ABSTRACT
Introduction
Mayaro fever is an emerging viral disease that manifests as an acute febrile illness. The disease is self-limiting, however joint pain can persist for months leading to chronic arthralgia. There is no specific treatment available, which ultimately leads to socioeconomic losses in populations at risk as well as strains to the public health systems.
Areas covered
We reviewed the candidate treatments proposed for Mayaro virus (MAYV) infection and disease, including antiviral compounds targeting viral or host mechanisms, and pathways involved in disease development and pathogenicity. We assessed compound screening technologies and experimental infection models used in these studies and indicated the advantages and limitations of available technologies and intended therapeutic strategies.
Expert opinion
Although several compounds have been suggested as candidate treatments against MAYV infection, notably those with antiviral activity, most compounds were assessed only in vitro. Compounds rarely progress toin vivo or preclinical studies, and such difficulty may be associated with limited experimental models. MAYV biology is largely inferred from related alphaviruses and reflected by few studies focusing on target proteins or mechanisms of action for MAYV. Therapeutic strategies targeting pathogenic inflammatory responses have shown potential against MAYV-induced disease in vivo, which might reduce long-term sequelae.
1. Introduction
Mayaro virus (MAYV) is an emerging arthropod-borne virus (arbovirus) circulating in South America, Central America, and the Caribbean [Citation1,Citation2]. Mayaro fever (MF) typically manifests as an unspecific febrile illness that can evolve into arthritogenic disease persisting for months following resolution of the infection [Citation3,Citation4]. MAYV was discovered in 1954 in Trinidad and Tobago [Citation2]. Over the past 70 years several outbreaks [Citation5–12] and epidemics with increasing frequency have been documented mostly in periurban or agricultural settings. The true burden of Mayaro disease is severely underestimated due to misdiagnosis to other arboviruses co-circulating in the region, as well as lack of accurate and specific diagnostics1. Critically, sustained transmission in urban centers would have a catastrophic public health and economic impact. Studies on the socioeconomic impact of chikungunya, a closely related arthritogenic disease also endemic in Latin America, indicated that the median economic cost of disease in Colombia is US$ 153 per adult and US$ 258 per child patient [Citation13]. Moreover, the average hospitalization cost for a chikungunya patient in Brazil may reach up to US$ 2400, overwhelming the public health system during epidemics [Citation14]. Chikungunya alone caused a loss of over 77.400 disability-adjusted life years (DALYs) in Brazil in 2016 alone, and a similar scenario caused by MF would be catastrophic [Citation15].
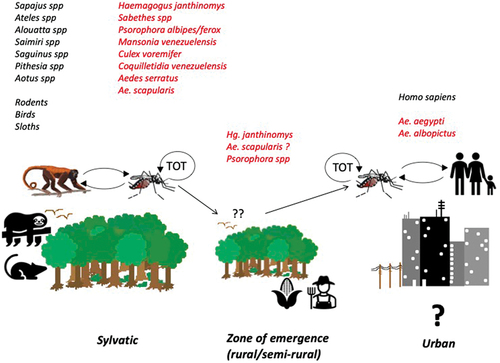
MAYV (Togaviridae, alphavirus) is an enveloped icosahedral virus approximately 70 nm in diameter, containing a single-stranded RNA genome of positive polarity that encodes non-structural (nsP1 to nsP4) and structural proteins (C, E3, E2, 6K, E1), which are involved in viral replication and assembly [Citation16]. MAYV is maintained in an enzootic, sylvatic cycle between arboreal mosquitos and non-human primates, and other possible hosts including sloths, birds, and rodents () [Citation17,Citation18]. Humans can acquire the infection when venturing into the forest or the forest-rural transition zones where enzootic vector(s) (e.g. Hg. janthinomys) often reach high densities and may also disperse long distances from the forest [Citation19,Citation20]. Recent studies suggest that MAYV may also be transmitted by Aedes mosquitoes, which in theory may facilitate urban outbreaks of Mayaro fever [Citation21,Citation22].
Figure 1. MAYV transmission cycles. The list of vertebrate species and other hosts (black), and possible vectors (red). TOT- Transovarial transmission.
MAYV is a neglected arbovirus for which there are no licensed vaccines or treatments approved for human use, or available serological diagnostic methods [Citation23]. Despite recent advances in our understanding of MAYV biology, mechanisms involved in infection and disease development are still poorly understood. Most data available for MAYV are inferred from research on similar alphaviruses, such as Chikungunya virus (CHIKV), Semliki Forest virus (SFV), Ross River virus (RRV), Venezuelan equine encephalitis virus (VEEV) and Sindbis virus (SINV). MAYV is closely related to arthritogenic alphaviruses such as CHIKV, O’nyong nyong virus (ONNV), and SFV, and shares Mxra8 as a cellular receptor for infection of vertebrate hosts [Citation24].
1.1. Replication cycle
Alphaviruses have an extensive host range and replicate in a wide array of vertebrate species. Importantly, the breadth and depth of the host range is likely facilitated through either the utilization of a variety of receptors or through a single ubiquitous receptor. MAYV enters the host cell through receptor-mediated endocytosis (), facilitated by the interaction of Mxra8 and the viral E2 glycoprotein [Citation24] in coated pits, leading to the formation of coated vesicles. Within the basic endosomal environment, the viral E1 protein undergoes conformational changes leading to virus fusion, capsid disassembly, and viral RNA (vRNA) release into the cytoplasm. Replication and transcription of the vRNA takes place on cellular membranes and the (+) single-stranded vRNA serves a dual purpose: (a) messenger RNA for the translation of the nonstructural proteins: and (b) as a template for the synthesis of the complimentary negative strand, which provides the template for the synthesis of new vRNA as well as the subgenomic RNA (sgRNA) () [Citation25]. Translation of the vRNA is initiated by a single starting codon and proceeds uninterrupted until encountering termination codons just downstream of the start of the subgenomic promoter. Processing of the polyprotein is facilitated by virus-encoded proteases into 4 polypeptides (nsP1-nsP4) which ultimately form the replicative complex. nsP1 possesses both guanine-7-methyltransferase and guanylyltransferase activities [Citation26,Citation27] as well as it has been implicated in the synthesis of the negative strand [Citation28]. The mechanism responsible for the capping of the vRNA is quite distinct from the mechanism that is used by the host cells and that is attractive antiviral target [Citation29] ( and section 2.1 below). nsP2 is a multifunctional protein that encodes for the helicase, and triphosphatase (RNA and nucleoside) as well as a cysteine protease that is critical for the processing of the nsP1-nsP4 polyprotein [Citation30]. Critically, nsP2 has been implicated in the shutoff of the Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling pathway, suggesting a role in the inhibition of the antiviral response [Citation31]. Both these properties offer an attractive target for antiviral intervention ( and section 2.1 below). While nsP3 is indispensable for viral replication and its interaction with several host proteins is well documented [Citation32–34], its function remains unknown. nsP4 sole purpose is to serve as the viral RNA-dependent RNA polymerase (RdRp) and could also serve as a target for the development of broad-spectrum antiviral compounds.
Figure 2. MAYV replication. The replication cycle and genomic structures. Possible targets for antiviral therapies are indicated in boxes. Cytopathic vacuoles I and II (CPV-I and CPV-II), endoplasmic reticulum (ER). Created with BioRender.com.
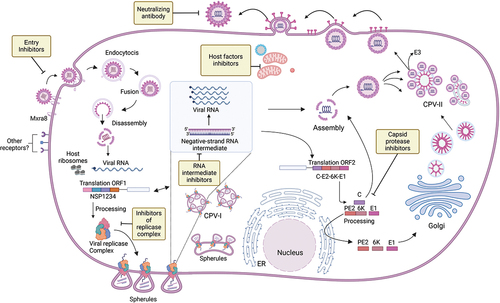
As soon as the sgRNA is formed early in MAYV infection, ribosomes initiate the translation of the subgenomic cassette, starting with the capsid which is cleaved from the nascent polyprotein by a virus-encoded serine protease. Translation proceeds with translocation of the polyprotein into the lumen of the endoplasmic reticulum, where oligosaccharide addition and proteolytic processing by a host-encoded signalase takes place, producing two type I transmembrane glycoproteins (heterodimer PE2, E1) and a membrane-embedded protein (6K) () [Citation35,Citation36]. Further host cell-specific oligosaccharide and post-translational modifications as well as cleavage of PE2 into E2 and E3 occur as the proteins move through the various departments of the Golgi secretory system and final embedding in the host membrane (). At the same time, the assembly of the nucleocapsid proceeds in the cytoplasm with the oligomerization of the capsid and packaging of the genomic RNA followed by the interaction with the host membrane populated by the viral glycoproteins, and release of the enveloped virion ().
Several compounds with antiviral activity against MAYV have been described and reviewed in recent years, but it is unclear why none progressed to clinical trials or approved for human use. We reviewed the candidate treatments for MAYV infection and disease, including antiviral compounds targeting viral or host mechanisms, and pathways involved in disease development and aggravation. We assessed compound screening technologies and experimental infection models used in these studies and highlighted the advantages and limitations of available technologies and intended therapeutic strategies, to identify and discuss the main obstacles to developing treatments against MAYV infection and disease.
2. Body
2.1. Therapeutic strategies against MAYV infection and disease
Most MAYV infections are asymptomatic [Citation11,Citation37], whereas symptomatic infections present with a number of nonspecific symptoms, notably fever, headaches, myalgia, arthralgia, rash, and retro-orbital pain [Citation11,Citation38]. To date there are no documented fatal outcomes that have been attributed to MAYV infection [Citation12], and patients do not require intensive care, even when disease severity leads to patient hospitalization. MF is usually self-limiting, but a significant proportion of patients progress to long-term arthritogenic disease with incapacitating joint pain [Citation39,Citation40]. Treatment is mostly supportive, aimed at ameliorating acute or chronic symptoms with analgesics, antipyretics, and non-steroidal anti-inflammatory drugs (NSAIDs), with limited efficacy [Citation41].
Acute viral infections present with a short viremic period that is initiated before the onset of disease that lasts up to five days [Citation2,Citation42]. Development of therapeutic interventions, e.g. vaccines or neutralizing antibodies, have been focused on prevention of Mayaro disease development [Citation23,Citation43]. Moreover, development of antivirals has been focused on the inhibition of viral replication (), targeting reduction of viremia during the acute phase of infection, and consequently reduction or prevention of chronic disease development. However, the transient nature of MAYV viremia has been a limiting factor, as the therapeutic window for antiviral compounds becomes restricted and viremia is declining by the time the patient manifests signs of disease and seeks treatment at healthcare facilities. This limitation has been also observed when evaluating the use and efficacy of antiviral compounds against CHIKV [Citation44], dengue [Citation45], influenza [Citation46]and SARS-CoV-2 infections [Citation47]. The viral and host factors contributing to the development of chronic sequelae following MAYV infections are not known, and critically, there is no evidence suggesting that antiviral therapies would be effective against chronic stages of MAYV infection. For example, CHIKV vRNA and proteins were found in perivascular synovial macrophages in an elderly patient suffering from chronic disease [Citation48]. Active viral replication in affected joints and its association with the development/persistence of alphavirus-induced arthritis has yet to be demonstrated in humans, although the persistence of viral infection in joints and muscle was also observed in a CHIKV infection model in cynomolgus macaques [Citation49]. Such a scenario is contrasted by chronic viral diseases, such as acquired immunodeficiency syndrome (AIDS) and hepatitis C, in which inhibition of viral replication and persistence is key for patient recovery, and antiviral compounds are the gold standard for treatment.
2.2. Compound screening technologies
In antiviral research, compound screening is usually performed using a common workflow, regardless of the method or technology employed. Initially, hit compound cytotoxicity and antiviral activity are evaluated, and should the hit compound pass minimal thresholds for both, the hit compound progresses in the workflow for further investigation, including testing on different cell lines. Ideally, reductionist experiments are performed to determine whether the hit compound targets a viral component, a host factor, or has the potential to affect multiple steps of viral replication or multiple molecular targets.
From the perspective of scalability in drug discovery, antiviral screening can be classified into three types: High-throughput screening (HTS) is a high-capacity, automated process used in early drug discovery to rapidly evaluate thousands to hundreds of thousands of compounds. Medium-throughput screening (MTS) occupies the middle ground, assessing hundreds to thousands of compounds, offering versatility and flexibility for research, assay formats, and readouts. Low-throughput screening (LTS) is focused and detail-oriented, ideal for smaller-scale, manual, or semi-automated experiments, often used during lead optimization and in-depth drug candidate analysis. The choice between these methods depends on research goals and resource availability, with HTS for the screening of large compound libraries, medium-throughput for balanced exploration, and low-throughput for meticulous, smaller-scale investigations.
Most compounds described as active against MAYV were identified and initially tested using low-throughput screening methods. The instrumentation needed for medium and high-throughput assays can be a limiting factor contributing to the prevalence of low-throughput screenings in MAYV studies. HTS has been used to screen and select potential compounds against CHIKV and VEEV [Citation50]. To our knowledge, HTS and MTS assays have yet to be developed in the search for antiviral compounds against MAYV. Moreover, while most antiviral compound screening studies tend to focus on virus-targeted compounds, screening technologies also allow the search for antiviral compounds targeting host proteins.
Still from the perspective of drug discovery, screening assays can vary in method and strategy, including in silico, biochemical, or phenotypic methods. In silico screening methods are necessarily target-based and allow the identification of potential drug candidates through the computational simulation of compound-protein interactions at the molecular level. An in silico screening campaign is typically initiated by the selection of a validated target protein associated with a key process in the viral replication cycle resulting in antiviral activity, and for which reliable high-resolution structural data must be available. Recently developed artificial intelligence (AI)-based protein structure prediction tools such as Alphafold2 have been used to generate structures for viral proteins that could be used for in silico screening campaigns, but such a strategy still needs to be tested. Subsequently, at the expense of considerable processing power (often in Graphics processing unit – GPU) interactions of the target protein with up to hundreds of thousands of compounds from virtual compound libraries are modeled and ranked, often in multiple poses or configurations for a given protein-compound pair. Such an approach has led to the discovery of Navitoclax, a Bcl-2 inhibitor used for the treatment of hematological malignancies [Citation51], but not for antiviral compounds so far. The advantages of in silico screening are cost-effectiveness and a focus on specific targets and mechanisms. In contrast, in silico screening assays require rare human resources trained in both computer and biological sciences and carry the highest rates of false-positive hit compounds in confirmatory testing [Citation52]. In silico screening campaigns are typically faster than biochemical or phenotypical assays (described below), but screening speed is determined by available processing power in GPU servers that can be expensive and difficult to maintain. The COVID-19 pandemic motivated several in silico screening campaigns aimed at the identification of antiviral compounds against SARS-CoV-2, with many false positive results [Citation53]. Nevertheless, computer-aided drug discovery has evolved significantly in recent years due to eased access to new virtual compound libraries with small molecules with drug-like properties, extensive databases on ligand structure and, chemical and pharmacological properties, binding affinities with known ligands, and 3D structural data for both protein targets and protein-inhibitor complexes [Citation54,Citation55].
Biochemical screening methods for antiviral compounds are also necessarily target-based and often focused on viral proteins. Since a biochemical screening assay is typically enzymatic, the assay is mechanistic by essence, e.g. by searching for inhibitors against the protease activity of alphavirus nsP2. By overcoming the challenge of seeking out a mechanism of action, such assays allow rational and precise approaches to antiviral drug discovery and development. While subsequent confirmation and validation tests in vitro indicate an elevated rate of hit compound false positives, such difficulties are mostly associated with compound metabolism and impaired crossing of biological membranes and compartments, which can be optimized during drug development.
Biochemical screening methods have been successfully used for the discovery of viral polymerase and protease inhibitors, such as remdesivir for the treatment of COVID-19 [Citation56]. Another nucleoside analog, molnupiravir, was identified and tested using recombinant SARS-CoV-2 RdRp and synthetic RNA in a biochemical RNA elongation assay [Citation57]. Nirmatrelvir was identified as a main-protease (MPro) inhibitor, and a new generation of MPro inhibitors against SARS-CoV-2 variants has been tested in biochemical assays, including the screening of 30,000 compounds using fluorescence resonance energy transfer (FRET) assay by Huang and collaborators [Citation58,Citation59].
Phenotypic screening methods are fundamental in the field of antiviral drug discovery and can complement in silico and biochemical studies. The use of cell culture systems allows a more comprehensive assessment of how potential drug candidates affect viral replication, which can be broken down into specific steps or assess the entire viral replication cycle within host cells [Citation60]. A key advantage of phenotypical screening campaigns is that antiviral activity can be assessed even when mechanistic or structural data are missing [Citation61,Citation62]. Within phenotypic screening campaigns, several types of assays can be employed: Reporter assays, whether based on replicons or recombinant viruses, are invaluable for their ability to introduce reporter genes that signal viral replication [Citation63,Citation64]. The establishment of a Hepatitis C virus (HCV) subgenomic replicon in Huh-7 cells was an important milestone for anti-HCV drugs [Citation65]. Cytopathic effect (CPE)-based assays take advantage of virus-induced CPE, which are signs of viral infection (and replication) observed with light or fluorescence microscopy in cell cultures. Monitoring CPE offers an insightful evaluation of the mode-of-action antiviral candidates on both viral replication and cells. Viral load-based assays are based on the sheer reduction of the viral load in a sample and may include quantitative reverse transcription polymerase chain reaction (RT-qPCR), tissue culture infectious dose (TCID50), or plaque-forming units (PFU) as possible readouts. These assays are not only fundamental in phenotypic screening but also frequently used as orthogonal assays for compound validation in low-throughput settings [Citation66,Citation67]. Immunofluorescence microscopy-based assays provide a visual perspective on the location and abundance of viral proteins within infected cells depending on the use of antibody labeling. These assays yield valuable insights into the effect of candidate drugs on viral protein expression, distribution, and localization, though commercialized options for antibodies against viral proteins are still lacking for viruses such as MAYV.
High-content screening (HCS) is a phenotypic assay in which cells are incubated with compounds and/or viruses, and by using various biochemical staining dyes, it is possible to assess multiple cell components simultaneously, such as cytoplasm, nucleus, and organelles, resulting in extensive data points per cell [Citation68]. This analysis often includes the fluorescent labeling of host and viral proteins, allowing automated image analysis to measure changes in cell phenotype and infection, and to distinguish infected from non-infected cells. Despite the need for data processing programs, this type of assay provides valuable information and allows for the reliable identification of antiviral compounds [Citation68,Citation69].
HCS assays require a significant understanding of virus biology in assay design and have contributed to the discovery and development of milestone treatments against Influenza (oseltamivir), HCV (Ribavirin and Sofosbuvir), and human immunodeficiency virus (HIV) (Tenofovir), a reverse transcriptase inhibitor widely used to treat AIDS [Citation70]. The main challenges of phenotypic screening are the high costs, due to maintaining and scaling up cell cultures for screening campaigns and the lack of information on molecular targets or mode-of-action behind identified hit compounds. In summary, while phenotypic screening methods present high hit validation rates, the substantial cost and lack of mechanistic insight remain inherent drawbacks in the antiviral drug discovery process.
The development of complementary tools such as infectious clones, purified target proteins, generation of reliable structural data, and the support of a team of medicinal chemists are key for continued success after an initial compound screening campaign, as shown for the development of treatments against other viral pathogens.
2.3. Antiviral compounds against MAYV infection
Antivirals play a crucial role in the management and control of viral infections. However, when it comes to MAYV, there is a lack of specific antiviral therapies due to the limited understanding of this virus. Nevertheless, research has identified a limited number of compounds with potential antiviral activity against MAYV, providing a foundation for further investigation and development of targeted therapies [Citation41].
Among these compounds described in the literature with anti-MAYV activity, in a recent study by Shini et al. found that lactoferrin has potent antiviral activity against MAYV by preventing viral entry into the cell [Citation71]. Cyclic ketones effectively reduced MAYV viral production [Citation72] and Quercetin, a flavonoid, also showed anti-MAYV activity and displayed better selectivity and potency compared to ribavirin [Citation73]. Thieno2,3-bpyridine derivatives were found to reduce viral production effectively at nontoxic concentrations [Citation74]. These studies highlight the potential of compounds in inhibiting MAYV replication and provide avenues for the development of antiviral strategies against MAYV. Additionally, repurposed drugs such as β-D-N4-hydroxycytidine (EIDD-1931), favipiravir, and suramin were identified as inhibitors of MAYV. Langendries et al. evaluated the activity of 12 compounds with described alphavirus activity, and only Favipiravir, Suramin, and EIDD-1931, which showed EC50 values of 79 μM, 124 μM, and 1.6 μM, respectively [Citation62].
Favipiravir is among the few compounds described in the literature that have been subjected to in vivo testing against MAYV [Citation75–77] (). Favipiravir has been approved for therapeutic use in Japan since 2014 for resistant cases of influenza and was selected through a high throughput screening (30,000 compounds) process based on plaque reduction assay [Citation78]. Favipiravir exhibits antiviral activity against several viruses, including alphaviruses, by affecting the viral RNA polymerase activity and inducing lethal mutagenesis [Citation79,Citation80]. Langendries et al. selected Favipiravir by measuring the inhibition of CPE in Vero cells using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium inner salt and phenazine methosulfate method. The study showed that Favipiravir inhibited the stages of viral replication related to RNA synthesis, specifically targeting nsP4 and promoting lethal mutagenesis as a possible mechanism of action [Citation62]. A subsequent study by Bengue et al. [Citation76] confirmed the antiviral activity of Favipiravir in Vero, BHK-21, and primary fibroblasts. Subsequently, an in vivo study using MAYV-infected C57BL/6 mice showed that Favipiravir was effective when administered before or simultaneously with the infection, resulting in reduced viral replication. However, in this model, once the infection was established, Favipiravir was unable to eliminate it and the protective effect was lost [Citation76].
Table 1. Animal models of MAYV infection.
Silymarin (the active ingredient in milk thistle) is the other compound tested in vivo against MAYV (). Silymarin has been widely described in the literature for various activities, including antioxidant [Citation81] and antiviral [Citation82]. Camini and colleagues found that silymarin could reduce reactive oxygen species (ROS) and oxidative stress caused by MAYV infection. Using HepG2 cells, the antiviral activity was evaluated through inhibition of CPE, resulting in an established EC50 value of 3.58 µg/mL. Treatment with Silymarin at 25 µg/mL reduced MAYV replication as well as a decrease in oxidative stress biomarkers malondialdehyde (MDA) and carbonyl protein, thus validating its antiviral activity [Citation83]. Ferraz and colleagues, showed that Silymarin reduced the viral load in the liver, spleen, footpad, thigh muscle, and brain of mice treated with silymarin (MAYV + SIL) compared to to the control group (MAYV only) [Citation77]. Despite promising results, the Food and Drug Administration (FDA) does not approve or recommend milk thistle as a treatment for any medical condition which indicates that further studies on silymarin toxicity and safety are necessary before its antiviral activity against MAYV can be further explored [Citation84].
2.4. Host strategies against MAYV infection and disease
While infection is a direct consequence of exposure to a pathogen, the disease may not derive solely from infection, especially when considering viral infections [Citation85]. A viral disease may also be a consequence of an excessive, uncontrolled, or misplaced inflammatory response that is not necessarily involved with controlling the viral pathogen [Citation86]. Conversely, antiviral effector mechanisms are not always coupled to pro-inflammatory responses [Citation87].
MF has an important immunopathogenic component that may provide new therapeutic strategies against the disease (). Replication of MAYV and other alphaviruses in infected cells leads to the formation of double-stranded vRNA intermediates that are potent inducers of type I interferon (IFN) responses [Citation88,Citation89], which ultimately restrict MAYV replication. The protective role of type I IFN responses in MAYV infection has been supported by in vivo studies using type I IFN receptor-deficient mice (IFNAR−/−), which are remarkably susceptible to disease [Citation3,Citation75]. Infection of adult IFNAR−/− mice with different strains of MAYV invariably results in systemic infection, with elevated viral loads in multiple tissues, which accelerates disease development (inflammation, tissue damage) and leads to death in a few days, even when inoculated with as little as 1 PFU per mouse. IFN-α expression has been observed in CHIKV-infected patients during the acute phase of infection [Citation90]. Possible expression of type I interferon in MF patients during the acute phase of infection, may represent an opportunity to reduce viremia levels in patients or to minimize disease progression into a chronic stage.
Other components of the innate immune response also participate in the early phases of MAYV infection and disease development. Castro-Jorge and colleagues [Citation91] showed that MAYV infection induces the expression and activation of the NLRP3 inflammasome in murine macrophages. MAYV infection in inflammasome-deficient mice () developed less footpad swelling, reduced expression of proinflammatory mediators, and leukocyte recruitment and pain, establishing a role of the NLRP3 inflammasome in MF pathogenesis. Moreover, corroborating previous evidence with related arthritogenic alphaviruses, authors reported higher levels of caspase1-p20, Interleukin (IL)-1β and IL-18 in the serum of MAYV-infected patients, thus supporting the participation of the NLRP3-inflammasome during MAYV infection in humans [Citation91]. Overall, these findings indicate that suppression of NLRP3 inflammasome activity in acute MF and MAYV-induced chronic arthralgia may be of benefit to patients, due to the reduction of pro-inflammatory cytokines.
Tissue-resident and monocyte-derived macrophages are considered target cells for MAYV infection and elevated expression of the chemoattractant CCL2, indicates that these leukocyte populations play a central role in Mayaro disease pathogenesis [Citation49,Citation92] Studies by Haist and colleagues showed that inflammatory CCR2+ monocytes are infected by RRV and CHIKV viruses, and that activation of type I IFN responses in this leukocyte population is important for control of the infection in vivo [Citation93]. Abrogation of CCR2 expression in mice resulted in an aggravated disease phenotype in a model of CHIKV infection, without affecting viral load in tissues [Citation94]. Conversely, treatment with bindarit, an inhibitor of CCL2 expression, prevented bone loss in experimental CHIKV infection [Citation95]. For MAYV infection, a preliminary study indicated that the CCR2-CCL2 axis is important for disease development, as CCR2 deficiency, silencing, or pharmacological blockade of CCR2 resulted in disease amelioration in mice [Citation96]. Similar examples were reported for the mediators MIF and IL-17 in experimental infections with RRV in mice, where blockade of MIF or the IL-17 receptor resulted in the reduction of myositis and arthralgia [Citation97,Citation98]
Neutrophils have also been implicated in the early response to MAYV infection, being recruited to MAYV-infected tissues in different in vivo models [Citation3,Citation75,Citation99]. The association between the expression of cytokines, such as CXCL1 and IL-1β, production of ROS and tissue damage in muscle and joints following the recruitment of neutrophils, suggests that neutrophils play a major role in disease development. However, Hiroki and collaborators reported that CHIKV induces the release of neutrophil extracellular traps (NETs) by human and mouse neutrophils and that Neutrophil Extracellular Traps (NETs) had a virucidal effect on CHIKV in vitro [Citation100]. Degradation of NETs in IFNAR−/− mice using DNAse resulted in increased susceptibility to infection, indicating that an effector mechanism by neutrophils was important for CHIKV neutralization. Although the role of NETs has not been investigated for other alphaviruses, it is likely that NETs may also neutralize MAYV and related alphaviruses and exert a protective role in vivo.
Regarding adaptive immune responses, T lymphocytes have been implicated in the pathogenesis of MAYV by contributing to clearance of MAYV from tissues [Citation75]. Although the role of T lymphocyte effector roles and memory in MAYV infection are largely unknown, the protective role of neutralizing antibody responses is straightforward and is based on functional and mechanistic evidence that has been elucidated for MAYV to some extent. Earnest and colleagues generated 18 neutralizing monoclonal antibodies against MAYV, some highly neutralizing at low nanomolar concentrations [Citation43]. Interestingly, in vivo experiments indicated that the most protective anti-MAYV antibodies were not the most potent neutralizing antibodies, but rather IgG2a subclass antibodies with moderate neutralizing activity in vitro. Protection from experimental MAYV infection in mice provided by monoclonal IgG2a antibodies MAY-115 and MAY-134 was associated with Fc effector functions and to the recruitment of neutrophils and monocytes to MAYV-infected sites. Importantly, the epitopes eliciting neutralizing activities were mapped to solvent-exposed domains in MAYV E1 and E2, whose structures were recently elucidated together with the MAYV particle [Citation16]. The structural elucidation of the MAYV virion allowed the identification and mapping of both MAYV-specific and alphavirus-conserved portions of the viral surface glycoproteins. This allowed for the elucidation of the mechanism of action of neutralizing antibodies, whose binding blocks the interaction of the cellular Mxra8 receptor with MAYV E2, as similarly demonstrated for CHIKV [Citation24].
If the search for MAYV antivirals is still in its infancy, the evidence for drugs that can modify or modulate the clinical aspects of the disease is even [Citation41]. Given the similarities between Mayaro and chikungunya arthritic disease [Citation101], we can speculate treatments that may be efficacious for CHIKV, especially anti-inflammatory and/or immunosuppressive drugs, and/or disease-modifying antirheumatic drugs could also be used to treat MAYV-induced arthritis [Citation102]. Although no clinical trials have been conducted, non-steroidal anti-inflammatory compounds, steroids, hydroxychloroquine and chloroquine, methotrexate, and immunobiological agents have shown promise in patients [Citation101–105]. One of the most controversial, yet effective, options for CHIKV is the use of anti-tumor necrosis factor (TNF) drugs. TNF-α is induced during CHIKV infection and has an antiviral effect yet plays a decisive role in disease resolution and pathogenesis [Citation106–108]. Mice infected with RRV (a closely related arthritogenic alphavirus), that received anti-TNF drug etanercept showed exacerbated disease and higher mortality than similarly infected untreated mice [Citation109]. On the other hand, in patients with chronic CHIKV-induced arthritis treatment with a TNF antagonist is recommended in line with treatment guidelines in Brazil [Citation110].
3. Conclusion
MAYV biology is not fully understood and possible antiviral or therapeutic strategies against MAYV infection and disease are likely being overlooked. A few bioactive compounds with antiviral activity against MAYV have been identified (silymarin, EIDD-1931, suramin, favipiravir), along with immunopathogenic mechanisms that could be targeted in the development of treatments against the disease (inflammasome, neutralizing antibodies, monocyte/macrophage recruitment and function). The use of cutting-edge compound screening technologies, structural biology, and the development of improved pre-clinical animal models for MAYV infection should facilitate and accelerate the development of treatments against MF. New viral and host molecular targets need to be identified, characterized, and validated so that a specific treatment against MAYV can become a reality in the following years.
4. Expert opinion
The accumulated evidence in the field of MAYV research indicates that the development of treatments against MAYV infection and MF is possible. A handful of antiviral compounds against MAYV infection have been identified together with immunopathogenic host mechanisms that can be targeted for the amelioration of disease. Although promising, a handful of bioactive molecules and host-targeted mechanisms are not sufficient for the successful development of an effective antiviral intervention in the long term. The discovery and development of treatments against MAYV have been limited by a lack of funding and initiatives originating in both academia and industry, which is typical for neglected infectious diseases. As such, significant advances in the identification and characterization of bioactive compounds can be achieved by drug repurposing, for which expensive and painstaking pharmacological optimization and clinical tests may have already been performed, and at least one mechanism of action may already be identified. Due to the obvious advantages in the use of time and resources, all possible options for drug repurposing should be exhausted before moving on to the discovery of new molecules, either in search for anti-MAYV or disease-modulating compounds, and then follow the path of classical drug discovery and development.
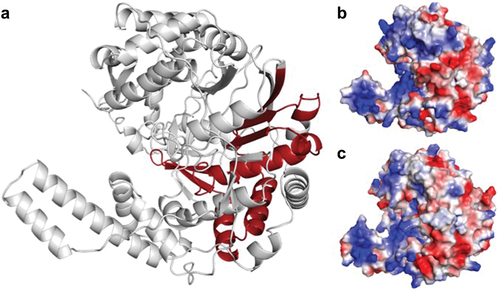
Regrettably, research leading to the identification of antiviral compounds and immunopathogenic host mechanisms has not progressed to date into preclinical studies. In our opinion, the lack of large-scale MAYV epidemics, the overall low number of confirmed human cases, and the underappreciated socioeconomic impact of MAYV in populations at risk have held back drug discovery. Importantly, our limited understanding of MAYV biology and disease pathogenesis is a major factor behind unsuccessful drug discovery so far. MAYV biology is largely unknown, and inferences from extensively studied related alphaviruses have been applied to MAYV. Unfortunately, inferences may not be precise enough or sufficient to elucidate MAYV biology, as illustrated in a comparison of predicted structures of MAYV and CHIKV nsP4 (). Such observation is reflected by the few studies available that focus on target proteins or mechanisms in MAYV infection. Sustainable research on MAYV is needed to identify, validate, characterize molecular targets, and define whether these targets are specific for MAYV or broad-acting against other alphaviruses.
Figure 3. Structural comparison between CHIKV and MAYV nsP4. (a) Predicted MAYV nsP4 structure with the RNA-dependent RNA polymerase domain in dark red, with predominant alpha-helices and loops. The overall structure of MAYV nsP4 is similar to CHIKV nsP4 (RMSD: 0.609). (b) MAYV and (c) CHIKV nsP4 predicted structures represented as volumes with surface charge distribution, (positive charges – blue, negative charges – light red). MAYV and CHIKV nsP4s have substantial differences in protein shape, volume, and charge distribution that might indicate different interacting partners and biological functions.
The use of cutting-edge technologies may provide the foundation for effective antiviral targets and countermeasures against MAYV. Such technologies should accelerate the development of MAYV treatments at different stages ranging from drug discovery to development and clinical testing. The use of algorithms for the prediction of protein structure and protein-protein interactions, such as Alphafold2 and Robetta, are powerful and accessible tools for the design and preliminary testing of hypotheses aimed at drug discovery and diagnosis. Cryo-electron microscopy (Cryo-EM) and Cryo-electron tomography (Cryo-ET) have revolutionized the field of structural biology and have been used for the elucidation of multiple structures of alphaviral proteins, alphavirus replication complexes, and alphavirus particles, including the MAYV mature particle [Citation16]. Such technologies provide high-resolution data on viral protein structure and organization, enzymatic activities, and protein-protein interactions that are essential to virus infection and/or replication and should facilitate the identification and characterization of new molecular targets for drug development and diagnostic methods and vaccine design. High-throughput screening assays based on artificial intelligence and machine learning, can accelerate the rate of discovery of bioactive compounds against MAYV, by expanding the sheer number of tested compounds and expanding chemical space. MAYV drug discovery initiatives would benefit from combining the strengths of target-based biochemical assays, which are inherently associated with a mechanism of action, and cell culture-based high content phenotypic assays, which involves biological activity in a living cell.
Animal models of MAYV infection and disease have been established solely on rodents and represent a bottleneck for our understanding of MAYV biology and pathogenicity (). The development of improved animal models is necessary, and models must allow the study of critical aspects, such as acute muscle and joint pain and the evolution from acute to chronic disease. Moreover, the use of imaging technologies such as intravital microscopy and synchrotron light-based techniques, such as X-ray microtomography should bring new insights to our understanding of disease pathogenesis, by allowing the visualization of inflammation in the tissues of living model organisms and allowing the 3D visualization of soft and hard tissues at cellular resolution, respectively. Should the solution require hosts other than rodents, e.g. non-human primates (NHPs), which are far more expensive and difficult to manage, the use of noninvasive X-ray tomography also allows 3D imaging of tissues in living hosts without injection of contrasting agents or surgery, thus minimizing animal stress and the number of individuals included in the experiment. Finally, although MAYV is known to be endemic in Latin America, the actual number of people affected by or at risk of MAYV infection remains unknown and estimates of the burden of MAYV disease are considered speculative at best. A low-cost and easy to use point-of-care diagnostic test would be a first critical step in understanding the true burden of the disease and especially in populations living in remote areas. Moreover, combination with existing sequencing and data management technologies should allow improved real-time monitoring of MAYV circulation.
An antiviral treatment is a necessary tool for the treatment of MAYV infections. Besides, therapeutic strategies targeting pathogenic inflammatory responses show greater potential for the treatment of disease, and reducing MAYV-induced morbidity in acute and chronic patients is key. Continuous exploration of host-targeted immunopathogenic mechanisms using in vivo infection models is essential for the discovery of strategies to prevent or reduce disease, and thus, reduce the socioeconomic burden of MAYV.
Article highlights
Mayaro virus is an emerging arbovirus associated with arthritogenic disease in humans.
There are no antiviral compounds or specific treatments available against Mayaro fever.
Anti-MAYV compounds have been discovered but never advanced to preclinical drug development or clinical testing.
Mechanistic insights and target validation are lacking in drug discovery against MAYV, the use of cutting-edge technologies is desirable.
Structural and biological differences between MAYV and other alphaviruses suggest specificity and indicate that more research on MAYV is necessary.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We would like to thank Eduardo H. Salviano Bezerra for his assistance with preparation.
Additional information
Funding
References
- Hotez PJ, Murray KO, Gubler DJ, et al. Dengue, West Nile virus, chikungunya, Zika—and now Mayaro? PLOS Negl Trop Dis. 2017;11(8):e0005462. doi: 10.1371/journal.pntd.0005462
- Diagne CT, Bengue M, Choumet V, et al. Mayaro virus pathogenesis and transmission mechanisms. Pathogens. 2020;9(9):738. doi: 10.3390/pathogens9090738
- de Carvalho AC, Dias CSB, Coimbra LD, et al. Characterization of systemic disease development and paw inflammation in a susceptible mouse model of mayaro virus infection and validation using X-ray synchrotron microtomography. Int J Mol Sci. 2023;24(5):4799. doi: 10.3390/ijms24054799
- Zaid A, Burt FJ, Liu X, et al. Arthritogenic alphaviruses: epidemiological and clinical perspective on emerging arboviruses. Lancet Infect Dis. 2021;21(5):e123–e133. doi: 10.1016/S1473-3099(20)30491-6
- Coimbra TLM, Santos CLS, Suzuki A, et al. Mayaro virus: imported cases of human infection in São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49(4):221–224. doi: 10.1590/S0036-46652007000400005
- de Paula Silveira-Lacerda E, Laschuk Herlinger A, Tanuri A, et al. Molecular epidemiological investigation of mayaro virus in febrile patients from Goiania City, 2017–2018. Infect Genet Evol. 2021;95:104981. doi: 10.1016/j.meegid.2021.104981
- Kazanji M, Bourreau E, Talarmin A, et al. Mayaro virus fever in French Guiana: isolation, identification, and seroprevalence. Am J Trop Med Hyg. 1998;59(3):452–456. doi: 10.4269/ajtmh.1998.59.452
- Torres JR, Russell KL, Vasquez C, et al. Family cluster of mayaro fever, Venezuela. Emerg Infect Dis. 2004;10(7):1304–1306. doi: 10.3201/eid1007.030860
- Mourão MPG, Bastos MDS, de Figueiredo RP, et al. Mayaro fever in the city of Manaus, Brazil, 2007–2008. Vector-Borne And Zoonotic Diseases. 2012;12(1):42–46. doi: 10.1089/vbz.2011.0669
- Forshey BM, Guevara C, Laguna-Torres VA, et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLOS Negl Trop Dis. 2010;4(8):e787. doi: 10.1371/journal.pntd.0000787
- Azevedo RSS, Silva EVP, Carvalho VL, et al. Mayaro fever virus, Brazilian Amazon. Emerg Infect Dis. 2009;15(11):1830–1832. doi: 10.3201/eid1511.090461
- Freitas RB, da Rosa JFT, LeDuc JW, et al. An outbreak of mayaro virus disease in Belterra, Brazil. Am J Trop Med Hyg [Internet]. 1981;30(3):674–681. doi: 10.4269/ajtmh.1981.30.674
- Alvis-Zakzuk NJ, Díaz-Jiménez D, Castillo-Rodríguez L, et al. Economic costs of Chikungunya virus in Colombia. Value Health Reg Issues. 2018;17:32–37. doi: 10.1016/j.vhri.2018.01.004
- de Margarette Oliveira de Andrade M, de Almeida Barreto FK, Coelho TMS, et al. Chikungunya in Brazil: an epidemic of high cost for private healthcare, 2017. Tropical Med Int Health. 2022;27(10):925–933. doi: 10.1111/tmi.13810
- Vidal ERN, Frutuoso LCV, Duarte EC, et al. Epidemiological burden of Chikungunya fever in Brazil, 2016 and 2017. Trop Med Int Health. 2022;27(2):174–184. doi: 10.1111/tmi.13711
- Ribeiro-Filho HV, Coimbra LD, Cassago A, et al. Cryo-EM structure of the mature and infective mayaro virus at 4.4 Å resolution reveals features of arthritogenic alphaviruses. Nat Commun. 2021;12(1):3038. doi: 10.1038/s41467-021-23400-9
- Pezzi L, Diallo M, Rosa-Freitas MG, et al. GloPID-R report on chikungunya, o’nyong-nyong and mayaro virus, part 5: entomological aspects. Antiviral Res. 2020;174:104670. doi: 10.1016/j.antiviral.2019.104670
- Caicedo E-Y, Charniga K, Rueda A, et al. The epidemiology of mayaro virus in the Americas: a systematic review and key parameter estimates for outbreak modelling. PLOS Negl Trop Dis. 2021;15(6):e0009418. doi: 10.1371/journal.pntd.0009418
- Causey OR, Laemmert HW, Kumm HW. Dispersion of forest mosquitoes in Brazil: further studies 1. Am J Trop Med Hyg. 1950;s1-30(2):301–312. doi: 10.4269/ajtmh.1950.s1-30.301
- Hendy A, Hernandez-Acosta E, Valério D, et al. Where boundaries become bridges: mosquito community composition, key vectors, and environmental associations at forest edges in the central Brazilian Amazon. PLOS Negl Trop Dis. 2023;17(4):e0011296. doi: 10.1371/journal.pntd.0011296
- Pereira TN, Carvalho FD, De Mendonça SF, et al. Vector competence of aedes aegypti, aedes albopictus, and Culex quinquefasciatus mosquitoes for mayaro virus. PLOS Negl Trop Dis. 2020;14(4):e0007518. doi: 10.1371/journal.pntd.0007518
- Cereghino C, Roesch F, Carrau L, et al. The E2 glycoprotein holds key residues for mayaro virus adaptation to the urban aedes aegypti mosquito. PLOS Pathog. 2023;19(4):e1010491. doi: 10.1371/journal.ppat.1010491
- Weise WJ, Hermance ME, Forrester N, et al. A novel live-attenuated vaccine Candidate for mayaro fever. PLOS Negl Trop Dis. 2014;8(8):e2969. doi: 10.1371/journal.pntd.0002969
- Zhang R, Kim AS, Fox JM, et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature. 2018;557(7706):570–574. doi: 10.1038/s41586-018-0121-3
- Mendonça DC, Reis E, Arias Nídia EC, et al. A study of the MAYV replication cycle: correlation between the kinetics of viral multiplication and viral morphogenesis. Virus Research. 2023;323:199002. doi: 10.1016/j.virusres.2022.199002
- Mi S, Durbin R, Huang HV, et al. Association of the sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989;170:385–391. doi: 10.1016/0042-6822(89)90429-7
- Ahola T. Semliki forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. Embo J. 1999;18(11):3164–3172. doi: 10.1093/emboj/18.11.3164
- Wang YF, Sawicki SG, Sawicki DL. Sindbis virus nsP1 functions in negative-strand RNA synthesis. J Virol. 1991;65(2):985–988. doi: 10.1128/jvi.65.2.985-988.1991
- Kaur R, Mudgal R, Narwal M, et al. Development of an ELISA assay for screening inhibitors against divalent metal ion dependent alphavirus capping enzyme. Virus Res. 2018;256:209–218. doi: 10.1016/j.virusres.2018.06.013
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994
- Abu Bakar F, Ng L. Nonstructural proteins of alphavirus—potential targets for drug development. Viruses. 2018;10(2):71. doi: 10.3390/v10020071
- Gao Y, Goonawardane N, Ward J, et al. Multiple roles of the non-structural protein 3 (nsP3) alphavirus unique domain (AUD) during Chikungunya virus genome replication and transcription. PLOS Pathog. 2019;15(1):e1007239. doi: 10.1371/journal.ppat.1007239
- Tossavainen H, Aitio O, Hellman M, et al. Structural basis of the high affinity interaction between the alphavirus nonstructural protein-3 (nsP3) and the SH3 domain of amphiphysin-2. J Biol Chem. 2016;291(31):16307–16317. doi: 10.1074/jbc.M116.732412
- Dominguez F, Shiliaev N, Lukash T, et al. NAP1L1 and NAP1L4 binding to hypervariable domain of Chikungunya virus nsP3 protein is bivalent and requires phosphorylation. J Virol. 2021;95(16). doi: 10.1128/JVI.00836-21
- Liljeström P, Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991;65(1):147–154. doi: 10.1128/jvi.65.1.147-154.1991
- Sefton BM. Immediate glycosylation of sindbis virus membrane proteins. Cell. 1977;10(4):659–668. doi: 10.1016/0092-8674(77)90099-X
- Watts DM, Russell KL, Wooster MT, et al. Etiologies of acute undifferentiated febrile illnesses in and near Iquitos from 1993 to 1999 in the Amazon River Basin of Peru. Am J Trop Med Hyg. 2022;107(5):1114–1128. doi: 10.4269/ajtmh.22-0259
- Tesh RB, Watts DM, Russell KL, et al. Mayaro virus disease: an emerging mosquito‐borne zoonosis in tropical South America. Clinical Infectious Diseases. 1999;28(1):67–73. doi: 10.1086/515070
- Anderson CR, Wattley GH, Ahin NW, et al. Mayaro virus: a new human disease agent. Am J Trop Med Hyg. 1957;6(6):1012–1016. doi: 10.4269/ajtmh.1957.6.1012
- Theilacker C, Held J, Allering L, et al. Prolonged polyarthralgia in a German traveller with mayaro virus infection without inflammatory correlates. BMC Infect Dis. 2013;13(1):369. doi: 10.1186/1471-2334-13-369
- Andreolla AP, Borges AA, Bordignon J, et al. Mayaro virus: the state-of-the-art for antiviral drug development. Viruses. 2022;14(8):1787. doi: 10.3390/v14081787
- Waggoner JJ, Rojas A, Mohamed-Hadley A, et al. Real-time RT-PCR for mayaro virus detection in plasma and urine. J Clin Virol. 2018;98:1–4. doi: 10.1016/j.jcv.2017.11.006
- Earnest JT, Basore K, Roy V, et al. Neutralizing antibodies against mayaro virus require fc effector functions for protective activity. J Exp Med. 2019;216(10):2282–2301. doi: 10.1084/jem.20190736
- Bartholomeeusen K, Daniel M, LaBeaud DA, et al. Chikungunya fever. Nat Rev Dis Primers. 2023;9(1):17. doi: 10.1038/s41572-023-00429-2
- Lim SP, Wang Q-Y, Noble CG, et al. Ten Years of dengue drug discovery: progress and prospects. Antiviral Res. 2013;100(2):500–519. doi: 10.1016/j.antiviral.2013.09.013
- Chan KKP, Hui DSC. Antiviral therapies for influenza. Curr Opin Infect Dis. 2023;36(2):124–131. doi: 10.1097/QCO.0000000000000910
- Rocco PRM, Silva PL, Cruz FF, et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58(1):2003725. doi: 10.1183/13993003.03725-2020
- Hoarau J-J, Jaffar Bandjee M-C, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust Host immune response. J Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255
- Labadie K, Larcher T, Joubert C, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Investig. 2010;120(3):894–906. doi: 10.1172/JCI40104
- Chung D-H, Jonsson CB, Tower NA, et al. Discovery of a novel compound with anti-venezuelan equine encephalitis virus activity that targets the nonstructural protein 2. PLOS Pathog. 2014;10(6):e1004213. doi: 10.1371/journal.ppat.1004213
- Sahin K, Orhan MD, Avsar T, et al. Hybrid in Silico and TR-FRET-Guided discovery of novel BCL-2 inhibitors. ACS Pharmacol Transl Sci. 2021;4(3):1111–1123. doi: 10.1021/acsptsci.0c00210
- Cerón‐Carrasco JP. When virtual screening yields inactive drugs: dealing with false theoretical friends. ChemMedchem. 2022;17(16):17. doi: 10.1002/cmdc.202200278
- Rossetti GG, Ossorio MA, Rempel S, et al. Non-covalent SARS-CoV-2 mpro inhibitors developed from in silico screen hits. Sci Rep. 2022;12(1):2505. doi: 10.1038/s41598-022-06306-4
- Gorgulla C, Boeszoermenyi A, Wang Z-F, et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature. 2020;580(7805):663–668. doi: 10.1038/s41586-020-2117-z
- Sadybekov AV, Katritch V. Computational approaches streamlining drug discovery. Nature. 2023;616(7958):673–685. doi: 10.1038/s41586-023-05905-z
- Kokic G, Hillen HS, Tegunov D, et al. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat Commun. 2021;12(1):279. doi: 10.1038/s41467-020-20542-0
- Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740–746. doi: 10.1038/s41594-021-00651-0
- Huang C, Shuai H, Qiao J, et al. A new generation mpro inhibitor with potent activity against SARS-CoV-2 omicron variants. Signal Transduct Target Ther. 2023;8(1):128. doi: 10.1038/s41392-023-01392-w
- Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19. Science (1979). 2021;374:1586–1593. Available from: https://www.science.org/doi/10.1126/science.abl4784
- Lohmann V. Hepatitis C virus cell culture models: an encomium on basic research paving the road to therapy development. Med Microbiol Immunol. 2019;208(1):3–24. doi: 10.1007/s00430-018-0566-x
- Sugasti-Salazar M, Llamas-González YY, Campos D, et al. Inhibition of p38 mitogen-activated protein kinase impairs mayaro virus replication in human dermal fibroblasts and HeLa cells. Viruses. 2021;13(6):1156. doi: 10.3390/v13061156
- Langendries L, Abdelnabi R, Neyts J, et al. Repurposing drugs for mayaro virus: identification of eidd-1931, favipiravir and suramin as mayaro virus inhibitors. Microorganisms. 2021;9(4):734. doi: 10.3390/microorganisms9040734
- Remenyi R, Gao Y, Hughes RE, et al. Persistent replication of a Chikungunya virus replicon in human cells is associated with presence of stable cytoplasmic granules containing non-structural protein 3. J Virol. 2018;92(16):JVI.00477–18. Available from: http://jvi.asm.org/lookup/doi/10.1128/JVI.00477-18
- Pohjala L, Utt A, Varjak M, et al. Inhibitors of alphavirus entry and replication identified with a stable chikungunya replicon cell line and virus-based assays. PLOS One. 2011 [cited 2015 Nov 23];6(12):e28923. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0028923
- Lohmann V, Körner F, Koch J-O, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science (1979). 1999;285(5424):110–113. doi: 10.1126/science.285.5424.110
- Leake CJ, Varma MGR, Pudney M. Cytopathic effect and plaque formation by arboviruses in a continuous cell line (XTC-2) from the toad xenopus laevis. J Gen Virol. 1977;35(2):335–339. doi: 10.1099/0022-1317-35-2-335
- Doms RW. Chapter 3 - basic concepts: a step-by-step Guide to viral infection. In: Katze M, Korth M Law G, editors. Viral Pathogenesis. Third ed. Boston: Academic Press; 2016. p. p. 29–40. Available from: https://www.sciencedirect.com/science/article/pii/B9780128009642000033
- Rietdijk J, Tampere M, Pettke A, et al. A phenomics approach for antiviral drug discovery. BMC Biol. 2021;19(1):156. doi: 10.1186/s12915-021-01086-1
- Cimini BA, Chandrasekaran SN, Kost-Alimova M, et al. Optimizing the cell painting assay for image-based profiling. Nat Protoc. 2023;18(7):1981–2013. doi: 10.1038/s41596-023-00840-9
- Richman DD, Nathanson N. Chapter 20 - antiviral therapy. In: Katze M, Korth M Law G, editors. Viral pathogenesis. Third ed. Boston: Academic Press; 2016. p. 271–287. Available from: https://www.sciencedirect.com/science/article/pii/B9780128009642000203
- Shini VS, Udayarajan CT, Nisha P. A comprehensive review on lactoferrin: a natural multifunctional glycoprotein. Food Funct. 2022;13(23):11954–11972. doi: 10.1039/D2FO02371G
- Fernandes LDS, Silva MD, Dias RS, et al. Evaluation of antiviral activity of cyclic ketones against mayaro virus. Viruses. 2021;13(11):2123. doi: 10.3390/v13112123
- dos Santos AE, Kuster RM, Yamamoto KA, et al. Quercetin and quercetin 3-O-glycosides from bauhinia longifolia (Bong.) Steud. show anti-mayaro virus activity. Parasites Vectors. 2014;7(1):130. doi: 10.1186/1756-3305-7-130
- Amorim R, de Meneses MDF, Borges JC, et al. Thieno[2,3-b]pyridine derivatives: a new class of antiviral drugs against mayaro virus. Arch Virol. 2017;162(6):1577–1587. doi: 10.1007/s00705-017-3261-0
- Figueiredo CM, Neris RDS, Gavino-Leopoldino D, et al. Mayaro virus replication restriction and induction of muscular inflammation in mice are dependent on age, type-I interferon response, and adaptive immunity. Front Microbiol. 2019;10:1–11. doi: 10.3389/fmicb.2019.02246
- Bengue M, Pintong AR, Liegeois F, et al. Favipiravir inhibits mayaro virus infection in mice. Viruses. 2021;13(11):2213–2217. doi: 10.3390/v13112213
- Ferraz AC, Almeida LT, da Silva Caetano CC, et al. Hepatoprotective, antioxidant, anti-inflammatory, and antiviral activities of silymarin against mayaro virus infection. Antiviral Res. 2021;194:105168. doi: 10.1016/j.antiviral.2021.105168
- Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005
- Franco EJ, Cella E, Tao X, et al. Favipiravir suppresses Zika Virus (ZIKV) through activity as a mutagen. Microorganisms. 2023;11(5):1342. doi: 10.3390/microorganisms11051342
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. Elsevier Inc.; 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512
- Köksal E, Gülçin İ, Beyza S, et al. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem. 2009;24(2):395–405. doi: 10.1080/14756360802188081
- Low ZX, OuYong BM, Hassandarvish P, et al. Antiviral activity of silymarin and baicalein against dengue virus. Sci Rep. 2021;11(1). doi: 10.1038/s41598-021-98949-y
- Camini FC, Silva TD, Caetano CDS, et al. Antiviral activity of silymarin against mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Research. 2018;158:8–12. doi: 10.1016/j.antiviral.2018.07.023
- Milk Thistle. StatPearls. NCBI Bookshelf [Internet]; [cited 2023 Aug 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541075/
- Marques RE, Marques PE, Guabiraba R, et al. Exploring the Homeostatic and sensory roles of the immune system. Front Immunol. 2016;7. doi: 10.3389/fimmu.2016.00125
- Rocha RF, Del Sarto JL, Marques RE, et al. Host target-based approaches against arboviral diseases. Biol Chem. 2018;399(3):203–217. doi: 10.1515/hsz-2017-0236
- Stoermer KA, Burrack A, Oko L, et al. Genetic ablation of Arginase 1 in macrophages and neutrophils enhances clearance of an Arthritogenic Alphavirus. J Immunol. 2012;189(8):4047–4059. doi: 10.4049/jimmunol.1201240
- Bae S, Lee JY, Myoung J. Chikungunya virus nsP2 impairs MDA5/RIG-I-Mediated induction of NF-κB promoter activation: a potential target for virus-specific therapeutics. J Microbiol Biotechnol. 2020;30(12):1801–1809. doi: 10.4014/jmb.2012.12005
- Hyde JL, Gardner CL, Kimura T, et al. A viral RNA structural element alters Host recognition of nonself RNA. Science (1979). 2014;343:783–787. doi: 10.1126/science.1248465
- Wauquier N, Becquart P, Nkoghe D, et al. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204(1):115–123. doi: 10.1093/infdis/jiq006
- De Castro-Jorge LA, De Carvalho RVH, Klein TM, et al. The NLRP3 inflammasome is involved with the pathogenesis of mayaro virus. PLOS Pathog. 2019;15(9):15. doi: 10.1371/journal.ppat.1007934
- Her Z, Malleret B, Chan M, et al. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184(10):5903–5913. doi: 10.4049/jimmunol.0904181
- Haist KC, Burrack KS, Davenport BJ, et al. Inflammatory monocytes mediate control of acute alphavirus infection in mice. PLOS Pathog. 2017;13(12):e1006748. doi: 10.1371/journal.ppat.1006748
- Poo YS, Nakaya H, Gardner J, et al. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol. 2014;88(12):6862–6872. doi: 10.1128/JVI.03364-13
- Chen W, Foo S-S, Taylor A, et al. Bindarit, an Inhibitor of Monocyte Chemotactic Protein Synthesis, protects against bone loss induced by Chikungunya virus infection. J Virol. 2015;89(1):581–593. doi: 10.1128/JVI.02034-14
- Santos FM, Costa VDM, Araújo SD, Heise MT, et al. Essential role of the CCL2–CCR2 axis in Mayaro virus-induced disease. Heise MT, editor. J Virol. 2024 [cited 2024 Feb 20];98. Available from 1). doi: 10.1128/jvi.01102-23
- Mostafavi H, Tharmarajah K, Vider J, et al. Interleukin-17 contributes to Ross River virus-induced arthritis and myositis. PLOS Pathog. 2022;18(2):e1010185. doi: 10.1371/journal.ppat.1010185
- Herrero LJ, Nelson M, Srikiatkhachorn A, et al. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. In: Proceedings of the National Academy of Sciences; Washington, DC; 2011. p. 12048–12053;108.
- Mota MDO, Costa VV, Sugimoto MA, et al. In-depth characterization of a novel live-attenuated mayaro virus vaccine candidate using an immunocompetent mouse model of mayaro disease. Sci Rep. 2020;10(1):5306. doi: 10.1038/s41598-020-62084-x
- Hiroki CH, Toller-Kawahisa JE, Fumagalli MJ, et al. Neutrophil extracellular traps effectively control acute chikungunya virus infection. Front Immunol. 2020;10:10. doi: 10.3389/fimmu.2019.03108
- Kumar R, Ahmed S, Parray HA, et al. Chikungunya and arthritis: an overview. Travel Med Infect Dis. 2021;44:102168. doi: 10.1016/j.tmaid.2021.102168
- Amaral JK, Bingham CO, Taylor PC, et al. Therapy for Chikungunya arthritis: a study of 133 Brazilian patients. Am J Trop Med Hyg. 2023;109(3):542–547. doi: 10.4269/ajtmh.23-0205
- Sales GMPG, Barbosa ICP, Canejo Neta LMS, et al. Treatment of chikungunya chronic arthritis: a systematic review. Rev Assoc Med Bras. 2018;64(1):63–70. doi: 10.1590/1806-9282.64.01.63
- Amaral J, Taylor P, Teixeira M, et al. The clinical features, Pathogenesis and methotrexate therapy of chronic chikungunya arthritis. Viruses. 2019;11(3):289. doi: 10.3390/v11030289
- Amaral JK, Bingham CO, Schoen RT. Successful methotrexate treatment of chronic chikungunya arthritis. JCR: J Clinic Rheumatol. 2020;26(3):119–124. doi: 10.1097/RHU.0000000000000943
- Teng T-S, Kam Y-W, Lee B, et al. A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J Infect Dis. 2015;211(12):1925–1935. doi: 10.1093/infdis/jiv049
- Wilson JAC, Prow NA, Schroder WA, et al. RNA-Seq analysis of chikungunya virus infection and identification of granzyme a as a major promoter of arthritic inflammation. PLOS Pathog. 2017;13(2):e1006155. doi: 10.1371/journal.ppat.1006155
- Moreira TP, Sousa CD, Melo Costa VD, et al. Tumour necrosis factor plays a deleterious role in the pathogenesis of chikungunya virus infection. Immunology. 2023;168(3):444–458. doi: 10.1111/imm.13583
- Zaid A, Rulli NE, Rolph MS, et al. Disease exacerbation by etanercept in a mouse model of alphaviral arthritis and myositis. Arthritis Rheum. 2011;63(2):488–491. doi: 10.1002/art.30112
- Marques CDL, Duarte ALBP, Ranzolin A, et al. Recommendations of the Brazilian Society of Rheumatology for the diagnosis and treatment of chikungunya fever. Part 2 – treatment. Rev Bras Reumatologia (English Edition). 2017;57:438–451. doi: 10.1016/j.rbre.2017.06.004

