kidneys for disease modeling and potentially replace lost kidney functions.
Areas covered: In this review, we will go through the different approaches that have been developed over the years to build kidneys. We will first present the current advances in xenotransplantation and generation of interspecies chimeras. Next, we will examine the attempts to create bioengineered kidneys with hemodialysis-derived implantable devices and decellularized organs. Finally, we will examine how organoids and microfluidic devices could answer important pathophysiological questions and model the path toward creating in vitro functional organs, for example through 3D bioprinting.
Expert opinion: While all the aforementioned approaches to create new kidneys are promising, their translation into clinical practice seems a long way off, except xenotransplantation. Nonetheless, these novel technologies already consent disease modeling and drug testing at 3D level. We will review the stages of progress toward patient therapy and advantages/drawbacks of the various strategies.
1. Introduction
End-stage kidney disease (ESKD) represents a major healthcare burden worldwide requiring costly renal replacement therapy in the form of dialysis or transplantation [Citation1]. However, kidney transplantation as a treatment option is limited by the shortage of healthy donors [Citation2]. In an attempt to increase the number of kidneys available for transplantation, the selection criteria have been expanded to include marginal kidneys, which are organs from suboptimal donors [Citation3]. In 2010, an estimated 5 to 10 million ESKD patients needed renal replacement therapy worldwide. However, only about two and a half million people received a kidney transplant, suggesting that at least 2 million people might have died prematurely because of the lack of donors [Citation4]. To account for organ shortage, in the last 20 years much effort has been put toward manufacturing bioengineered kidneys that would be able to replace entirely or to complement the organ and improve the renal function, with the final objective to free patients from the burden of current renal replacement therapies, dialysis or kidney transplant. Indeed, restoring as little as 10% of the renal function would allow the patients in ESKD to avoid dialysis, increasing significantly the quality of life [Citation5].
The kidney offers a major challenge to organ (re)generation scientists due to structural and functional reasons. The kidney has a composite embryonic origin – from the metanephros, after degeneration of the pronephros and mesonephros, and from different progenitor lineages (nephron, ureteric, stromal and endothelial) – and an extremely complex organ anatomy, that is a unique epithelial, endothelial and interstitial architecture integrated with a continent excretion pathway. In respect to its function, the kidney has very elaborate and energy-consuming, massive and together finely tuned, functional processes. Besides its filtration (glomerular) function, the kidney also regulates homeostasis, hormones production, reabsorbs fluid and noble solutes into the blood stream and secretes toxic and unnecessary ions and molecules. Accordingly, the evolution in the field of kidney bioengineering has yet to match advances in other simpler organs [Citation6–Citation10]. Despite this, from cell-based microsystems to macroscopic devices, the bioengineered kidney scenario is increasingly expanding and sustains high interest and expectations in the field of regenerative medicine.
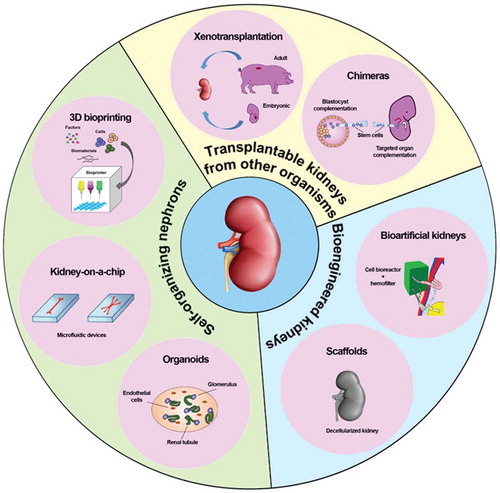
In this review, we will focus on existing bioengineering approaches to kidney (re)generation from an historical perspective, starting with the first xenogeneic experimental efforts (xenotransplantation and chimeras), through the different attempts to build a new whole organ (bioartificial portable and implantable kidneys and recellularized scaffolds), ending with the latest advances in creating in vitro functional nephrons (kidney organoids and kidney-on-a-chip) and organs (3D bioprinting) (, ).
Table 1. Summary of advantages, disadvantages, and clinical relevance of each bioengineering approaches to kidney (re)generation.
2. Transplantable kidney from another organism
2.1. Xenotransplantation of adult organs or embryonic tissues
Cross-species transplantation, or xenotransplantation, offers an attractive possibility to overcome the shortage of kidneys from deceased and living donors. Patients unable to obtain an allograft may benefit significantly from xenotransplantation, on various levels: avoidance of ethical issues such as coercion or payment of living donors, reduction of stress from long wait for a suitable donor, lower complication and costs related to long-term dialysis. Additionally, xenotransplantation may provide a chance to receive kidneys to patients who are highly HLA sensitized [Citation11].
The first attempt by Reemtsma et al. to graft kidneys from a non-human primate (NHP) goes back to 1963, when 13 patients with ESKD were transplanted chimpanzee kidneys. All hosts died from rejection or infection, between 11 days and 2 months post-surgery, with a patient who exceptionally survived for 9 months [Citation12]. However, it rapidly appeared that NHPs are not the best source of organs for transplant, mostly due to limited availability, poor breeding potential and slow growth, risk of transfer of infection, high maintenance costs and mixed public opinion [Citation13]. On the other hand, pigs seem better suited to satisfy all the requirements, and their renal function is similar to humans. With the advances in genetic engineering and cloning technologies, they quickly became a preferred source of organs for transplant. These new techniques are allowing the researchers to lift the pathobiological barriers of pig kidney transplantation, and while wild type pig kidneys fail within minutes in NHPs, humanized pig kidneys that express a single human complement-regulatory protein have functioned for 3 months [Citation14].
An important breakthrough in the field of xenotransplantation is the production of the α − 1,3-galactosyltransferase knockout (GalT-KO) pig in 2002 [Citation15,Citation16]. In 2005, the Boston group showed their initial results demonstrating an 83-day survival of a baboon bearing a life-supporting GalTKO pig kidney graft without rejection, when a vascularized donor pig thymus was co-transplantated from the same GalT-KO pig [Citation17]. They have recently demonstrated further survival of baboons of up to 193 days without rejection in 2018 [Citation18], which is comparable to the results of recent trials using multi-transgenic pigs as donors. Genetic modifications performed in the years 2010s, i.e. deletion of galactose-α 1,3-galactose, a pig antigen expressed on the graft, together with complement-regulatory proteins and/or coagulation-regulatory proteins, have extended the life of the graft up to more than a year [Citation19–Citation21]. Therefore, although recent advances in gene-editing technology have allowed multiple transgenic pigs to act as organ donors for xenotransplantation, determining which genes to add to the donors as well as the clinically applicable immunosuppressive regimen would be the next step toward clinical applicability.
In particular, these regimens are heavier than the ones used for allotransplants, increasing the risk of recipient death, or involve drugs not yet tested on humans [Citation22]. This aspect is being improved thanks to the latest advances in genome editing, in particular, the CRISPR/Cas9 approach, which, applied to xenotransplantation, have considerably sped progress toward clinical relevance, as reviewed elsewhere [Citation23]. Briefly, a whole panel of genes vital to improving xenograft survival rate have been modified using this technique, including cytidine monophosphate-N-acetylneuraminic acid hydroxylase, B1,4N-acetylgalactosaminyltransferase, isoglobotrihexosylceramide synthase, class I MHC, von Willebrand factor and C3. The risk of transmission of a porcine infectious disease to the recipient, and subsequent health and legal issues, is another problem faced by xenotransplantation. Particular concerns involve porcine endogenous retroviruses (PERVs), which are integrated into the pig genome and are present within all transplanted tissues, but have shown no human transmission so far [Citation22,Citation24]. However, PERVs can be inactivated using CRISPR-Cas9 [Citation25] or by currently available anti-retroviral drugs [Citation26], bringing clinical trials a step closer.
Importantly, ethical issues linked with xenotransplantation remain to be addressed. This topic has been comprehensively reviewed by Mann et al. [Citation27], and include cultural and religious concerns, as well as the need to respect both animal rights and human dignity. And finally, regulation of clinical xenotransplantation must be undertaken [Citation28].
Xenotransplantation of embryonic kidney offers a series of advantages over adult kidney. The kidney primordium is genetically preprogrammed to develop a functional kidney. It requires multiple organogenesis steps to be functional in embryogenesis through adulthood. Indeed, metanephroi from pig embryos transplanted into the omentum of unilaterally nephrectomized adult pigs or mice that received costimulatoring blocking agents (anti-CD45RB, anti-CD154, and anti-CD11a) developed into an enlarged, vascularized structure formed of mature tubules and glomeruli [Citation29]. Another advantage is the fact that the primordia attracts the host vasculature and is therefore less susceptible to humoral rejection [Citation30]. Human metanephroi transplanted into immunodeficient mice exhibited rapid growth and development. Embryonic kidney was shown to be less immunogenic than adult organ when transplanted in fully immunocompetent hosts [Citation31]. Further analysis indicated no risk of malignant transformation. Altogether, embryonic tissues represent a valid alternative to adult tissue as a source of organ for xenotransplantation.
2.2. Chimeras
Chimeras are defined as organisms composed of a mixture of cell populations originated from different organisms. They necessitate the combination of donor cells, which can be of embryonic, fetal or adult origin, and a host, which provides the physiological environment and life support to the donor cells. Donor and host can be or not of the same species [Citation32]. While used for years as experimental models to study the pathophysiology of different organs, recent technological breakthroughs opened the door to potential applications for organ generation. Two different approaches have been developed, blastocyst complementation and targeted organ complementation [Citation32].
2.2.1. Blastocyst complementation
The blastocyst complementation is a technique originally developed by Chen et al. in 1993 [Citation33] to assay gene function in lymphocyte development. It consists in injecting embryonic stem cells (ESCs) into blastocysts, the initial embryonic stage following fertilization, and to transfer the embryo into the uteri of a foster mother. It has been applied to a wide range of tissues over the years, including thymic epithelia [Citation34], heart [Citation35], germ cells [Citation36], hepatocytes [Citation37] pancreas [Citation38] and lungs [Citation39]. Usui and colleagues used this system to compensate for the developmental defect of the Sall1−/- mice, in which kidneys do not form [Citation40]. Sall1 is expressed during embryonic development in epithelial cellular lineages originating from the metanephric mesenchyme and renal stroma, and mice deficient for Sall1 die right after birth from kidney agenesis. The authors injected wild type murine ESCs or induced pluripotent stem cells (iPSCs) into blastocysts form Sall1−/- mice and observed the bilateral formation of kidneys entirely formed by the injected pluripotent stem cell (PSC)-derived cells, with the exception of structures that do not depend on Sall1 expression to develop, such as collecting ducts from ureteric buds and microvascular endothelial cells. This proof-of-principle study shows that this technique could be used to generate donor PSCs-derived kidneys, but that in order to generate an entire organ from PSCs-derived cells all renal lineages must be absent from the blastocyst.
Several seminal studies have shown that it was possible to recreate interspecies chimeras, where PSCs from mice were implanted in blastocysts from rat, or vice versa, to regenerate pancreas [Citation38,Citation41], thymus [Citation42] or even kidney [Citation43]. However, attempts to generate organs from human PSC-derived chimeras using a mouse as a host were unsuccessful, as the authors failed to see efficient incorporation of naive human cells into mouse embryos [Citation44–Citation46]. Similarly, human PSCs robustly engrafted in large animal species – pig and cattle – pre-implantation blastocysts, but show limited contribution to post-implantation pig embryos [Citation47]. While this approach would allow the generation of human organs in animals whose organ size, anatomy, and physiology are closer to humans, the technique is still in its early stages and host endothelial cells in the blastocyst-complemented organ continues to be a problem.
2.2.2. Targeted organ complementation
In order to avoid any possible risk of human contribution to gametes or neural tissue, and subsequent ethical concerns, various research groups proposed to generate specifically an organ, either by allowing PSCs to differentiate only into the organ of interest [Citation48], or by using committed progenitors or organ buds instead of PSCs [Citation49]. Rat nephron progenitor cells (NPCs) injected into the fetal kidney of a NPC-deficient mouse led to the formation of nephrons [Citation50], suggesting that this NPC replacement strategy could potentially be applied to the development of human-animal chimeric kidney. In their 2019 article, Yamanaka et al. transplanted allogenic mouse renal progenitor cells, a heterogeneous population containing Six2-positive NPCs, into the nephrogenic zone of mouse embryos [Citation51]. They observed the formation of transplant-derived, vascularized and functioning glomeruli. In a second set of experiments, rat renal progenitor cells implanted into kidney-deficient embryos led to the formation of new nephrons connected to the host uretic bud. Although exogenous donor cells substantially formed part of the kidney, including glomeruli and vasculatures, there was a substantial amount of host-derived cells in the chimeric kidney. Thus, this organ generation system still requires immunosuppressive drugs for autologous kidney transplantation, at least briefly. However, these results show that a kidney can regenerate from exogenous renal progenitors and promise new avenues for renal organ regenerative medicine.
3. Building bioengineered kidneys
Organ bioengineering emerged from the need to explore new paths in order to obtain alternative sources of transplantable organs. Scientists faced this challenge by assembling cells, biologically relevant molecules, and scaffolds into functional organs.
3.1. Bioartificial kidney
The idea of developing alternative solutions to renal replacement therapy while ameliorating traditional dialysis performance via the implementation of bioengineering technology was conceived in the late nineties [Citation52]. Humes et al. developed a multi-fiber bioreactor in which synthetic hollow fibers of a high-flux hemofiltration cartridge were seeded with porcine primary tubular epithelial cells, thus forming a hybrid system. This new bioartificial Renal Assist Device (RAD) was capable of mimicking native kidneys' tubular functions by providing active transport (differential reabsorption and secretion), metabolic functions and endocrine functions for the first time [Citation53]. This new technology, using both porcine and human primary tubular epithelial cells (renal tubule progenitors harvested from kidney transplant discards and expanded), when applied in series to a hemofilter, was able to improve acute hemodialysis performance in uremic dogs [Citation54], paving the way to promising clinical trials in the intensive care unit clinical setting [Citation55,Citation56]. The RAD is, up to now, the only bioartificial kidney device successfully tested in humans. Despite this success, cell sourcing, device manufacturing times and costs, delicate storage requirements and distribution issues proved to be important limitations to an extensive use of this device for acute and chronic renal patients. To try to overcome these problems, the same research group developed a Bioartificial Renal Epithelial Cell System (BRECS) [Citation57,Citation58]. The BRECS technology, based on niobium-coated carbon and cryopreservable polycarbonate seeded with human renal tubular epithelial cells derived from adult progenitor cells, recently demonstrated efficacy when applied in series to a hemofilter in a porcine septic shock model [Citation59] and as a wearable device connected to a peritoneal dialysis circuit in an anephric sheep model [Citation60]. Although promising, the BRECS technology is yet to be applied in clinical trials. In the meantime, in an effort to create a fully functional bioartificial kidney, Jansen and colleagues developed bioengineered kidney tubules capable of uremic toxins removal via active transport processes. This result was achieved by culturing human conditionally immortalized proximal tubular epithelial cells (PTECs), enriched with specific transporters, on traditional hemofilters [Citation61].
The pioneering work by Humes et al. has opened a new perspective not only for improving renal replacement treatment, but also for moving dialysis patients outside the clinic. Indeed, the growing evidence for the efficacy and safety of longer and more frequent dialysis treatment has led a multitude of studies and new prototypes, the so-called Portable and Wearable Artificial Kidneys (PAK, WAK). The development and challenges of such highly engineered, non-cell based, technologies go beyond the scope of this review and have been recently reviewed elsewhere [Citation62–Citation64]. Combining the first experiences with RAD bioengineering and new advances in miniaturization technology, a project for an Implantable RAD, or implantable bioartificial kidney, was described by Fissel and Roy [Citation65,Citation66]. The two main challenges that hinder engineering of an implanted system are i) to reduce the large size and replicate the high permeability coefficient of conventional hemofilters, and ii) to overcome the need for a great amount of dialyzate. The first challenge was tackled by the use of microelectromechanical system technology and the creation of silicon nanopore membranes capable of designing highly uniform pores [Citation67]. This technology guaranteed higher permeability and selectivity, mimicking the glomerulus basement membrane structure, and allowed to both reduce the filter size and the required pressure ahead of the filter taking advantage only of the arterial-venous pressure differential, with no need of a mechanical pump [Citation66,Citation67]. This silicon nanotechnology has been successfully tested in large animals [Citation68,Citation69]. The second challenge could be tackled by placing a system of selective reabsorption in series the filtering unit permitted, by mimicking the nephron anatomy, to overcome the need for a large volume of dialysate. This could be possible by taking advantage of the bioartificial kidney technology developed by Humes et al. in the RAD and the BRECS, by seeding and differentiating human epithelial cells over silicon and thin-film material substrates and microelectromechanical system materials [Citation70]. Despite limitations and challenges, mostly represented by high costs of production and storage, the ability to reabsorb a great volume of water and solutes from the filtrate, and the durability of the implanted device, this bioengineered artificial kidney could represent a feasible alternative to renal replacement therapy and transplantation.
3.2. Kidney-derived scaffolds
The generation of kidney-derived scaffolds may represent a valid tool to create bioartificial kidneys. These biological scaffolds are obtained upon removal of cellular components through detergents and enzymes without affecting the extracellular matrix (ECM), a process termed ‘decellularization’, which has been developed within the past decade [Citation71,Citation72]. The resulting kidney acellular matrix is subsequently recellularized through different cell seeding strategies. Importantly, biological scaffolds permit signal exchange between the matrix and the cells to induce migration, proliferation, and differentiation. Despite those unique aspects of the decellularization-recellularization technology, the number of donor cells is limited from the point of view of costs and its technological incompleteness. Achieving tissue/organ function genuinely requires increasing its scale and advancement of the recellularization-technology. The scaffold provides the environment to promote such a cellular function. However, the chemical-treated scaffold does not necessarily guarantee the presumable repopulation of the donor cells that support renal organ function on the scaffold. The repopulation of billions of properly aligned cells to achieve kidney function that is better than dialysis demands further technological breakthroughs and innovations.
While the clinical use of decellularized scaffolds has been documented for some organs, like bladder [Citation10] and dermis [Citation73], kidney engineering is still at its early stage. This is mostly due to the complexity of the renal structure, composed of more than 26 different types of highly specialized cells [Citation74]. This is translated into a lack of satisfying recellularization protocol to repopulate the whole kidney. In fact, for efficient kidney regeneration, both the parenchyma and the vasculature need to be entirely reconstructed with, ideally, patient-derived cells that would not trigger immune rejection. So far, the most promising results for kidney recellularization have been obtained by using either epithelial cells in combination with endothelial cells or PSCs that can differentiate toward every other cell type. Song et al. showed encouraging results by infusing human umbilical venous endothelial cells (HUVECs) and rat neonatal kidney cells (NKC) in a rat kidney scaffold. After cell infusion and maintenance in a whole-organ bioreactor, the newly regenerated epithelium appeared to resemble the native nephron. Importantly, the recellularized kidney was able to produce rudimentary urine both in vitro and, following transplantation into rats, in vivo [Citation75]. The other appealing source to repopulate the biological scaffolds is represented by PSCs as they can potentially differentiate into any of the adult renal cell types [Citation76]. Ross et al. observed that, after infusion through the renal artery, murine ESCs engrafted mainly the vascular and glomerular structures of decellularized rat kidneys. Notably, only murine ESCs in direct contact with the basement membrane showed signs of differentiation while the others became apoptotic, thereby forming lumens, thus suggesting that the extracellular matrix directs commitment of pluripotent cells [Citation77]. More recently, Bonandrini et al. developed an optimized protocol for rat whole-kidney scaffolds recellularization, by infusing murine ESCs through the renal artery under controlled pressure perfusion with recirculating medium for up to 72 h. Nevertheless, murine ESCs were mainly distributed in the glomerular capillaries and the vasculature and only occasionally reached the tubular structures [Citation78]. Attempts have been made also using human-derived ESCs and human iPSCs. Using acellular scaffolds derived from rhesus monkey kidneys, Batchelder et al. and Nakayama et al. showed that human ESCs differentiate toward renal lineage and formed tubular structures [Citation79–Citation81]. Remarkably, the age of the donor was shown to affect the grafting success [Citation81]. To avoid the in vivo differentiation process of PSCs, which could be partial, in 2016 Du et al. injected into mouse decellularized kidney Pax2+ renal progenitor cells and endothelial cells derived from human iPSCs [Citation82]. Following implantation for 12 weeks of the recellularized kidney into immunodeficient mice, the authors observed effective repopulation of the glomeruli only in the presence of both Pax2+ and endothelial cells concluding that endothelial cells are required for cellular assembly of the glomerular structures while they do not affect tubule repopulation [Citation82]. In addition, the presence of endothelial cells positively influenced the filtration potential of the glomerular units measured by employing a bioreactor in vitro. More recently, Ciampi et al. showed efficient repopulation of kidney vasculature in all the compartments, from glomerular capillaries to peritubular capillaries and small vessels, using iPSC-derived endothelial cells [Citation83]. The detection of fenestrated endothelium in glomerular capillaries, but not in the vascular capillaries, clearly suggested site-specific endothelial cell specialization.
One of the many hurdles to translating such recellularized scaffolds into clinical practice is represented by the need to achieve an efficient repopulation of the kidney. In an attempt to overcome this obstacle, different delivery routes have been tested. When cells are infused via the renal artery, they can only reach glomerular capillaries [Citation77,Citation84], while the infusion through the renal vein [Citation84] allowed the cells to spread in the peritubular capillary, at the cortical and medullary level, but only focally. To improve the engraftment, Song et al. delivered endothelial cells through the renal artery and epithelial cells through the ureter while maintaining negative pressure outside the kidney scaffold [Citation75]. This protocol permitted the repopulation of the tubular structures, although it was limited. In order to further increase the degree of recellularization, a specialized bioreactor was designed to infuse cells through the renal artery at high pressure [Citation85]. In this case, the authors were successful in recellularizing about 50% of the renal volume. However, the observed cell translocation to the peritubular structures was likely the result of capillary and tubular membrane rupture due to the high pressure applied, an observation confirmed also by Ciampi et al. [Citation83].
Despite the encouraging results reported so far, we are still far from translating this technology into clinical settings due to uncertainty of complete decellularization methods without disrupting the remaining matrix [Citation86], and/or the host immunological response to the decellularized scaffold [Citation87].
4. Development of self-organizing nephrons
While creating a whole replacement kidney remains a technical challenge, the current technologies led to the development of nephron parts, that can serve as a tool to understand renal (patho)physiology or eventually could be used as the elementary units of bigger structures.
4.1. Kidney organoids
Kidney organoids are self-organizing 3D aggregations derived from ESCs or iPSCs that respond to environmental cues. The generation of kidney organoids was first reported in 2014 and a whole body of work has been published since then, as comprehensively reviewed by Little and Combes [Citation88] and by Nishinakamura [Citation89]. Kidney organoids represent a remarkable tool, in particular for disease modeling, as illustrated by two recent reports that underline their potential for personalized medicine. In the first one, the authors generate human iPSCs able to form organoids from the urine of pediatric patients affected with congenital anomalies of the kidney and urinary tract (CAKUT) [Citation90]. This approach validates the use of urine as a reliable source of iPSCs from infants and children with kidney diseases. In the second one, kidney organoids were obtained from iPSCs produced from the somatic cells of a patient with hereditary c-met-mutated papillary renal cell carcinoma, and represent the first proof of concept of a ‘hereditary renal cancer in a dish’ model [Citation91]. Several major pitfalls still hamper the use of organoids as transplantable organs. First, while kidney organoids are composed of glomeruli and structures resembling proximal and distal tubules, their organization does not mimic one of mammalian kidneys. Although some glomeruli present endothelial cells, the majority of them lack a vascular network. However, organoids implanted under the renal capsule of an immunodeficient mouse become vascularized, and endothelial cells have been found to originate either from the host [Citation92] or from the graft [Citation93]. Independently of their origin, the neovessels are much smaller than renal arteries, and could compromise blood filtration by the organoids [Citation89]. Secondly, single cell RNA sequencing of kidney organoids has revealed that the organoids are more immature than the adult kidney [Citation94], being more similar to embryonic kidneys from the first trimester of gestation [Citation95]. Maturation, as well as size increase, would therefore be essential to obtain transplantable kidneys [Citation89]. Recently, Homan and colleagues developed an in vitro method for culturing human PSC-derived kidney organoids on printed millifluidic chips under high fluidic shear stress, which allowed the formation of a glomerular vasculature within the organoids and an improved morphological maturation of the glomerular and tubular cells [Citation96]. Whether or not the microvascular networks present within these kidney organoids are readily perfusable is still unknown. Nonetheless, this method represents a significant advancement in the field of kidney organoids. Then, Taguchi and Nishinakamura generated murine higher-order kidney organoids, that presented branching ureter with nephron progenitor niches and differentiated nephron components [Citation97]. Unfortunately, they failed to form higher-order organoids from human iPSCs, mitigating the enthusiasm for this new protocol. Finally, organoids require a urinary exit tract to be connected to the host’s ureter or bladder. Possible solutions involve surgery to fashion a urinary drainage system from embryonic ureter and/or bladder tissues [Citation98]. Alternatively, the lower urinary tract could be generated from human stem cells, such as in the method proposed by Suzuki et al. for the directed differentiation of human iPSCs into mature stratified bladder urothelium [Citation99]. Meanwhile, several research groups are studying the molecular cues that direct the development of the ureter and urinary bladder. For example bone morphogenetic protein 4-soaked beads placed near an organoid broke the symmetry of the system, causing a nearby collecting duct to develop into an ureter-like ‘trunk’, while away from the bead collecting duct branching and nephron formation were not disturbed [Citation100]. On a side note, considerable variations between experiments, clones, as well as research groups, have been reported, which could partly be related to the several protocols currently used to obtain kidney organoids [Citation101]. The whole field would greatly benefit from a standardization of the methods. Overall, organoids represent an advance in the development of self-organizing nephrons.
4.2. Kidney-on-a-chip
4.2.1. Glomerulus-on-a-chip
The main function of the glomerulus is to filter fluids and electrolytes from the blood and to prevent the loss of proteins [Citation102]. This activity occurs at the level of the glomerular filtration barrier (GFB) and is coordinated by the interaction of two highly specialized glomerular cells, the fenestrated endothelium and the podocytes, which are separated by a thin layer of glomerular basement membrane (GBM) [Citation102]. Conventional tissue culture methods fail to reproduce the structural and functional characteristics of the glomerulus [Citation103] and systems-level analysis of podocyte biology and kidney disease mechanisms largely rely on animal studies [Citation104]. However, the process often fails to predict human responses because traditional animal models do not accurately mimic human pathophysiology; meanwhile, a staggering number of animals are used [Citation104]. For these reasons, there is a broad need for alternative ways to model human diseases in vitro to accelerate the development of new drugs and advance personalized medicine. In the past 5 years, the development of human organs-on-a-chips, in which microscale engineering technologies enable the recapitulation of the microarchitecture and functions of living human organs, has opened entirely new possibilities to create in vitro models that reconstitute more complex 3D organ-level structures and to integrate crucial dynamic mechanical cues as well as chemical signals [Citation105].
Zhou et al. used the glomerulus-on-a-chip concept to develop a model of hypertensive glomerulopathy, composing a glomerulus-on-a-chip microdevice with conditionally immortalized glomerular endothelial cells and mouse podocyte precursor cells co-cultured on opposite sides of a laminin-coated PDMS membrane [Citation106]. The physiological and pathological glomerular microenvironment was then established by supplying perfusion flow in the upper microchannel and regulating mechanical forces (e.g. glomerular capillary pressure, shear force, and stretch stress) to act on the membrane [Citation106]. Wang et al. subsequently reported the use of a glomerulus-on-a-chip microdevice lined by isolated primary glomerular microtissues that experience fluid flow for studying early-stage diabetic nephropathy, providing further proof of principle that these devices could serve as disease models of glomerulopathy [Citation107].
In 2019, Petrosyan et al. described a glomerulus-on-a-chip constituted by human podocytes of different origin (primary-, immortalized-, and amniotic fluid-derived podocytes) and human glomerular endothelial cells co-cultured in a three-channel version of the OrganoPlate® in the absence of an artificial membrane separating them [Citation108]. This system reproduced a functional GFB that can perform differential clearance of albumin and inulin and was disrupted following exposure to puromycin aminonucleoside (PAN). Intriguingly, when exposed to sera from patients with anti-podocyte autoantibodies, the chips showed albuminuria proportional to patients’ proteinuria, phenomenon not observed with sera from healthy controls or individuals with primary podocyte defects. The authors also validated the chip as a disease-modeling platform for diabetic nephropathy and genetic diseases affecting the podocytes and for drug testing [Citation108].
The development of a functional glomerulus-on-a-chip had been hindered by the lack of functional podocytes until the work of Musah et al., who obtained terminally differentiated podocytes inducing direct differentiation of human iPSCs with high efficiency within 26 days under chemically defined conditions [Citation109]. The iPSC-derived podocytes recapitulated the cell/cell interface and the molecular filtration properties of the glomerular capillary wall when co-cultured with primary human glomerular endothelial cells in a microfluidic device [Citation110]. In this device, the GBM was mimicked by using a porous and flexible polydimethylsiloxane (PDMS) membrane functionalized with the protein laminin and cyclic mechanical strain was applied to cell layers by stretching the flexible PDMS membrane, using vacuum. The glomerulus-on-a-chip was not designed to engineer the whole kidney, but it represents an advanced standard for modeling the GFB, thanks to the possibility to modulate both physical forces and chemical stimuli controlling glomerular cell functions.
4.2.2. Tubule-on-a-chip
The main tubular functions are categorized as excretion of endogenous and exogenous waste products, reabsorption of compounds from the glomerular filtrate and regulation of water and electrolytes. For an in-depth understanding of renal tubular physiology, engineered platforms able to reproduce functional portions of the nephron are increasingly expanding. The first rudimental device has been developed in 2001 [Citation111]. Over the last 20 years, the bioengineering research has led to the development of several on-chip platforms that have been proved to be useful elements to modeling of human kidney disease in vitro and to predicting drug toxicity [Citation112,Citation113]. A tubule-on-a-chip is a device where tubular cells are cultured in a three-dimensional (3D) channel, reproducing the microenvironment of human kidney tubule and its functions of reabsorption and secretion at the same microscale as living cellular milieu. Several investigators have successfully tested different synthetic materials in combination with various natural polymer-based devices to better mimic the natural physiological scaffolds [Citation112–Citation115]. A critical issue in designing and developing tubule-on-a-chip is the cell source. The majority of current tubule-on-a-chip systems utilizes immortalized cell lines such as canine (Madin-Darby canine kidney, MDCK) tubular epithelial cells, the porcine LLC-PK1 (Lilly Laboratories cell, porcine kidney) cells, and the immortalized human renal tubular cell line HK-2 (human kidney 2). Unfortunately, none of these cell lines fully recapitulates the primary cell phenotype, nor do they display proximal tubular functions because of considerable phenotypic and genetic divergences [Citation116]. Primary human PTECs are the most promising in terms of functionality, even if this model is hampered by donor variability, a limited proliferation capacity and cell dedifferentiation upon prolonged culture [Citation115]. More robust cell models that ensure constant availability and a stable phenotype are preferred over primary cells for applications of high-throughput screening. To this aim, engineered cells such as the conditionally immortalized human PTECs might be promising suitable models for the implementation of tubule-on-a-chip technology [Citation114]. Embryonic stem-cell-derived human PTECs are also promising but have not been sufficiently characterized in microfluidic systems [Citation117]. Renal progenitor cells represent an attractive option because they can be easily isolated from either renal tissue or urine, expanded in culture and differentiated in both tubular cells and podocytes [Citation118,Citation119]. Two independent studies developed microfluidic tubule-on-a-chips, starting from renal progenitor cells isolated from human kidneys and urine. The use of renal progenitors isolated from patients may pave the way to the development of personalized-disease-modeling [Citation120,Citation121].
Differently from static culture systems, a fundamental advance in tubule-on-a-chip technology was the microfluidic device that provided a mechanical stimulus, the fluid shear stress (FSS), that affects the cellular structure and the expression of proteins linked to specific tubular functions [Citation122–Citation124]. More importantly, the generation of leak-tight, polarized kidney tubules enabled to recapitulate physiological (trans-epithelial) activity including cellular uptake of albumin [Citation120,Citation121,Citation125], secretory clearance of albumin-bound uremic toxins [Citation61], transportation of sodium/potassium, urea, creatinine, glucose and bicarbonate, and vitamin D activation [Citation115]. In addition, microsensors to measure transepithelial electrical resistance may be embedded in tubule-on-a-chips, improving real-time assessment of tubular physiology in response to environmental changes [Citation125]. This is helpful when microfluidic systems are used to model human kidney disease in vitro. A first attempt of modeling human kidney disease was the study of the pathological role of human PTECs in the development of kidney fibrosis during proteinuric nephropathy [Citation126]. The epithelial cells exposed to serum proteins showed apoptosis or epithelial-mesenchymal-transition (EMT) similar to in vivo processes [Citation126]. A further disease model regarded the study of the mechanisms of stone formation in the tubule in real time after injection of CaCl2 and Na3PO4 into the device [Citation127]. In the future, it is expected that tubule-on-a-chip would be used to model various kidney tubular diseases.
In contrast to proximal tubule, few studies have examined the physiology of distal tubular and cortical collecting duct cells cultured in microfluidic devices [Citation128]. However, in order to be physiologically and pathophysiologically relevant, microfluidic devices must integrate cell–cell interactions, such as those in vivo in the nephron. Although Weinberg et al. proposed the first computational model for a nephron including the four major components (the glomerulus, proximal tubule, loop of Henle and connector), a true kidney-on-a-chip model in vitro has yet to be achieved [Citation129]. The first attempt was a microfluidic system that consists of a peristaltic micropump (heart), a dialysis component that mimics glomerular filtration and a tubular secretion component [Citation130]. Moreover, complex metabolic interactions were reconstructed by using dedicated organ-on-a-chip platforms. A recent example is the use of a multi-compartment microfluidic chip to recapitulate hepatic vitamin D metabolism and renal bio-activation [Citation131]. However, many challenges have to be overcome because the structural and functional complexity of kidney make the development of a true kidney-on-a-chip more than just a sum of individual nephron components.
Recently, Peired et al. developed a preclinical model of renal papillary tumor, by growing renal progenitor cells (RPCs) infected with NOTCH1-overexpressing lentivirus [Citation132] in a tubule-on-a-chip generating tumor-like masses [Citation133].
The most diffusely and appealing application of tubule-on-a-chip is drug-induced nephrotoxicity screening [Citation134–Citation136]. This technology was helpful to reproduce the in vivo cisplatin and gentamicin toxicity, demonstrating that the once-a-day bolus dosing method was less nephrotoxic than the continuous infusion method [Citation137]. Moreover, the combination of tubule-on-a-chip systems with innovative detection methods to analyze novel biomarkers specific for nephrotoxicity identifies this technology as a fully compatible platform with automation and high content screening equipment [Citation134–Citation141]. However, the goal standard in understanding nephrotoxicity is the integration of multiple cell types from different organs. This strategy is crucial in evaluating secondary drug toxicities resulting from drug metabolism. An example is ifosfamide, that is not nephrotoxic, but chloroacetaldeyde, a hepatic metabolite of ifosfamide, is the major cause of ifosfamide-induced nephrotoxicity. Co-culture of HepaRG hepatocytes and MDCK cells reproduced the effect of ifosfamide metabolism on nephrotoxicity and confirmed the systemic interaction of the liver and kidney [Citation142]. Following this, multi-organ-on-a-chip models comprising intestine (human enterocytes), liver (human hepatocytes), skeletal muscle (human myocytes), skin (human biopsy), nervous system (iPSC-derived human neurons and astrocytes) and kidney (human PTECs) were developed to assess drug absorption, distribution, metabolism, and excretion as well as multiorgan toxicity [Citation143–Citation145].
Overall, the tubule-on-a-chip technology has shown strong promises in mimicking the complexity of native tissues in vitro and ex vivo, showing recent significant advances to study the kidney and its diseases.
4.2.3. 3D bioprinting
A step further in the direction of reproducing in vitro the tissue complexity is represented by 3D bioprinting. Bioprinting consists in the layer-by-layer deposition of cells and supporting components into complex 3D functional living tissues. So far, 3D bioprinting has been applied to the generation of skin, cartilage, bone, and vascular tissue to be transplanted in reconstructive surgery [Citation146–Citation148]. However, bioprinting of more complex tissues, like the kidney, presents several challenges: 1. the technical difficulty to reproduce the complex renal architecture; 2. the choice of cell types; 3. the choice of proper biomaterials that permit the preservation of renal structure and functionality [Citation149–Citation153]. Because of these difficulties, currently 3D printing technology is principally employed for the generation of portions of the nephron that would allow the development of a more accurate model from an architectural and functional point of view. King et al. used a 3D bioprinting platform to create an interstitial interface containing human renal fibroblasts, HUVEC and human PTECs. This model demonstrated to be able to maintain human PTECs morphology, viability and function for at least 2 weeks in culture [Citation154]. The tissues also showed to be sensible to cisplatin-induced nephrotoxicity, that was reverted by inhibiting the cationic uptake transporter OCT2, confirming the successful mimicking of native tissue. Subsequently, the Lewis bioprinting team worked to create a 3D kidney tissue able to replicate human kidney physiology [Citation155]. Briefly, their multi-material 3D printing platform permitted the creation of a perfusable, convoluted proximal tubule by printing the fugitive ink within an engineered extracellular matrix composed of a gelatin–fibrin hydrogel, housed within a customized perfusion chip. The ink was then removed, establishing an open lumen that was then seeded with immortalized human PTECs. This fabrication method allowed the formation of 3D proximal tubule models with customized diameter, length, and curvature lined with confluent layer of epithelium that preserved viability for up to 2 months. Moreover, the immortalized human PTECs showed morphological (dense brush border) and functional (albumin uptake) properties which were enhanced if compared to the same cells growing in 2D and comparable to native proximal tubule epithelial cells. In addition, when treated with different concentration of Cyclosporine A, a high number of cells died determining the disruption of the epithelial barrier function. In 2019, to better mimic the microenvironment of a native kidney tissue, the same team created a vascularized proximal tubule model [Citation156]. These 3D renal tissues were made up of neighboring ducts that were seeded with immortalized human PTECs and glomerular microvascular endothelial cells, incorporated in a permeable-engineered extracellular matrix, and independently addressed using a closed-loop perfusion system. Both epithelium and endothelium showed a healthy and mature phenotype and exhibited active reabsorption of solutes via tubular – vascular exchange. These characteristics permitted the authors to evaluate the epithelium-endothelium cross-talk in basal and disease conditions (hyperglycemic state).
Recently, Higgins et al. used the 3D bioprinting technology to produce kidney organoids in a way that was reproducible, rapid and transferable between cell lines [Citation157]. This technique will greatly facilitate high content compound screening. The studies mentioned above demonstrated that the 3D bioprinting is a reliable tool for creation of in vitro kidney disease model and drug testing. There is great hope that the 3D bioprinting technology would be applied to the construction of fully functional whole kidneys, resolving the issue of organ availability for renal transplantation.
4D bioprinting is a new technology characterized by a fourth dimension, ‘time’, that is incorporated within the 3D bioprinting [Citation158]. Utilizing a particular type of hydrogel responsive to external stimuli as physical (e.g. water, temperature, light, electric field, and magnetic field); chemical (e.g. pH value and ion concentration) or biological (e.g. glucose and enzymes) ones, the printed material can modify reversibly its shape and better mimic the physiological dynamic changes of native tissue. However, despite its enormous potential, the 4D bioprinting is still in the stage of proof-of-concept study.
5. Conclusion
Huge advances in kidney bioengineering are generating new opportunities to develop renal replacement therapies. Although these techniques represent attractive strategies, the complexity of the organ from a structural and functional point of view precludes their immediate clinical application. Nevertheless, the microscale engineering technology combined with stem cell technology (organoids, microfluidic devices and 3D bioprinting of nephron portions) are creating interesting possibilities to investigate the physiology and the pathophysiology of the kidney. Collectively, these kidney bioengineering techniques allow to envision the creation of new organs in the future, and at present to generate advanced experimental models of renal diseases and tools to address drug toxicity. This can be tailored to the individual patient in the context of personalized medicine, providing new solutions for a steadily increasing number of chronic and ESKD patients ().
6. Expert opinion
Bioengineered kidneys hold promises for future clinical practice. While not tested yet in clinical settings, xenotransplantation of pig kidney may be a strategy for renal replacement therapy. Once removed the last immunological barriers, spatial and temporal issues related to transplantation would be reduced, as pig kidneys may be prepared on site and on demand. The development of personalized interspecies chimaeras would significantly reduce the issues of immune rejection. However, no viable human-animal chimera has been produced so far, highlighting the technical challenge involved. Because of their similarity to humans and despite legal and ethical issues, the development of human organs in NHP would alleviate many technical difficulties.
Bioengineered artificial kidneys represent an alternative approach to renal replacement therapy and transplantation. The combination of engineering, biomaterial science, cell biology, and reconstructive microsurgery led to the development of the RAD, the only bioartificial kidney device successfully tested in humans. The RAD could not only improve renal replacement therapy, but also move dialysis patients outside the clinic. Nevertheless, given the complexity of renal architecture and matrix composition, moving from RAD to whole kidney generation is virtually impossible with the current technologies. Biological scaffolds derived from allogenic or xenogeneic kidneys would permit to circumvent these issues. As custom bioreactors enable complete kidney decellularization, the remaining hurdle is obtaining fully functional recellularized scaffolds with the reconstitution of the entire nephron structure to restore renal function, neovascularization, and nervous innervation. Collectively, these approaches could produce a virtually unlimited supply of organs that could potentially permit to lower the staggering human and societal costs of ESKD patients worldwide.
However, creating a de novo, fully functional, bioartificial kidney that could replace the native kidney with efficiency and safety remains an unmet, although plausible goal. Breakthroughs in stem cell technology, involving iPSCs or renal progenitors, paved the way to recreating the complex architecture of the adult organ with kidney organoids, which revealed themselves optimal tool for disease modeling and drug testing. However, their small size, poor vascularization and lack of drainage system currently impede their translation into the clinic. Meanwhile, advances in bioengineering and cell biology allowed the production of microfluidics-based systems that enable to control cell microenvironments, reproducing accurately the physiology of nephron portions. These technologies have been successfully applied to renal disease modeling and drug toxicity testing, using patient-specific cells, showing their potential to reduce patient morbidity and mortality associated with unpredicted adverse drug reactions. Still, drug-induced nephrotoxicity screening needs standardized microfluidic tubule-on-a-chip models compatible with automation and high throughput screening equipment. The combination of microfluidics with bioprinting could be a valid approach to support pharmaceutical research, to reduce animal experimentation, to limit the costs of development of new and safer drugs, and to form the basis to eventually engineer a whole kidney.
Article highlights
Human tissue bioengineering is a field of great fascination for worldwide researchers. Huge advances in kidney bioengineering are generating new opportunities to develop renal replacement therapies. However, the complexity of this organ from a structural and functional point of view precludes their immediate clinical application.
Xenotransplantation offers an attractive possibility to overcome the shortage of kidneys. However, major challenges must be addressed prior to the transition from pre-clinical studies to clinical trials, such as immunosuppressive regimens, the risk of transmission of infectious disease to the recipient and legal issues.
Kidney bioengineering offers alternative solutions to renal replacement therapy. However, assembling cells, biologically relevant molecules and scaffolds into functional organs represents technical challenges that remained to be solved.
While creating a whole replacement kidney remains a technical challenge, the current technologies led to the development of nephron parts, organoids and kidney-on-a-chip, combined or not with 3D bioprinting, that can serve as a tool to understand kidney (patho)physiology or eventually could be used as the elementary units of bigger structures.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015 Nov;26(11):2621–2633.
- Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. 2019 Feb;19(Suppl 2):19–123.
- Rouchi AH, Mahdavi-Mazdeh M. When is transplantation with a “marginal kidney” justifiable? Ann Transplant. 2016 Jul;26(21):463–468.
- Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015 May 16;385(9981):1975–1982.
- Locatelli F, Buoncristiani U, Canaud B, et al. Dialysis dose and frequency. Nephrol Dial Transplant. 2005 Feb;20(2):285–296.
- Sladkova M, Alawadhi R, Jaragh Alhaddad R, et al. Segmental additive tissue engineering. Sci Rep. 2018 Jul 18;8(1):10895.
- Zhou H, Kitano K, Ren X, et al. Bioengineering human lung grafts on porcine matrix. Ann Surg. 2018 Mar;267(3):590–598.
- Marino D, Luginbuhl J, Scola S, et al. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med. 2014 Jan 29;6(221):221ra14.
- Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010 Feb;139(2):431–6, 436 e1–2.
- Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006 Apr 15;367(9518):1241–1246.
- Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006 Aug 15;82(3):314–319.
- Reemtsma K, McCracken BH, Schlegel JU, et al. Heterotransplantation of the kidney: two clinical experiences. Science. 1964 Feb 14;143(3607):700–702.
- Cooper DKC, Gaston R, Eckhoff D, et al. Xenotransplantation-the current status and prospects. Br Med Bull. 2018 Mar 1;125(1):5–14.
- Baldan N, Rigotti P, Calabrese F, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004 Apr;4(4):475–481.
- Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002 Mar;20(3):251–255.
- Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002 Feb 8;295(5557):1089–1092.
- Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005 Jan;11(1):32–34.
- Rivard CJ, Tanabe T, Lanaspa MA, et al. Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation – an experimental study. Transpl Int. 2018 Oct;31(10):1164–1177.
- Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015 Jul-Aug;22(4):302–309.
- Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015 May-Jun;22(3):221–230.
- Kim SC, Mathews DV, Breeden CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 2019 Aug;19(8):2174–2185.
- Shaw BI, Kirk AD. Kidney xenotransplantation: steps toward clinical application. Clin J Am Soc Nephrol. 2019 Apr 5;14(4):620–622.
- Naeimi Kararoudi M, Hejazi SS, Elmas E, et al. Clustered regularly interspaced short palindromic repeats/Cas9 gene editing technique in xenotransplantation. Front Immunol. 2018;9:1711.
- Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant. 2017 Dec;22(6):513–521.
- Niu D, Wei HJ, Lin L, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017 Sep 22;357(6357):1303–1307.
- Argaw T, Colon-Moran W, Wilson C. Susceptibility of porcine endogenous retrovirus to anti-retroviral inhibitors. Xenotransplantation. 2016 Mar;23(2):151–158.
- Mann SP, Sun R, Hermeren G. Ethical considerations in crossing the xenobarrier. Methods Mol Biol. 2019;2005:175–193.
- Schuurman HJ. Regulatory aspects of clinical xenotransplantation. Int J Surg. 2015 Nov;23(Pt B):312–321.
- Rogers SA, Talcott M, Hammerman MR. Transplantation of pig metanephroi. Asaio J. 2003 Jan-Feb;49(1):48–52.
- Takeda S, Rogers SA, Hammerman MR. Differential origin for endothelial and mesangial cells after transplantation of pig fetal renal primordia into rats. Transpl Immunol. 2006 Jan;15(3):211–215.
- Dekel B, Burakova T, Ben-Hur H, et al. Engraftment of human kidney tissue in rat radiation chimera: II. Human fetal kidneys display reduced immunogenicity to adoptively transferred human peripheral blood mononuclear cells and exhibit rapid growth and development. Transplantation. 1997 Dec 15;64(11):1550–1558.
- Wu J, Greely HT, Jaenisch R, et al. Stem cells and interspecies chimaeras. Nature. 2016 Dec 1;540(7631):51–59.
- Chen J, Lansford R, Stewart V, et al. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532.
- Muller SM, Terszowski G, Blum C, et al. Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood vessel architecture. Proc Natl Acad Sci U S A. 2005 Jul 26;102(30):10587–10592.
- Fraidenraich D, Stillwell E, Romero E, et al. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004 Oct 8;306(5694):247–252.
- Ueno H, Turnbull BB, Weissman IL. Two-step oligoclonal development of male germ cells. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):175–180.
- Espejel S, Roll GR, McLaughlin KJ, et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010 Sep;120(9):3120–3126.
- Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010 Sep 3;142(5):787–799.
- Mori M, Furuhashi K, Danielsson JA, et al. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat Med. 2019 Nov;25(11):1691–1698.
- Usui J, Kobayashi T, Yamaguchi T, et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012 Jun;180(6):2417–2426.
- Yamaguchi T, Sato H, Kato-Itoh M, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017 Feb 9;542(7640):191–196.
- Isotani A, Hatayama H, Kaseda K, et al. Formation of a thymus from rat ES cells in xenogeneic nude mouse<–>rat ES chimeras. Genes Cells. 2011 Apr;16(4):397–405.
- Goto T, Hara H, Sanbo M, et al. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat Commun. 2019 Feb 5;10(1):451.
- Theunissen TW, Friedli M, He Y, et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell. 2016 Oct 6;19(4):502–515.
- Theunissen TW, Powell BE, Wang H, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014 Oct 2;15(4):471–487.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013 Dec 12;504(7479):282–286.
- Wu J, Platero-Luengo A, Sakurai M, et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017 Jan 26;168(3):473–486 e15.
- Kobayashi T, Kato-Itoh M, Nakauchi H. Targeted organ generation using Mixl1-inducible mouse pluripotent stem cells in blastocyst complementation. Stem Cells Dev. 2015 Jan 15;24(2):182–189.
- Li Z, Araoka T, Wu J, et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell. 2016 Oct 6;19(4):516–529.
- Fujimoto T, Yamanaka S, Tajiri S, et al. In vivo regeneration of interspecies chimeric kidneys using a nephron progenitor cell replacement system. Sci Rep. 2019 May 6;9(1):6965.
- Yamanaka S, Saito Y, Fujimoto T, et al. Kidney regeneration in later-stage mouse embryos via transplanted renal progenitor cells. J Am Soc Nephrol. 2019 Sep 23;30:2293–2305.
- MacKay SM, Funke AJ, Buffington DA, et al. Tissue engineering of a bioartificial renal tubule. Asaio J. 1998 May-Jun;44(3):179–183.
- Humes HD, MacKay SM, Funke AJ, et al. Tissue engineering of a bioartificial renal tubule assist device: in vitro transport and metabolic characteristics. Kidney Int. 1999 Jun;55(6):2502–2514.
- Humes HD, Fissell WH, Weitzel WF, et al. Metabolic replacement of kidney function in uremic animals with a bioartificial kidney containing human cells. Am J Kidney Dis. 2002 May;39(5):1078–1087.
- Tumlin J, Wali R, Williams W, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008 May;19(5):1034–1040.
- Humes HD, Weitzel WF, Bartlett RH, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004 Oct;66(4):1578–1588.
- Pino CJ, Westover AJ, Buffington DA, et al. Bioengineered renal cell therapy device for clinical translation. Asaio J. 2017 May/Jun;63(3):305–315.
- Buffington DA, Pino CJ, Chen L, et al. Bioartificial renal epithelial cell system (BRECS): a compact, cryopreservable extracorporeal renal replacement device. Cell Med. 2012 Jan;4(1):33–43.
- Westover AJ, Buffington DA, Johnston KA, et al. A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock. J Tissue Eng Regen Med. 2017 Mar;11(3):649–657.
- Johnston KA, Westover AJ, Rojas-Pena A, et al. Development of a wearable bioartificial kidney using the bioartificial renal epithelial cell system (BRECS). J Tissue Eng Regen Med. 2017 Nov;11(11):3048–3055.
- Jansen J, Fedecostante M, Wilmer MJ, et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep. 2016 May 31;6:26715.
- Dang BV, Taylor RA, Charlton AJ, et al. Towards portable artificial kidneys: the role of advanced microfluidics and membrane technologies in implantable systems. IEEE Rev Biomed Eng. 2019 Aug 5;1.
- Salani M, Roy S, Fissell WHT. Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis. 2018 Nov;72(5):745–751.
- van Gelder MK, Mihaila SM, Jansen J, et al. From portable dialysis to a bioengineered kidney. Expert Rev Med Devices. 2018 May;15(5):323–336.
- Roy S, Goldman K, Marchant R, et al. Implanted renal replacement for end-stage renal disease. Panminerva Med. 2011 Sep;53(3):155–166.
- Fissell WH, Roy S. The implantable artificial kidney. Semin Dial. 2009 Nov-Dec;22(6):665–670.
- Fissell WH, Dubnisheva A, Eldridge AN, et al. High-performance silicon nanopore hemofiltration membranes. J Memb Sci. 2009 Jan 5;326(1):58–63.
- Kim S, Feinberg B, Kant R, et al. Diffusive silicon nanopore membranes for hemodialysis applications. PLoS One. 2016;11(7):e0159526.
- Kensinger C, Karp S, Kant R, et al. First implantation of silicon nanopore membrane hemofilters. Asaio J. 2016 Jul-Aug;62(4):491–495.
- Fissell WH, Manley S, Westover A, et al. Differentiated growth of human renal tubule cells on thin-film and nanostructured materials. Asaio J. 2006 May-Jun;52(3):221–227.
- Hussein KH, Saleh T, Ahmed E, et al. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. J Biomed Mater Res A. 2018 Jul;106(7):2034–2047.
- Figliuzzi M, Bonandrini B, Remuzzi A. Decellularized kidney matrix as functional material for whole organ tissue engineering. J Appl Biomater Funct Mater. 2017 Nov 10;15(4):e326–e333.
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995 Jun;21(4):243–248.
- Nishinakamura R. Stem cells in the embryonic kidney. Kidney Int. 2008 Apr;73(8):913–917.
- Song JJ, Guyette JP, Gilpin SE, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013 May;19(5):646–651.
- Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005 Dec;16(12):3527–3534.
- Ross EA, Williams MJ, Hamazaki T, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009 Nov;20(11):2338–2347.
- Bonandrini B, Figliuzzi M, Papadimou E, et al. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A. 2014 May;20(9–10):1486–1498.
- Batchelder CA, Martinez ML, Tarantal AF. Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS One. 2015;10(12):e0143849.
- Nakayama KH, Lee CC, Batchelder CA, et al. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8(5):e64134.
- Nakayama KH, Batchelder CA, Lee CI, et al. Renal tissue engineering with decellularized rhesus monkey kidneys: age-related differences. Tissue Eng Part A. 2011 Dec;17(23–24):2891–2901.
- Du C, Narayanan K, Leong MF, et al. Functional kidney bioengineering with pluripotent stem-cell-derived renal progenitor cells and decellularized kidney scaffolds. Adv Healthc Mater. 2016 Aug;5(16):2080–2091.
- Ciampi O, Bonandrini B, Derosas M, et al. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Sci Rep. 2019 May 29;9(1):8001.
- Remuzzi A, Figliuzzi M, Bonandrini B, et al. Experimental evaluation of kidney regeneration by organ scaffold recellularization. Sci Rep. 2017 Mar 7;7:43502.
- Caralt M, Uzarski JS, Iacob S, et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant. 2015 Jan;15(1):64–75.
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011 Apr;32(12):3233–3243.
- Stahl EC, Bonvillain RW, Skillen CD, et al. Evaluation of the host immune response to decellularized lung scaffolds derived from alpha-Gal knockout pigs in a non-human primate model. Biomaterials. 2018;187:93–104.
- Little MH, Combes AN. Kidney organoids: accurate models or fortunate accidents. Genes Dev. 2019 Oct 1; 33(19–20):1319–1345.
- Nishinakamura R. Human kidney organoids: progress and remaining challenges. Nat Rev Nephrol. 2019 Oct;15(10):613–624.
- Mulder J, Sharmin S, Chow T, et al. Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatr Res. 2019 Oct 19.
- Hwang JW, Desterke C, Feraud O, et al. iPSC-derived embryoid bodies as models of C-met-mutated hereditary papillary renal cell carcinoma. Int J Mol Sci. 2019 Sep 30;20:19.
- van den Berg CW, Ritsma L, Avramut MC, et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018 Mar 13;10(3):751–765.
- Tanigawa S, Islam M, Sharmin S, et al. Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Reports. 2018 Sep 11;11(3):727–740.
- Wu H, Uchimura K, Donnelly EL, et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018 Dec 6;23(6):869–881 e8.
- Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015 Oct 22;526(7574):564–568.
- Homan KA, Gupta N, Kroll KT, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019 Mar;16(3):255–262.
- Taguchi A, Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell. 2017 Dec 7; 21(6):730–746 e6.
- Yokote S, Matsunari H, Iwai S, et al. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):12980–12985.
- Suzuki K, Koyanagi-Aoi M, Uehara K, et al. Directed differentiation of human induced pluripotent stem cells into mature stratified bladder urothelium. Sci Rep. 2019 Jul 19;9(1):10506.
- Mills CG, Lawrence ML, Munro DAD, et al. Asymmetric BMP4 signalling improves the realism of kidney organoids. Sci Rep. 2017 Nov 1;7(1):14824.
- Phipson B, Er PX, Combes AN, et al. Evaluation of variability in human kidney organoids. Nat Methods. 2019 Jan;16(1):79–87.
- Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013 Aug;9(8):470–477.
- Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu Rev Med. 2013;64:357–366.
- Ortiz A, Sanchez-Nino MD, Izquierdo MC, et al. Translational value of animal models of kidney failure. Eur J Pharmacol. 2015 Jul 15;759:205–220.
- Rothbauer M, Rosser JM, Zirath H, et al. Tomorrow today: organ-on-a-chip advances towards clinically relevant pharmaceutical and medical in vitro models. Curr Opin Biotechnol. 2019;55:81–86.
- Zhou M, Zhang X, Wen X, et al. Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci Rep. 2016 Aug 25;6:31771.
- Wang L, Tao T, Su W, et al. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip. 2017 May 16;17(10):1749–1760.
- Petrosyan A, Cravedi P, Villani V, et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun. 2019 Aug 13;10(1):3656.
- Musah S, Dimitrakakis N, Camacho DM, et al. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc. 2018 Jul;13(7):1662–1685.
- Musah S, Mammoto A, Ferrante TC, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1.
- Essig M, Terzi F, Burtin M, et al. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol. 2001 Oct;281(4):F751–62.
- Ashammakhi N, Wesseling-Perry K, Hasan A, et al. Kidney-on-a-chip: untapped opportunities. Kidney Int. 2018 Dec;94(6):1073–1086.
- Nieskens TT, Wilmer MJ. Kidney-on-a-chip technology for renal proximal tubule tissue reconstruction. Eur J Pharmacol. 2016 Nov;5(790):46–56.
- Ng CP, Zhuang Y, Lin AWH, et al. A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int J Tissue Eng. 2013;2013:10.
- Weber EJ, Chapron A, Chapron BD, et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016 Sep;90(3):627–637.
- Jenkinson SE, Chung GW, van Loon E, et al. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012 Dec;464(6):601–611.
- Narayanan K, Schumacher KM, Tasnim F, et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013 Apr;83(4):593–603.
- Lazzeri E, Ronconi E, Angelotti ML, et al. Human urine-derived renal progenitors for personalized modeling of genetic kidney disorders. J Am Soc Nephrol. 2015 Aug;26(8):1961–1974.
- Ronconi E, Sagrinati C, Angelotti ML, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009 Feb;20(2):322–332.
- Sciancalepore AG, Sallustio F, Girardo S, et al. A bioartificial renal tubule device embedding human renal stem/progenitor cells. PLoS One. 2014;9(1):e87496.
- Schutgens F, Rookmaaker MB, Margaritis T, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019 Mar;37(3):303–313.
- Vriend J, Peters JGP, Nieskens TTG, et al. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim Biophys Acta Gen Subj. 2019 Sep 11;1864(1):129433.
- Raghavan V, Rbaibi Y, Pastor-Soler NM, et al. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8506–8511.
- Maggiorani D, Dissard R, Belloy M, et al. Shear stress-induced alteration of epithelial organization in human renal tubular cells. PLoS One. 2015;10(7):e0131416.
- Ferrell N, Ricci KB, Groszek J, et al. Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng. 2012 Mar;109(3):797–803.
- Zhou M, Ma H, Lin H, et al. Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials. 2014 Feb;35(5):1390–1401.
- Wei Z, Amponsah PK, Al-Shatti M, et al. Engineering of polarized tubular structures in a microfluidic device to study calcium phosphate stone formation. Lab Chip. 2012 Oct 21;12(20):4037–4040.
- Baudoin R, Griscom L, Monge M, et al. Development of a renal microchip for in vitro distal tubule models. Biotechnol Prog. 2007 Sep-Oct;23(5):1245–1253.
- Weinberg E, Kaazempur-Mofrad M, Borenstein J. Concept and computational design for a bioartificial nephron-on-a-chip. Int J Artif Organs. 2008 Jun;31(6):508–514.
- Sakuta Y, Takehara I, Tsunoda KI, et al. Development of a microfluidic system comprising dialysis and secretion components for a bioassay of renal clearance. Anal Sci. 2018 Sep 10;34(9):1073–1078.
- Theobald J, Abu El Maaty MA, Kusterer N, et al. In vitro metabolic activation of vitamin D3 by using a multi-compartment microfluidic liver-kidney organ on chip platform. Sci Rep. 2019 Mar 15;9(1):4616.
- Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010 Sep;28(9):1674–1685.
- Peired AJ, Antonelli G, Angelotti ML, et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci Transl Med. 2020.
- Vriend J, Nieskens TTG, Vormann MK, et al. Screening of drug-transporter interactions in a 3d microfluidic renal proximal tubule on a chip. Aaps J. 2018 Jul 26;20(5):87.
- Lee J, Kim S. Kidney-on-a-chip: a new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr Drug Metab. 2018;19(7):577–583.
- Wilmer MJ, Ng CP, Lanz HL, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016 Feb;34(2):156–170.
- Kim S, LesherPerez SC, Kim BC, et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016 Mar 24;8(1):015021.
- Adler M, Ramm S, Hafner M, et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol. 2016 Apr;27(4):1015–1028.
- Jang KJ, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb). 2013 Sep;5(9):1119–1129.
- Sakolish CM, Philip B, Mahler GJ. A human proximal tubule-on-a-chip to study renal disease and toxicity. Biomicrofluidics. 2019 Jan;13(1):014107.
- Sakolish C, Weber EJ, Kelly EJ, et al. Technology transfer of the microphysiological systems: a case study of the human proximal tubule tissue chip. Sci Rep. 2018 Oct 5;8(1):14882.
- Choucha-Snouber L, Aninat C, Grsicom L, et al. Investigation of ifosfamide nephrotoxicity induced in a liver-kidney co-culture biochip. Biotechnol Bioeng. 2013 Feb;110(2):597–608.
- Vernetti L, Gough A, Baetz N, et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep. 2017 Feb 8;7:42296.
- Maschmeyer I, Lorenz AK, Schimek K, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015 Jun 21;15(12):2688–2699.
- Ramme AP, Koenig L, Hasenberg T, et al. Autologous induced pluripotent stem cell-derived four-organ-chip. Future Sci OA. 2019 Sep 10;5(8):FSO413.
- Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012 Nov;1(11):792–802.
- Cui X, Breitenkamp K, Finn MG, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012 Jun;18(11–12):1304–1312.
- Poldervaart MT, Wang H, van der Stok J, et al. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS One. 2013;8(8):e72610.
- Datta S, Das A, Chowdhury AR, et al. Bioink formulations to ameliorate bioprinting-induced loss of cellular viability. Biointerphases. 2019 Oct 14;14(5):051006.
- Vijayavenkataraman S, Yan WC, Lu WF, et al. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296–332.
- Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018 May 1;6(5):915–946.
- Ong CS, Yesantharao P, Huang CY, et al. 3D bioprinting using stem cells. Pediatr Res. 2018 Jan;83(1–2):223–231.
- Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013 Jan;101(1):272–284.
- King SM, Higgins JW, Nino CR, et al. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol. 2017;8:123.
- Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016 Oct 11;6:34845.
- Lin NYC, Homan KA, Robinson SS, et al. Renal reabsorption in 3D vascularized proximal tubule models. Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5399–5404.
- Higgins JW, Chambon A, Bishard K, et al. Bioprinted pluripotent stem cell-derived kidney organoids provide opportunities for high content screening. bioRxiv. 2018;505396.
- Yang Q, Gao B, Xu F. Recent advances in 4D bioprinting. Biotechnol J. 2019 Sep 4:e1900086.