ABSTRACT
Introduction
CLEC10A is a C-type lectin receptor that specifically marks the conventional dendritic cell subsets two and three (cDC2 and DC3). It has a unique recognition profile of glycan antigens, with terminal N-Acetylgalactosamine residues that are frequently present in the tumor microenvironment. Even though CLEC10A expression allows for precise targeting of cDC2 and DC3 for the treatment of cancer, CLEC10A signaling has also been associated with anti-inflammatory responses that would promote tumor growth.
Areas covered
Here, we review the potential benefits and drawbacks of CLEC10A engagement in the tumor microenvironment. We discuss the CLEC10A-mediated effects in different cell types and incorporate the pleiotropic effects of IL-10, the main anti-inflammatory response upon CLEC10A binding.
Expert opinion
To translate this to a successful CLEC10A-mediated immunotherapy with limited tumor-promoting capacities, finding the right ligand presentation and adjuvant combination will be key.
1. Introduction
The cell surface of both prokaryotic and eukaryotic cells is covered by a dense layer of glycans, the glycocalyx, that functions as a protective barrier and mediates cell adhesion and cell or protein interactions [Citation1]. This layer of glycans is generated through a process called glycosylation. Glycosylation is one of the most common protein and lipid modifications in the cell and it regulates protein folding, stability, and function [Citation1]. Glycans are attached to proteins in the ER and Golgi to the hydroxyl group of a serine, threonine or tyrosine (O-glycosylation) or to the amide group of an asparagine (N-glycosylation) and extended by sequential addition of monosaccharides by a large set of glycosyltransferases [Citation1]. Like many cellular processes, glycosylation is altered during tumorigenesis. Common alterations include aberrant expression of truncated O-glycans, increased sialylation, and fucosylation, altered branching of N-glycans and altered glycosaminoglycans [Citation1,Citation2]. This distorted glycosylation can modify the activity of receptor tyrosine kinases, cell adhesion, or the interaction with the extracellular matrix, promoting tumor progression and invasiveness [Citation2].
In line with the abundance of glycans on the outer surface of cells, the immune system harbors many glycan-binding pattern recognition receptors. Among these are the myeloid C-type lectin receptors (CLRs), part of the C-type lectin domain subfamily of receptors that often bind sugars in a calcium-dependent manner [Citation3,Citation4]. CLRs can induce responses via common signaling motifs, modulate the signaling by heterologous receptors or facilitate endocytosis and antigen presentation of a wide range of targets [Citation5]. A common paradigm for CLR signaling is that responses via an individual CLR can display enormous flexibility. First, signaling can be influenced by differential ligand binding, caused by changes in ligand structure, affinity, avidity, presentation, or size [Citation5]. This is most studied for DC-SIGN (dendritic cell-specific ICAM-3-grabbing non-integrin), a CLR that shows differential responses upon fucose or mannose binding [Citation5]. Secondly, for the same receptor, the signaling cascade that is evoked can differ between cell types. For example, Dectin-1 activates different intracellular signaling molecules in DCs and macrophages [Citation5,Citation6]. Lastly, a variety of cellular responses can be induced upon ligand binding, generally cytokine and chemokine production, phagocytosis, and the respiratory burst [Citation5]. Because of their abundance and versatility, CLRs are important for the recognition of pathogens but they can also facilitate immune escape by pathogens and tumors [Citation3,Citation4].
This review focuses on the CLEC10A receptor (also known as CD301, macrophage galactose-type lectin (MGL) or DC-asialoglycoprotein receptor (DC-ASGPR)), a CLR that specifically recognizes terminal N-Acetylgalactosamine (GalNAc) residues [Citation4,Citation7–9]. CLEC10A ligands are rare in healthy human tissues but are expressed by some viruses, bacteria, and parasites [Citation4,Citation10]. Glycans with exposed GalNAc residues are also a common feature on carcinoma cell surfaces [Citation2]. In this context, CLEC10A can bind to truncated O-glycans, such as the Thomsen-nouveau (Tn) antigen (αGalNAc-Ser/Thr) and sialyl-Tn (sTn, Neu5Acα2-6GalNAcα-O-Ser/Thr) [Citation11]. Human CLEC10A is expressed by multiple cell types, namely monocyte-derived dendritic cells (moDCs) [Citation9] and alternatively-activated macrophages with a more M2-like phenotype [Citation12,Citation13]. Besides moDCs, human dendritic cells consist of CD11c− plasmacytoid DCs and CD11c+ conventional DCs (cDCs). The second group can be further subdivided into cDC1, that are defined by the expression of CD141 and CLEC9A, and the CD1c+ cDC subsets two and three (cDC2 and DC3) [Citation14]. The last two subsets differ in their expression of CD5, CD14 and CD163 [Citation15]. CLEC10A is also specifically expressed on the cDC2 and DC3 subsets [Citation14,Citation15]. Both cDC2 and DC3s are regarded as potent CD4+ T cell activators, which is crucial for the generation of an effective and sustained anti-tumor CD8+ T cell response [Citation14,Citation15].
The specific expression of CLEC10A on cDC2 and DC3 creates an unique opportunity to target and harness these cells for the treatment of cancer. While CLEC10A targeting induces antigen presentation [Citation16] and production of functional antibodies [Citation17], the stimulation generally results in increased production of the anti-inflammatory cytokine interleukin (IL) 10 [Citation18,Citation19], suggesting that concomitant CLEC10A signaling might be undesirable. Possible CLEC10A signaling plasticity, as seen for other CLRs, might complicate the targeting even further. Therefore, in this review, we will explore the potential beneficial and detrimental effects of CLEC10A signaling in the tumor microenvironment (TME). After an in-depth discussion of CLEC10A ligand binding and signaling in moDCs, we will incorporate the CLEC10A-mediated effects in cDC2s and DC3, to investigate cell type dependent signaling and explore the possible use of CLEC10A in anti-tumor vaccination strategies. We will also examine the pleiotropic effects of IL-10, the main cellular response, to answer this question.
2. Structural insights into the ligand binding specificity of CLEC10A
For decades, CLEC10A has been reported to harbor specificity toward terminal GalNAc residues. Specifically, glycan microarrays, surface plasmon resonance and cellular binding assays using the extracellular domain (ECD) of CLEC10A have demonstrated the high binding specificity of this lectin for terminal α- and β-GalNAc residues presented on O-glycan Tn-structures, helminth N-glycans fucosylated and non-fucosylated LacdiNAc (GalNAcβ1 − 4GlcNAc), and glycosphingolipid structures GM2 and GD2 [Citation7,Citation8]. However, complete, and integrative Nuclear Magnetic Resonance (NMR) and X-Ray crystallography studies were only possible after the expression and purification of the carbohydrate recognition domain (CRD) of CLEC10A. These studies allowed full description of the molecular basis for the recognition of the minimal GalNAc moiety by CLEC10A CRD () [Citation20,Citation21,Citation23]. The binding process was revealed to be in the slow exchange regime in the chemical shift timescale by 1H,15N-heteronuclear single quantum coherence spectroscopy (HSQC) based titration of the CLEC10A CRD with the minimal epitope α-MeGalNAc () [Citation20]. The 1H,15N-HSQC shows a separate peak for each N-H bond in amino acid in the CLEC10A CRD, which can shift upon binding of the α-MeGalNAc. Besides a remarkable chemical shift perturbation (CSP) on the Q267 and D269 residues involved in Ca2+ coordination, a significant CSP was observed for other residues (Y236, W271, H286, N292, and D294) and several resonances appear after ligand addition, which suggests dramatic conformational changes on CLEC10A structure upon GalNAc binding (). Additionally, NMR data in tandem with molecular dynamics simulation of the CRD of CLEC10A highlight an impressive decrease on flexibility of the long loop of CLEC10A (L5) upon α-MeGalNAc engagement () [Citation20]. The binding mechanism of CLEC10A is different of that observed for other C-type lectins, such as DC-SIGN. DC-SIGN displays low affinity for its minimal epitope (KD in mM range) [Citation24], while CLEC10A shows specificity and affinity (KD in low µM range) toward its minimal epitope GalNAc [Citation23].
Figure 1. Insights into the molecular recognition of the minimal GalNAc moiety by the CLEC10A CRD domain by NMR and X-Ray crystallography. (a) 1H,15N-HSQC spectra of CLEC10A in the apo state (blue) and with the addition of 0.5 (black) and 5 equivalents (red) of α-MeGalNAc at 600 MHz and 310 K. The residues with asterisks (*) correspond to those that are not present in the apo state and appear in the bound state. In this technique, the resonances of the most affected residues by the presence of α-MeGalNAc are perturbed. (b) Atomic fluctuation (Cα) analysis of CLEC10A CRD domain in the apo state (left) and bound to GalNAc (right) derived from 100 ns molecular dynamics (MD) simulations [Citation20]. The data correspond to the average protein structure derived from the MD simulations. (c) Chemical shift perturbation (CSP) analysis to quantify the perturbation in the resonances observed in A. Two cut-off lines at 0.04 ppm and 0.08 ppm shown in black and blue dash lines, respectively, and are used to differentiate strong and moderate shift in the presence of α-MeGalNAc. (d) X-ray crystallography structure (PDB code: 6PY1) [Citation21] of CLEC10A CRD domain complexed with α-MeGalNAc with the most affected residues determined in panel C highlighted in red. An expansion of the binding-site of CLEC10A (gray carbons) and α-GalNAc (yellow carbons) in stick representation containing the Q267/P268/D269 and W291/N292/D293 motifs and the residues Y236, H286, H284, W271 and E280. The calcium ion (Ca2+) that coordinates OH3 and OH4 of α-GalNAc is shown with a purple sphere. The 3D models in panels b and d were created using Pymol 2.4.1 [Citation22].
![Figure 1. Insights into the molecular recognition of the minimal GalNAc moiety by the CLEC10A CRD domain by NMR and X-Ray crystallography. (a) 1H,15N-HSQC spectra of CLEC10A in the apo state (blue) and with the addition of 0.5 (black) and 5 equivalents (red) of α-MeGalNAc at 600 MHz and 310 K. The residues with asterisks (*) correspond to those that are not present in the apo state and appear in the bound state. In this technique, the resonances of the most affected residues by the presence of α-MeGalNAc are perturbed. (b) Atomic fluctuation (Cα) analysis of CLEC10A CRD domain in the apo state (left) and bound to GalNAc (right) derived from 100 ns molecular dynamics (MD) simulations [Citation20]. The data correspond to the average protein structure derived from the MD simulations. (c) Chemical shift perturbation (CSP) analysis to quantify the perturbation in the resonances observed in A. Two cut-off lines at 0.04 ppm and 0.08 ppm shown in black and blue dash lines, respectively, and are used to differentiate strong and moderate shift in the presence of α-MeGalNAc. (d) X-ray crystallography structure (PDB code: 6PY1) [Citation21] of CLEC10A CRD domain complexed with α-MeGalNAc with the most affected residues determined in panel C highlighted in red. An expansion of the binding-site of CLEC10A (gray carbons) and α-GalNAc (yellow carbons) in stick representation containing the Q267/P268/D269 and W291/N292/D293 motifs and the residues Y236, H286, H284, W271 and E280. The calcium ion (Ca2+) that coordinates OH3 and OH4 of α-GalNAc is shown with a purple sphere. The 3D models in panels b and d were created using Pymol 2.4.1 [Citation22].](/cms/asset/1c664747-b9f6-43b2-a997-c6247be87935/iett_a_2374743_f0001_oc.jpg)
The purified CRD domain of CLEC10A also permitted the elucidation of the crystal structure of the CLEC10A/GalNAc complex () [Citation21]. This X-Ray crystallography structure clearly pinpoints the coordination of Ca2+ through the OH3 and OH4 from the GalNAc, the residues Q267 and D269 of the QPD motif, N292 and D293 of WND motif along with E280 residue [Citation21]. This is a common structural feature among all C-type lectins. Additionally, the X-Ray structure of the complex highlighted the relevance of the Y236, W271 and H286 residues in the engagement of GalNAc in agreement with NMR deducing data (). The binding mode orientation suggested by the X-Ray crystal was also inferred in solution by two techniques commonly used for this: saturation-transfer difference NMR and transfer-rotating-frame nuclear Overhauser effect spectroscopy (TR-ROESY). There were observable exchange cross-peaks in the TR-ROESY spectra of α-MeGalNAc in presence of the CLEC10A CRD domain, which correspond to binding [Citation21,Citation23].
From NMR and X-Ray structural data, it was deduced that CLEC10A may accommodate GalNAc-containing ligands with chemical modifications at C1 and C6 of GalNAc, which explains the recognition of sTn [Citation11] and Tn-glycopeptides [Citation25] and more complex GalNAc-containing structures, such as GM2, GD2 [Citation20] and LacdiNAc [Citation23]. Noteworthy, NMR data suggest that the conformation and dynamics of the CRD of CLEC10A change upon the binding of various ligands containing a terminal GalNAc residue: such as blood group A antigen, Forssman antigen, GM2 and asialoGM2 [Citation20]. Besides, depending on the GalNAc-containing ligand, a different engagement of H286 residue was deduced and its involvement might be crucial for the binding of the peptide backbone or longer oligosaccharides [Citation7]. Although these differences have not been confirmed by X-Ray complex structures, they could point toward signaling flexibility by the receptor and might explain the capacity of CLEC10A to produce distinct immune responses (tolerance or immunity) depending on the specific structure of CLEC10A ligand.
Biophysical and structural biology experiments have described the CLEC10A ECD as a homotrimer [Citation26,Citation27]. The CLEC10A trimerization is mediated by coiled coil formation of neck domain of the ECD, while the CRDs are reported to behave as independent domains [Citation26,Citation27]. This receptor presentation at the surface of dendritic cells and macrophages is a common feature of C-type lectins, and a way to increase the avidity toward its ligands. The specificity of CLEC10A homotrimer is dictated by the CRD domain, however investigations using the CLEC10A ECD suggest that CLEC10A preferentially binds glycopeptides with more than two Tn residues on the peptide structure [Citation28,Citation29].
3. Signaling via CLEC10A
Most studies that deciphered CLEC10A-mediated signaling have used moDCs in vitro and not M2 macrophages. We will discuss their findings before examining the role of CLEC10A signaling in the TME. CLEC10A lacks classical signaling motifs in its intracellular domain, yet it does evoke rapid internalization after ligand binding via an YENF signaling motif [Citation16,Citation19]. Besides endocytosis, CLEC10A stimulation with an antibody or Tn-glycosylated peptides leads to the phosphorylation of and signaling via ERK1/2 [Citation18,Citation30–32], p90RSK, and CREB [Citation18,Citation30] (). The CLEC10A-mediated signaling pathway could include Syk, PLCγ2, PKCδ, MEK1/2, JNK, Akt, β-catenin, GSK-3a/b, and p38, as phosphorylation of these molecules has also been detected () [Citation30,Citation31]. Since Syk activates NF-κB via PKCδ for other CLRs [Citation5], CLEC10A-mediated signaling by itself could induce NF-κB activation. The cleavage of NF-κB, which is necessary for the activation, was seen after stimulation using an CLEC10A antibody or glycopeptide by Napoletano et al. [Citation32], but not by Gu et al. [Citation30]. Moreover, blocking NF-κB activation only prevented some of the CLEC10A-mediated cytokine production, as it reduced IL-10 but not TNF-α production [Citation18]. The cross-talk with toll-like receptors (TLRs) might be necessary for sufficient NF-κB activation, but this remains uninvestigated. The activation of phosphatases that inhibit TLR signaling for other CLRs, such as SHP-1 or SHP-2 [Citation5], has also not been analyzed.
Figure 2. CLEC10A stimulation of human monocyte-derived dendritic cells. (a) Without toll-like receptor (TLR) activation, CLEC10A stimulation using anti-CLEC10A antibodies, Tn-glycosylated peptides or Tn-dendrimers induces a signaling cascade comprising of ERK1/2, p90RSK, and CREB, with possible contributions of Syk, PLCγ2, PKCδ, p38, MEK1/2, JNK, Akt, and β-catenin [Citation30,Citation32]. This signaling cascade activates a distinct transcriptional program [Citation33]. Furthermore, the CLEC10A stimulation reduces the glycolysis in monocyte-derived dendritic cells [Citation33]. The stimulated dendritic cells produce increased amounts of IL-10, IL-6 and TNF-α and reduce the proliferation of target CD4+ T cells [Citation30]. (b) In combination with TLR stimulation, the CLEC10A-stimulated signaling cascade includes p38, MEK1/2, ERK1/2, p90RSK, and CREB [Citation18]. The transcription of the cytokine genes is initiated and this leads to increased production and secretion of IL-10, IL-6 and IL-8 [Citation18,Citation19]. Besides reducing their proliferation, the IL-10 drives the differentiation of CD4+ T cells into type 1 regulatory T (Tr1) cells. These cells produce IL-10 themselves and can repress the activation of responder CD4+ T cells [Citation31]. (c) CD45 on activated CD4+ and CD8+ T cells can directly bind CLEC10A, which induces apoptosis in the T cells [Citation12,Citation34].
![Figure 2. CLEC10A stimulation of human monocyte-derived dendritic cells. (a) Without toll-like receptor (TLR) activation, CLEC10A stimulation using anti-CLEC10A antibodies, Tn-glycosylated peptides or Tn-dendrimers induces a signaling cascade comprising of ERK1/2, p90RSK, and CREB, with possible contributions of Syk, PLCγ2, PKCδ, p38, MEK1/2, JNK, Akt, and β-catenin [Citation30,Citation32]. This signaling cascade activates a distinct transcriptional program [Citation33]. Furthermore, the CLEC10A stimulation reduces the glycolysis in monocyte-derived dendritic cells [Citation33]. The stimulated dendritic cells produce increased amounts of IL-10, IL-6 and TNF-α and reduce the proliferation of target CD4+ T cells [Citation30]. (b) In combination with TLR stimulation, the CLEC10A-stimulated signaling cascade includes p38, MEK1/2, ERK1/2, p90RSK, and CREB [Citation18]. The transcription of the cytokine genes is initiated and this leads to increased production and secretion of IL-10, IL-6 and IL-8 [Citation18,Citation19]. Besides reducing their proliferation, the IL-10 drives the differentiation of CD4+ T cells into type 1 regulatory T (Tr1) cells. These cells produce IL-10 themselves and can repress the activation of responder CD4+ T cells [Citation31]. (c) CD45 on activated CD4+ and CD8+ T cells can directly bind CLEC10A, which induces apoptosis in the T cells [Citation12,Citation34].](/cms/asset/610e1129-1dbe-4a2d-86b6-153c1dd34dd7/iett_a_2374743_f0002_oc.jpg)
CLEC10A stimulation without accompanying TLR stimulation causes a decrease in glycolysis [Citation33], but does not activate moDCs nor induce robust cytokine production [Citation18,Citation33]. However, in combination with TLR triggering, an increased production of IL-10, tumor necrosis factor (TNF) α, and IL-6 by moDCs is observed () [Citation18,Citation30]. The increase in IL-10 is regulated on a transcriptional level, while the increase in TNFα might involve increased mRNA stability [Citation18]. Nevertheless, moDC activation without additional TLR stimulation has also been reported [Citation30,Citation32], which might be influenced by the specificity or concentration of the CLEC10A antibody used as ligand or the protocol used for moDC differentiation and stimulation [Citation18,Citation30–32].
To investigate the effect CLEC10A stimulation has on the adaptive immune response, the capacity of CLEC10A-stimulated DCs to stimulate T cells has been studied. Because of the increased IL-10 production, CLEC10A-stimulated moDCs have a reduced capacity to promote CD4+ T cell proliferation () [Citation12,Citation30]. In interferon (IFN) α-stimulated moDCs, CLEC10A stimulation enhances the percentage of IL-10 producing type 1 regulatory T (Tr1) cells via IL-10, which can inhibit the proliferation of responder CD4+ T cells ()) [Citation31]. Furthermore, CLEC10A can inhibit T cells through the recognition of GalNAc moieties on CD45 on T cells, reducing their T cell receptor signaling and enhancing T cell death ()) [Citation12,Citation34]. Since the expression of Tn-antigen on CD45 was upregulated on CD4+ and CD8+ T cells after T cell receptor activation [Citation35], this mainly led to the apoptosis of these activated CD4+ and CD8+ T cells. Next to increased moDC activation, Napoletano et al. [Citation32] also found increased CD4+ T cell proliferation for moDCs stimulated with an CLEC10A antibody but not with mucin (MUC) 1-Tn peptides. Despite these contradicting results, overall, CLEC10A-stimulated moDCs appear to inhibit CD4+ T cell responses.
Similar to other CLRs, there are multiple indications that CLEC10A displays ligand-dependent signaling in moDCs. Two CLEC10A ligands (α-GalNAc and asialoGM2) induced different transcriptional programs in moDCs even in the absence of TLR triggering [Citation33], suggesting that the precise structure of GalNAc-containing ligands is crucial for signaling induction. Regarding the nature of the ligand and its effect on signaling, van Vliet et al. [Citation5] showed that, compared to other bacterial lipooligosaccharide formulations, CLEC10A-binding Neisseria gonorrhoeae lipooligosaccharide decreased IL-10 production, instead of increasing it. This led to the induction of a T helper (Th) 2 and potentially a Th17 response [Citation10]. Additionally, pathogens and cells usually express many different sugar moieties simultaneously that can target multiple glycan-binding receptors. Consequently, a combination of ligands can have different effects than an isolated ligand [Citation3]. Investigating the effects or combinations of different ligands may be necessary to choose the optimal candidate for harnessing CLEC10A-mediated signaling and DC targeting.
3.1. CLEC10A-mediated effects in the tumor micro-environment
Ligands for CLEC10A are highly expressed in many tumor types, including breast cancer [Citation36], ovarian cancer [Citation29], cervical cancer [Citation37], colorectal cancer [Citation38–40], and glioblastoma [Citation41]. Additionally, high expression of Tn or sTn antigen has been found in, for example, renal cell carcinoma [Citation42], and bladder cancer [Citation43]. In the colon, pre-neoplastic lesions already display increased Tn expression [Citation44]. In tumors, CLEC10A can bind to extracellular membrane-bound and secreted proteins, such as mucins, extracellular matrix components [Citation29,Citation36], cell surface receptors [Citation38,Citation45], integrins, and human leucocyte antigen (HLA) molecules [Citation38], although exact ligands vary between tumor types. Nevertheless, high Tn or sTn levels are not directly correlated to CLEC10A ligand expression as CLEC10A recognizes a broader range of GalNAc-containing structures [Citation7,Citation11].
The effect of CLEC10A ligand expression, either Tn/sTn antigen or other GalNAc-containing structures, on tumor progression and prognosis varies. High CLEC10A binding of tumor tissue has been associated with a worse outcome in cervical cancer [Citation37] and colorectal cancer [Citation39], while a similar association exists for high Tn and/or high sTn for renal cell carcinoma [Citation42]. Furthermore, CLEC10A ligand positive cervical carcinomas show increased lymph node metastasis and CD163+CD14− myeloid cell infiltration [Citation37]. High expression of all CLEC10A ligands also correlates to the BRAFV600E mutation in colorectal cancer [Citation39]. In colorectal cancer cell lines, this mutation increases the activity of N-Acetylgalactosaminyltransferase-3, responsible for the generation of Tn antigen [Citation46]. Alternatively, high Tn antigen expression was associated with a subset of colorectal cancer patients with deficient mismatch repair, reduced CD8 T cell infiltration, and PD-L1 expression [Citation47]. The authors suggest that these patients might show a poor response to immune checkpoint inhibition therapy [Citation47]. In contrast, CLEC10A ligand positivity correlates with increased survival in breast cancer [Citation36]. In this tumor type, the expression of all CLEC10A ligands was increased by DNA damage and ROS production, and dependent on hormone levels. CLEC10A ligand expression was therefore proposed as a signal for cell stress or cell death [Citation36]. An increase in C1GALT1, the enzyme responsible for elongating the Tn antigen and thus reducing Tn expression, was found in bladder cancer. High C1GALT1 levels were associated with a worse prognosis and increased the proliferation, migration, and colony formation of bladder cancer cell lines [Citation43].
CLEC10A itself is expressed on CD163+ myeloid cells in the TME of cervical, glioblastoma, and colorectal tumors [Citation37,Citation40,Citation41]. Single-cell RNA sequencing datasets have revealed CLEC10A mRNA in cDC2s, as well as mRNA expression in a small percentage of tumor-associated macrophages in multiple tumor types [Citation48–51]. However, studies into the function of these tumor-associated cells and their effect on tumor progression are limited. Most studies correlate the CLEC10A levels to survival outcome based on the bulk mRNA data in the Cancer Genome Atlas (TCGA) or similar datasets, sometimes supplemented with some immunohistochemical experiments to show expression on protein level. An increase in CLEC10A protein expression was seen in glioblastoma patients and in silico analysis confirmed that CLEC10A expression increased with the tumor stage, as defined by the World Health Organization [Citation41]. High CLEC10A mRNA levels correlated with a worse overall survival in the TCGA data for glioblastoma [Citation41]. For many other tumor types, CLEC10A mRNA levels were lower in the tumor compared to the adjacent (normal) tissue [Citation52,Citation53]. In contrast to glioblastoma, the CLEC10A mRNA decreased with the tumor stage for head and neck squamous cell carcinoma [Citation54], adrenocortical carcinoma, and renal cell carcinoma [Citation52]. Looking at multiple tumor types pooled together, high CLEC10A mRNA levels were associated with increased immune infiltration and a good prognosis [Citation52], although the differences are small [Citation53]. Although significant, CLEC10A mRNA levels only weakly correlate to overall immune infiltration [Citation54,Citation55]. To summarize, correlations between CLEC10A expression and disease prognosis differ between tumor types, but these indications are mainly based bulk RNA sequencing data and not confirmed on protein level.
There are two homologues of CLEC10A in the mouse, CD301a (mMGL1) and CD301b (mMGL2), of which only CD301b recognizes GalNAc and galactose-containing structures [Citation56]. Although CLEC10A and CD301b have overlapping, yet not identical sub-specificities [Citation11,Citation56], mouse studies investigating the effects of CD301b in the TME can be useful to speculate about the CLEC10A-mediated effects in human cancers. Lung tumors with high Tn expression show faster growth because of CD301b-mediated immunosuppression and stimulation of angiogenesis [Citation57]. Interestingly, differences in Tn antigen expression and CD301b recognition between cell lines influences the activation and cytokine production of bone marrow-derived dendritic cells [Citation58], suggesting there might also be ligand-dependent signaling for CD301b. The location and not the amount of glycans on MUC6 was previously shown to be an important determinant for the CD301b-mediated effects [Citation59], supporting this concept. Tn-high tumors also show increased neutrophil and macrophage infiltration, including myeloid-derived suppressor cells (MDSCs) [Citation41,Citation57,Citation60,Citation61]. In addition, the infiltration of Tregs was augmented, while the CD8+ T cell infiltration was reduced [Citation57,Citation60]. These changes induced increased production of the anti-inflammatory cytokines IL-10 and TGF-β in the TME and hampered an effective anti-tumor immune response [Citation57,Citation60]. There has been no definite clarification of mechanistic link between CLEC10A-tumor interactions and the differentiation of Tregs and MDSCs in the TME, but it likely involves IL-10. Thus, in mice, CD301b mainly seems to have an immunosuppressive role in the TME.
4. CLEC10A-mediated activation of CD1c+ DCs
The unique expression of CLEC10A on cDC2 and DC3 enables targeting to and the manipulation of these DC subsets. Its presence on both subsets allows for harnessing specific functions of both subsets and investigation of the effect of CD1c+ DCs as a whole. The CD1c+ cDC2 and DC3 also express FcεR1a, but differ in their expression of CD5, CD14 and CD163 [Citation15]. The subset-defining markers and general functions of cDC2 and DC3 are reviewed in more detail elsewhere [Citation62,Citation63]. Still, cDC2 and DC3 have distinct roles in the anti-tumor immune response that must be taken into consideration when exploring the potential beneficial and detrimental effects of CLEC10A signaling in the TME. Moreover, since there are many phenotypic differences between moDCs and CD1c+ DCs [Citation62], CLEC10A-mediated signaling might vary between these cell types.
In vitro experiments have revealed both similarities and differences between moDCs and primary cDCs in response to CLEC10A activation. Similar to moDCs, CLEC10A stimulation of CD1c+ DCs leads to rapid internalization of the receptor and, in combination with a TLR ligand, to increased production of IL-10, TNFα, IL-6 and IL-8 [Citation19,Citation30,Citation64]. The CLEC10A-stimulated cDCs also have a slightly diminished capacity to stimulate CD4+ T cell proliferation [Citation30]. However, the cDC experiments failed to show phosphorylation of the signaling molecules proposed by moDC experiments: ERK, JNK, p38, NF-κB, and CREB [Citation19]. Further research should be performed to determine how translatable the results obtained in moDCs are to the primary cDCs, especially regarding the ligand-specific effects of, for example, α-GalNAc versus asialoGM2.
Similar to CLEC10A mRNA levels, the presence of CLEC10A-positive CD1c+ DCs in the TME and their functioning depends on the tumor type. With a few exceptions, high levels of intratumoral cDC2s or DC3s are mostly associated with a good outcome. For example, a high number of CD1c+ DCs in the TME is associated with a good prognosis in breast cancer [Citation65,Citation66] and glioblastoma [Citation67], but with a poorer survival in melanoma [Citation68]. Although the presence of cDC2s and DC3s in the tumor might be a positive factor, the immunosuppressive TME often appears to impede cDC activation and function. There are changes in gene expression [Citation66,Citation69], maturation marker expression and cytokine production [Citation67,Citation68]. Whether this is related to CLEC10A signaling, which could continuously occur in the TME, is currently unclear.
DCs can also be harnessed for the treatment of cancer patients. Both moDCs and primary cDCs have been tested in DC vaccination strategies, where the patient’s own DCs are expanded ex vivo and primed with antigens before being re-injected. Trials where a patient’s own cDC2s are injected show modest clinical responses with mainly low-grade adverse effects [Citation70–72]. The cDC2s used in these studies were loaded with a mixture of tumor-associated antigenic peptides, which can only be used for patients with certain HLA haplotypes [Citation71]. Loading using Tn-glycopeptides provides a more specific targeting of DCs via CLEC10A and may result in faster uptake and antigen processing. Tn-glycosylation of proteins would generate a vaccine applicable to patients with varying HLA haplotypes. Indeed, moDCs loaded with Tn-MUC1 generated substantial anti-MUC1-Tn CD4+ and CD8+ T cell responses in non-metastatic prostate cancer patients [Citation73]. Direct vaccination of patients with Tn-glycopeptides also elicits anti-Tn responses via CLEC10A [Citation17]. In breast cancer patients, vaccination, using Tn antigens coupled to a tetanus toxin CD4+ T cell epitope and an adjuvant, lead to the generation of anti-Tn antibodies that are capable of mediating complement-dependent cytotoxicity but not antibody-dependent cellular cytotoxicity [Citation17]. Furthermore, conjugation of a high-affinity glycocluster targeting CLEC10A to MUC1 and tetanus toxoid as adjuvant promoted the uptake of MUC1 glycopeptides and improved the elicited antibody response [Citation74]. Thus, CLEC10A may be employed for direct DC targeting in the TME and could lead to further refinements of DC vaccination therapies.
Current focus in the field is the blocking of unwanted immune interactions in the TME, collectively known as immune checkpoints, using blocking antibodies. Blocking antibodies targeting CLEC10A could be used to prevent the high IL-10 production in the TME. However, all antibodies that have been described to block CLEC10A, are able to activate the receptor as well [Citation18,Citation30–32]. Furthermore, after internalization, CLEC10A releases its ligand in early endosomes [Citation16], while the receptor recycles back to the cell membrane [Citation75]. Thus, to effectively block CLEC10A signaling, high levels of antibody would need to be continuously supplied to the TME, which would make the treatment expensive and cumbersome. Alternatives to blocking the receptor need the be explored as potential immune checkpoint inhibition therapies.
5. Pleiotropic effects of IL-10 in the TME
Increased IL-10 production is one of the most consistently seen outcomes of CLEC10A stimulation [Citation18,Citation19,Citation30,Citation33]. IL-10 is generally considered an anti-inflammatory cytokine whose presence accelerates tumor growth. Indeed, in a meta-analysis, high serum levels of IL-10 correlate with a worse overall survival for both hematological and solid tumors [Citation76]. Similarly, individuals with an IL-10 promotor haplotype associated with high IL-10 production have an increased risk of developing cancer [Citation77]. In the TME specifically, IL-10 production by tumor-associated macrophages is associated with a worse survival in, for example, gastric cancer [Citation78]. Indeed, as mentioned before (), the CLEC10A-dependent IL-10 reduced CD4+ T cell proliferation and led to the generation of regulatory Tr1 cells, which in turn also secreted increased levels of IL-10 [Citation30,Citation31].
However, IL-10 is a pleiotropic cytokine that can also exert pro-inflammatory effects on natural killer (NK) and CD8+ T cells [Citation79], which could positively influence the anti-tumor immune response. In breast cancer, for example, high production of IL-10 in the tumor or stroma was associated with increased survival and less metastasis formation [Citation80]. Furthermore, patients with mutations in the IL-10 or IL-10 receptor region develop both autoimmune diseases and B cell lymphomas [Citation81], so IL-10 seems to have some protective role in cancer development as well.
In mice, the anti-metastatic effect of IL-10 on breast tumors was mediated by NK cells [Citation82]. This might be an indirect effect, as IL-10 leads to downregulation of major histocompatibility complex (MHC) I expression on tumor cells [Citation82]. Since MHC-I is a ligand for inhibitory receptors on NK cells, lower MHC-I increases NK cell activation and cytotoxicity in an antibody-independent manner, also called the ‘missing-self hypothesis’ [Citation83]. However, IL-10 also affects NK cells directly. First, IL-10 stimulation increases transcriptional programs associated with apoptosis, NK cell activation and migration [Citation84]. Second, recombinant IL-10 stimulates glycolysis and oxidative phosphorylation in NK cells, which, in turn, augments IFNγ production and NK cell activation [Citation85]. These effects boost the NK cell killing of MHC-I negative Daudi [Citation84] and K562 [Citation85] cells. In mice, regulatory DCs that display high IL-10 production via ERK1/2, stimulate NK cell activation and their cytotoxicity against allogeneic DCs and YAC-1 targets cells [Citation86]. These regulatory DCs recapitulate the phenotype seen after CLEC10A stimulation and thus, it is tempting to speculate that CLEC10A-licensed DCs might enhance NK cell cytotoxicity in a similar manner.
While IL-10 produced after CLEC10A stimulation can decrease CD4+ T cell proliferation [Citation30,Citation31], it might boost CD8+ T cell responses. In vivo, IL-10 administration to target the CD8+ T cells increases tumor control [Citation87,Citation88]. The timing of IL-10 administration appears to be crucial for its effects: IL-10 exerts suppressive effects if administered before DC vaccination but augments the anti-tumor response if it is given as a booster [Citation89]. In vitro, IL-10 reduces monocyte and cDC activation, but it increases the cytotoxicity and granzyme B production by CD8+ T cells, and the proliferation of naïve CD8+ T cells [Citation90,Citation91]. Furthermore, IL-10 enhances the survival, proliferation, and granzyme B production of terminally exhausted CD8+ T cells (Lag-3+PD-1+ or Tim-3+PD-1+ CD8+ T cells) [Citation87,Citation92,Citation93]. Because of these promising in vitro and in vivo data indicating that IL-10 augments CD8+ T cell responses, several clinical trials have started investigating IL-10 administration for the treatment of cancer. Despite initial promising results [Citation94], these trials witnessed an increase in treatment-related adverse effects without any clinical benefit [Citation95,Citation96]. Although the IL-10 produced after CLEC10A activation might stimulate some cell types, its anti-inflammatory effects on others are difficult to overcome in the TME.
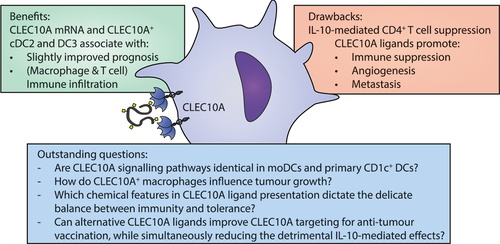
6. Conclusion
Overall, CLEC10A targeting in the TME could exert both positive and negative influences on tumor treatment, which are summarized in . In vitro, CLEC10A stimulation of moDCs and CD1c+ cDCs leads to IL-10 mediated suppression of CD4+ T cell responses. Along this line, the presence of CLEC10A ligands in the tumor promotes invasion and metastasis, infiltration of immunosuppressive cells, and supports angiogenesis. The presence of elevated CLEC10A mRNA levels, however, has a small but positive influence on the disease outcome and is associated with increased immune infiltration in certain tumor types. This could be the result of an increase in CLEC10A-expressing cDC2s or DC3s in the TME, whose numbers are generally positively associated with tumor prognosis and improved immune infiltration. Nevertheless, cDC2 and DC3 functioning is often hampered in the immunosuppressive TME. Lastly, the pleiotropic cytokine IL-10 can boost the cytotoxicity of NK cells and CD8+ T cells; however these effects seem to be overruled by IL-10 mediated suppression of DCs, macrophages, and CD4+ T cells, as these preclinical results could not be recapitulated in clinical trials. Although there are no conclusive results indicating that CLEC10A has advantageous effects in the TME, targeting tumor antigens to DCs via CLEC10A has been successful.
7. Expert opinion
Cancer immunotherapy focuses on boosting the patient’s own anti-tumor immune response. Strategies that target glycans can be widely applicable, as many tumor types share common glycan alterations. Targeting tumor vaccines to DCs via CLEC10A has already been achieved with Tn-glycosylated vaccines [Citation17,Citation74]. Altering the affinity of the sugar moiety for CLEC10A improved the vaccine uptake and subsequent antibody responses [Citation74]. Nevertheless, translating in vivo findings to human disease is challenging, as expression patterns and carbohydrate specificities of mouse and human CLEC10A homologues are not identical [Citation56]. Further advancements can be achieved by focusing research on the outstanding questions, as listed in .
A structure-immune relationship analysis is required to unravel the key chemical modifications required to generate ligands that induce potent CLEC10A signaling or that trigger only certain signaling routes downstream of CLEC10A. In this perspective, a ligand capable of inducing CLEC10A-mediated endocytosis without the concomitant IL-10 induction would benefit the CLEC10A targeting, while reducing the potential detrimental effects of IL-10. Ligands that block the receptor, without inducing its signaling, could also be utilized as immune checkpoint inhibitors. It has been hypothesized that high CLEC10A receptor occupancy leads to tolerogenic responses, while low occupancy would lead to activating signals [Citation97]. Therefore, tweaking the receptor occupancy of CLEC10A might improve anti-tumor immunity. Conversely, selective high-avidity targeting of the CLEC10A receptor could be harnessed to dampen unwanted allergic or autoimmune responses, enforcing CLEC10A’s role in the resolution of neuroinflammation [Citation13].
CLEC10A targeting can be further improved by taking ligand-dependent signaling into consideration [Citation20,Citation33]. Many CLRs display ligand and cell type-dependent signaling, implying that this is a general feature of these receptors [Citation5,Citation6,Citation98]. The structure of the CLEC10A CRD is dynamic undergoing significant conformational changes upon ligand binding [Citation7,Citation21] and experiencing a distinct degree of interactions depending on the ligand [Citation20,Citation23], suggesting that the signal strength or even the signaling downstream of CLEC10A could be modified by the interacting ligand. CLEC10A also engages the protein backbone carrying the Tn antigen [Citation7], however, the preference or specificity for particular protein-binding motif is currently unknown and should be investigated. So far, CLEC10A signaling has been mainly studied in isolated systems, involving one cell type and one ligand. Obviously, tumor cells and pathogens contain a multitude of carbohydrate epitopes at their cell surface that combined fine-tune immunity through engagement of multiple lectins simultaneously. For example, a prominent CLEC10A ligand, sTn, can bind Siglec-15 and mediates immunosuppressive effects through this receptor as well [Citation99]. How CLEC10A cooperates with other lectins and at what level it converges or antagonizes pattern recognition receptor signaling are outstanding aspects that need exploration before CLEC10A can be exploited to manipulate the immune system.
Finally, the cell type that expresses CLEC10A needs consideration. Most studies regarding CLEC10A function have focused on (monocyte-derived) DCs, while research into alternatively-activated (M2-like) macrophages is virtually absent. The limited data available shows that CLEC10A stimulation on macrophages also increased IL-10 production [Citation13] and enhanced bacterial phagocytosis [Citation100]. In the TME, CLEC10A is expressed on myeloid cells, which are partly of macrophage origin [Citation48–51]. As macrophages are among the most abundant cell types present in the TME, contributing to tumor cell survival, angiogenesis, metastasis, and immune evasion [Citation101], this cell type should not be disregarded in future studies. Overall, finding the right CLEC10A-positive APC to target and the optimal ligand, in combination with the right adjuvant to overcome the immunosuppressive TME, are needed to advance the field and key to a successful CLEC10A-mediated immunotherapy in cancer.
Article highlights
CLEC10A is an anti-inflammatory receptor, present on dendritic cells and macrophages, that specifically binds to terminal N-Acetylgalactosamine sugar residues.
Ligands for CLEC10A are commonly overexpressed in the tumor microenvironment.
While the presence of intratumoral CLEC10A-positive dendritic cells is associated with a better outcome for patients, the presence of CLEC10A ligands promotes tumor progression.
Delivering tumor vaccines to intratumoral dendritic cells via CLEC10A is a promising therapeutic tool against cancer.
Strategies to disrupt aberrant CLEC10A/tumor-associated glycan interactions can potentially alleviate anti-tumor suppressive immune responses.
Abbreviation
| ASGPR | = | Asialoglycoprotein receptor |
| cDC | = | Conventional dendritic cell |
| CLR | = | C-type lectin receptor |
| CRD | = | Carbohydrate recognition domain |
| CSP | = | Chemical shift perturbation |
| DC-SIGN | = | Dendritic cell-specific ICAM-3-grabbing non-integrin |
| ECD | = | Extracellular domain |
| GalNAc | = | N-Acetylgalactosamine |
| HLA | = | Human leucocyte antigen |
| HSQC | = | Heteronuclear single quantum coherence spectroscopy |
| IFN | = | Interferon |
| IL | = | Interleukin |
| KD | = | Dissociation constant |
| MDSC | = | Myeloid-derived suppressor cell |
| MGL | = | Macrophage galactose-type lectin |
| MHC | = | Major histocompatibility complex |
| moDC | = | Monocyte-derived dendritic cell |
| MUC | = | Mucin |
| NK | = | Natural killer (cell) |
| NMR | = | Nuclear Magnetic Resonance |
| TCGA | = | The Cancer Genome Atlas |
| Th | = | T helper cell |
| TLR | = | Toll-like receptor |
| TME | = | Tumor microenvironment |
| Tn | = | Thomsen-nouveau |
| TNF | = | Tumor necrosis factor |
| Tr1 | = | Type 1 regulatory T (cell) |
| Treg | = | Regulatory T cell |
| TR-ROESY | = | Transfer-Rotating-frame nuclear Overhauser effect spectroscopy |
| sTn | = | Sialyl-Tn |
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Schjoldager KT, Narimatsu Y, Joshi HJ, et al. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020 Dec;21(12):729–749. doi: 10.1038/s41580-020-00294-x
- Rodrigues JG, Balmana M, Macedo JA, et al. Glycosylation in cancer: selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018 Nov;333:46–57. doi: 10.1016/j.cellimm.2018.03.007
- Fischer S, Stegmann F, Gnanapragassam VS, et al. From structure to function - ligand recognition by myeloid C-type lectin receptors. Comput Struct Biotechnol J. 2022;20:5790–5812. doi: 10.1016/j.csbj.2022.10.019
- Valverde P, Martinez JD, Canada FJ, et al. Molecular recognition in C-type lectins: the cases of DC-SIGN, langerin, MGL, and L-sectin. Chembiochem. 2020 May 19;21(21):2999–3025. doi: 10.1002/cbic.202000238
- Del Fresno C, Iborra S, Saz-Leal P, et al. Flexible signaling of myeloid c-type lectin receptors In immunity and inflammation. Front Immunol. 2018;9:804. doi: 10.3389/fimmu.2018.00804
- Goodridge HS, Shimada T, Wolf AJ, et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol Res. 2009 Jan 15;182(2):1146–1154. doi: 10.4049/jimmunol.182.2.1146
- Marcelo F, Supekar N, Corzana F, et al. Identification of a secondary binding site in human macrophage galactose-type lectin by microarray studies: implications for the molecular recognition of its ligands. J Biol Chem. 2019 Jan 25;294(4):1300–1311. doi: 10.1074/jbc.RA118.004957
- van Vliet SJ, van Liempt E, Saeland E, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005 May;17(5):661–669. doi: 10.1093/intimm/dxh246
- Higashi N, Fujioka K, Denda-Nagai K, et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002 Jun 7;277(23):20686–20693. doi: 10.1074/jbc.M202104200
- van Vliet SJ, Steeghs L, Bruijns SC, et al. Variation of neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced t helper responses. PLOS Pathog. 2009 Oct;5(10):e1000625. doi: 10.1371/journal.ppat.1000625
- Mortezai N, Behnken HN, Kurze AK, et al. Tumor-associated Neu5Ac-Tn and Neu5Gc-Tn antigens bind to C-type lectin CLEC10A (CD301, MGL). Glycobiology. 2013 Jul;23(7):844–852. doi: 10.1093/glycob/cwt021
- van Vliet SJ, Gringhuis SI, Geijtenbeek TB, et al. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006 Nov;7(11):1200–1208. doi: 10.1038/ni1390
- Ilarregui JM, Kooij G, Rodríguez E, et al. Macrophage galactose-type lectin (MGL) is induced on M2 microglia and participates in the resolution phase of autoimmune neuroinflammation. J Neuroinflamm. 2019 Jun 27;16(1):130. doi: 10.1186/s12974-019-1522-4
- Villani AC, Satija R, Reynolds G, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Sci. 2017 Apr 21;356(6335):eaah4573. doi: 10.1126/science.aah4573
- Bourdely P, Anselmi G, Vaivode K, et al. Transcriptional and functional analysis of CD1c(+) human dendritic cells identifies a CD163(+) subset priming CD8(+)CD103(+) T cells. Immunity. 2020 Aug 18;53(2):335–52 e8. doi: 10.1016/j.immuni.2020.06.002
- van Vliet SJ, Aarnoudse CA, Broks-van den Berg VC, et al. MGL-mediated internalization and antigen presentation by dendritic cells: a role for tyrosine-5. Eur J Immunol. 2007 Aug;37(8):2075–2081. doi: 10.1002/eji.200636838
- Rosenbaum P, Artaud C, Bay S, et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol Immun. 2020 May;69(5):703–716. doi: 10.1007/s00262-020-02503-0
- van Vliet SJ, Bay S, Vuist IM, et al. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-alpha secretion. J Leukoc Biol. 2013 Aug;94(2):315–323. doi: 10.1189/jlb.1012520
- Heger L, Balk S, Luhr JJ, et al. CLEC10A Is a specific marker for human CD1c(+) dendritic cells and enhances their toll-like receptor 7/8-induced cytokine secretion. Front Immunol. 2018;9:744. doi: 10.3389/fimmu.2018.00744
- Diniz A, Coelho H, Dias JS, et al. The plasticity of the carbohydrate recognition domain dictates the exquisite mechanism of binding of human macrophage galactose-type lectin. Chem – A Eur J. 2019;25(61):13945–13955. doi: 10.1002/chem.201902780
- Gabba A, Bogucka A, Luz JG, et al. Crystal structure of the carbohydrate recognition domain of the human macrophage galactose C-type lectin bound to GalNAc and the tumor-associated Tn antigen. Biochemistry. 2021 May 4;60(17):1327–1336. doi: 10.1021/acs.biochem.1c00009
- Schrodinger L. The PyMOL molecular graphics system. Version 2.4.1. [software]. 2010 [cited 2024 May 17]. Available from: https://pymol.org/
- Lima CDL, Coelho H, Gimeno A, et al. Structural insights into the molecular recognition mechanism of the cancer and pathogenic epitope, LacdiNAc by immune-related lectins. Chem A Eur J. 2021 May 20;27(29):7951–7958. doi: 10.1002/chem.202100800
- Martínez JD, Valverde P, Delgado S, et al. Unraveling sugar binding modes to DC-SIGN by employing fluorinated carbohydrates. Molecules. 2019 Jun 25;24(12):2337. doi: 10.3390/molecules24122337
- Artigas G, Monteiro JT, Hinou H, et al. Glycopeptides as targets for dendritic cells: exploring MUC1 glycopeptides binding profile toward macrophage galactose-type Lectin (MGL) orthologs. J Med Chem. 2017 Nov 09;60(21):9012–9021. doi: 10.1021/acs.jmedchem.7b01242
- Abbas M, Maalej M, Nieto-Fabregat F, et al. The unique three-dimensional arrangement of macrophage galactose lectin enables E. coli LipoPolySaccharides recognition through two distinct interfaces. bioRxiv. 2023:2023.03.02.530591.
- Jégouzo SA, Quintero-Martínez A, Ouyang X, et al. Organization of the extracellular portion of the macrophage galactose receptor: a trimeric cluster of simple binding sites for N-acetylgalactosamine. Glycobiology. 2013 Jul;23(7):853–864. doi: 10.1093/glycob/cwt022
- Beckwith DM, FitzGerald FG, Rodriguez Benavente MC, et al. Calorimetric analysis of the interplay between synthetic Tn antigen-presenting MUC1 glycopeptides and human macrophage galactose-type lectin. Biochemistry. 2021 Feb 23;60(7):547–558. doi: 10.1021/acs.biochem.0c00942
- Napoletano C, Steentoff C, Battisti F, et al. Investigating patterns of immune interaction in ovarian cancer: probing the O-glycoproteome by the macrophage galactose-like C-type lectin (MGL). Cancers (Basel). 2020 Oct 1;12(10):2841. doi: 10.3390/cancers12102841
- Gu C, Wang L, Zurawski S, et al. Signaling cascade through DC-ASGPR induces transcriptionally active CREB for IL-10 induction and immune regulation. J Immunol. 2019 Jul 15;203(2):389–399. doi: 10.4049/jimmunol.1900289
- Li D, Romain G, Flamar AL, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012 Jan 16;209(1):109–121. doi: 10.1084/jem.20110399
- Napoletano C, Zizzari IG, Rughetti A, et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur J Immunol. 2012 Apr;42(4):936–945. doi: 10.1002/eji.201142086
- Zaal A, Li RJE, Lubbers J, et al. Activation of the C-type lectin MGL by terminal GalNAc ligands reduces the glycolytic activity of human dendritic cells. Front Immunol. 2020;11:305. doi: 10.3389/fimmu.2020.00305
- Zizzari IG, Martufi P, Battisti F, et al. The macrophage galactose-type C-type lectin (MGL) modulates regulatory T cell functions. PLOS ONE. 2015;10(7):e0132617. doi: 10.1371/journal.pone.0132617
- van Vliet SJ, Vuist IM, Lenos K, et al. Human T cell activation results in extracellular signal-regulated kinase (ERK)-calcineurin-dependent exposure of Tn antigen on the cell surface and binding of the macrophage galactose-type lectin (MGL). J Biol Chem. 2013 Sep 20;288(38):27519–27532. doi: 10.1074/jbc.M113.471045
- Kurze AK, Buhs S, Eggert D, et al. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun Signal. 2019 Aug 27;17(1):107. doi: 10.1186/s12964-019-0420-9
- Sahasrabudhe NM, van der Horst JC, Spaans V, et al. MGL ligand expression is correlated to lower survival and distant metastasis in cervical squamous cell and adenosquamous carcinoma. Front Oncol. 2019;9:29. doi: 10.3389/fonc.2019.00029
- Pirro M, Rombouts Y, Stella A, et al. Characterization of macrophage galactose-type lectin (MGL) ligands in colorectal cancer cell lines. Biochim Biophys Acta Gen Subj. 2020 Apr;1864(4):129513. doi: 10.1016/j.bbagen.2020.129513
- Lenos K, Goos JA, Vuist IM, et al. MGL ligand expression is correlated to BRAF mutation and associated with poor survival of stage III colon cancer patients. Oncotarget. 2015 Sep 22;6(28):26278–26290. doi: 10.18632/oncotarget.4495
- Saeland E, van Vliet SJ, Bäckström M, et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immun. 2007 Aug;56(8):1225–1236. doi: 10.1007/s00262-006-0274-z
- Dusoswa SA, Verhoeff J, Abels E, et al. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc Natl Acad Sci. 2020;117(7):3693–3703. doi: 10.1073/pnas.1907921117
- NguyenHoang S, Liu Y, Xu L, et al. High truncated-O-glycan score predicts adverse clinical outcome in patients with localized clear-cell renal cell carcinoma after surgery. Oncotarget. 2017 Oct 3;8(45):80083–80092. doi: 10.18632/oncotarget.15900
- Tan Z, Jiang Y, Liang L, et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHp1bp3/miR-1-3p axis in bladder cancer. J Exp Clin Cancer Res. 2022 Jul 21;41(1):228. doi: 10.1186/s13046-022-02438-7
- Dombek GE, Ore AS, Cheng J, et al. Immunohistochemical analysis of Tn antigen expression in colorectal adenocarcinoma and precursor lesions. BMC Cancer. 2022 Dec 7;22(1):1281. doi: 10.1186/s12885-022-10376-y
- Pirro M, Schoof E, van Vliet SJ, et al. Glycoproteomic analysis of MGL-binding proteins on acute T-cell leukemia cells. J Proteome Res. 2019 Mar 1;18(3):1125–1132. doi: 10.1021/acs.jproteome.8b00796
- Sahasrabudhe NM, Lenos K, van der Horst JC, et al. Oncogenic BRAFV600E drives expression of MGL ligands in the colorectal cancer cell line HT29 through N-acetylgalactosamine-transferase 3. Biol Chem. 2018 Jun 27;399(7):649–659. doi: 10.1515/hsz-2018-0120
- Matsumoto T, Okayama H, Nakajima S, et al. Tn antigen expression defines an immune cold subset of mismatch-repair deficient colorectal cancer. IJMS. 2020 Nov 29;21(23):9081. doi: 10.3390/ijms21239081
- Chen YP, Yin JH, Li WF, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020 Nov;30(11):1024–1042. doi: 10.1038/s41422-020-0374-x
- Pombo Antunes AR, Scheyltjens I, Lodi F, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021 Apr;24(4):595–610. doi: 10.1038/s41593-020-00789-y
- Wu SZ, Al-Eryani G, Roden DL, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021 Sep;53(9):1334–1347. doi: 10.1038/s41588-021-00911-1
- Sun Y, Wu L, Zhong Y, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021 Jan 21;184(2):404–21.e16. doi: 10.1016/j.cell.2020.11.041
- Qin Y, Wang L, Zhang L, et al. Immunological role and prognostic potential of CLEC10A in pan-cancer. Am J Transl Res. 2022;14(5):2844–2860.
- Tang S, Zhang Y, Lin X, et al. CLEC10A can serve as a potential therapeutic target and its level correlates with immune infiltration in breast cancer. Oncol Lett. 2022 Aug;24(2):285. doi: 10.3892/ol.2022.13405
- Zou M, Wu H, Zhou M, et al. High expression of CLEC10A in head and neck squamous cell carcinoma indicates favorable prognosis and high-level immune infiltration status. Cell Immunol. 2022 Feb;372:104472. doi: 10.1016/j.cellimm.2021.104472
- Pang Z, Chen X, Wang Y, et al. Comprehensive analyses of the heterogeneity and prognostic significance of tumor-infiltrating immune cells in non-small-cell lung cancer: development and validation of an individualized prognostic model. Int Immunopharmacol. 2020 Sep;86:106744. doi: 10.1016/j.intimp.2020.106744
- Singh SK, Streng-Ouwehand I, Litjens M, et al. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Mol Immunol. 2009 Mar;46(6):1240–1249. doi: 10.1016/j.molimm.2008.11.021
- da Costa V, van Vliet SJ, Carasi P, et al. The Tn antigen promotes lung tumor growth by fostering immunosuppression and angiogenesis via interaction with macrophage galactose-type lectin 2 (MGL2). Cancer Lett. 2021 Oct 10;518:72–81. doi: 10.1016/j.canlet.2021.06.012
- da Costa V, Mariño KV, Rodríguez-Zraquia SA, et al. Lung tumor cells with different Tn antigen expression present distinctive immunomodulatory properties. Int J Mol Sci. 2022 Oct 10;23(19):12047. doi: 10.3390/ijms231912047
- Freire T, Lo-Man R, Bay S, et al. Tn glycosylation of the MUC6 protein modulates its immunogenicity and promotes the induction of Th17-biased T cell responses. J Biol Chem. 2011 Mar 11;286(10):7797–7811. doi: 10.1074/jbc.M110.209742
- Festari MF, da Costa V, Rodríguez-Zraquia SA, et al. The tumor-associated Tn antigen fosters lung metastasis and recruitment of regulatory T cells in triple negative breast cancer. Glycobiology. 2022 Apr 21;32(5):366–379. doi: 10.1093/glycob/cwab123
- Cornelissen LAM, Blanas A, Zaal A, et al. Tn antigen expression contributes to an immune suppressive microenvironment and drives tumor growth in colorectal cancer. Front Oncol. 2020;10:1622. doi: 10.3389/fonc.2020.01622
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018 May;154(1):3–20. doi: 10.1111/imm.12888
- Heger L, Hofer TP, Bigley V, et al. Subsets of CD1c(+) DCs: dendritic cell versus monocyte lineage. Front Immunol. 2020;11:559166. doi: 10.3389/fimmu.2020.559166
- Niveau C, Sosa Cuevas E, Roubinet B, et al. Melanoma tumour-derived glycans hijack dendritic cell subsets through C-type lectin receptor binding. Immunology. 2024;171(2):286–311. doi: 10.1111/imm.13717
- Iwanowycz S, Ngoi S, Li Y, et al. Type 2 dendritic cells mediate control of cytotoxic T cell resistant tumors. JCI Insight. 2021 Sep 8;6(17):e145885. doi: 10.1172/jci.insight.145885
- Michea P, Noel F, Zakine E, et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol. 2018 Aug;19(8):885–897. doi: 10.1038/s41590-018-0145-8
- Adhikaree J, Franks HA, Televantos C, et al. Impaired circulating myeloid CD1c+ dendritic cell function in human glioblastoma is restored by p38 inhibition - implications for the next generation of DC vaccines. Oncoimmunology. 2019;8(7):e1593803. doi: 10.1080/2162402X.2019.1593803
- Sosa Cuevas E, Ouaguia L, Mouret S, et al. BDCA1(+) cDc2s, BDCA2(+) pDCs and BDCA3(+) cDc1s reveal distinct pathophysiologic features and impact on clinical outcomes in melanoma patients. Clin Transl Immunol. 2020;9(11):e1190. doi: 10.1002/cti2.1190
- Zilionis R, Engblom C, Pfirschke C, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019 May 21;50(5):1317–34.e10. doi: 10.1016/j.immuni.2019.03.009
- van Beek JJP, Florez-Grau G, Gorris MAJ, et al. Human pDCs are superior to cDc2s in attracting cytolytic lymphocytes in melanoma patients receiving DC vaccination. Cell Rep. 2020 Jan 28;30(4):1027–38 e4. doi: 10.1016/j.celrep.2019.12.096
- Westdorp H, Creemers JHA, van Oort IM, et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J Immunother Cancer. 2019 Nov 14;7(1):302. doi: 10.1186/s40425-019-0787-6
- Schwarze JK, Awada G, Cras L, et al. Intratumoral combinatorial administration of CD1c (BDCA-1)(+) myeloid dendritic cells plus ipilimumab and avelumab in combination with intravenous low-dose nivolumab in patients with advanced solid tumors: a phase IB clinical trial. Vaccines (Basel). 2020 Nov 10;8(4):670. doi: 10.3390/vaccines8040670
- Scheid E, Major P, Bergeron A, et al. Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res. 2016 Oct;4(10):881–892. doi: 10.1158/2326-6066.CIR-15-0189
- Gabba A, Attariya R, Behren S, et al. MUC1 glycopeptide vaccine modified with a GalNAc glycocluster targets the macrophage galactose C-type lectin on dendritic cells to elicit an improved humoral response. J Am Chem Soc. 2023 Jun 21;145(24):13027–13037. doi: 10.1021/jacs.2c12843
- García-Vallejo JJ, Bloem K, Knippels LM, et al. The consequences of multiple simultaneous C-type lectin-ligand interactions: DCIR alters the endo-lysosomal routing of DC-SIGN. Front Immunol. 2015;6:87. doi: 10.3389/fimmu.2015.00087
- Zhao S, Wu D, Wu P, et al. Serum IL-10 predicts worse outcome in cancer patients: a meta-analysis. PLOS ONE. 2015;10(10):e0139598. doi: 10.1371/journal.pone.0139598
- Zhang K, Zhang L, Wang X, et al. The IL-10 promoter haplotype and cancer risk: evidence from a meta-analysis. Fam Cancer. 2012 Sep;11(3):313–319. doi: 10.1007/s10689-012-9533-7
- Zhang H, Li R, Cao Y, et al. Poor clinical outcomes and immunoevasive contexture in intratumoral IL-10-producing macrophages enriched gastric cancer patients. Ann Surg. 2022 Apr 1;275(4):e626–35. doi: 10.1097/SLA.0000000000004037
- Mannino MH, Zhu Z, Xiao H, et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015 Oct 28;367(2):103–107. doi: 10.1016/j.canlet.2015.07.009
- Li Y, Gao P, Yang J, et al. Relationship between IL-10 expression and prognosis in patients with primary breast cancer. Tumour Biol. 2014 Nov;35(11):11533–11540. doi: 10.1007/s13277-014-2249-6
- Neven B, Mamessier E, Bruneau J, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013 Nov 28;122(23):3713–3722. doi: 10.1182/blood-2013-06-508267
- Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell Immunol. 1997 Aug 25;180(1):55–61. doi: 10.1006/cimm.1997.1176
- Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124
- Mocellin S, Panelli M, Wang E, et al. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes Immun. 2004 Dec;5(8):621–630. doi: 10.1038/sj.gene.6364135
- Wang Z, Guan D, Huo J, et al. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 signaling. Front Immunol. 2021;12:619195. doi: 10.3389/fimmu.2021.619195
- Qian C, Jiang X, An H, et al. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells (diffDcs), leading to NK-cell activation. Blood. 2006 Oct 1;108(7):2307–2315. doi: 10.1182/blood-2006-03-005595
- Guo Y, Xie YQ, Gao M, et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. 2021 Jun;22(6):746–756. doi: 10.1038/s41590-021-00940-2
- Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011 Dec 13;20(6):781–796. doi: 10.1016/j.ccr.2011.11.003
- Fujii S, Shimizu K, Shimizu T, et al. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001 Oct 1;98(7):2143–2151. doi: 10.1182/blood.v98.7.2143
- Nizzoli G, Larghi P, Paroni M, et al. IL-10 promotes homeostatic proliferation of human CD8(+) memory T cells and, when produced by CD1c(+) DCs, shapes naive CD8(+) T-cell priming. Eur J Immunol. 2016 Jul;46(7):1622–1632. doi: 10.1002/eji.201546136
- Gorby C, Sotolongo Bellón J, Wilmes S, et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci Signal. 2020 Sep 15;13(649):eabc0653. doi: 10.1126/scisignal.abc0653
- Sun Q, Zhao X, Li R, et al. STAT3 regulates CD8+ T cell differentiation and functions in cancer and acute infection. Journal Of Experimental Medicine. 2023 Apr 3;220(4):e20220686. doi: 10.1084/jem.20220686
- Naing A, Infante JR, Papadopoulos KP, et al. PEGylated IL-10 (pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018 Nov 12;34(5):775–91.e3. doi: 10.1016/j.ccell.2018.10.007
- Naing A, Papadopoulos KP, Autio KA, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016 Oct 10;34(29):3562–3569. doi: 10.1200/JCO.2016.68.1106
- Spigel D, Jotte R, Nemunaitis J, et al. Randomized phase 2 studies of checkpoint inhibitors alone or in combination with pegilodecakin in patients with metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J Thorac Oncol. 2021 Feb;16(2):327–333. doi: 10.1016/j.jtho.2020.10.001
- Hecht JR, Lonardi S, Bendell J, et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol. 2021 Apr 1;39(10):1108–1118. doi: 10.1200/JCO.20.02232
- Hoober JK, Eggink LL. Glycomimetic peptides as therapeutic tools. Pharmaceutics. 2023 Feb 17;15(2):688. doi: 10.3390/pharmaceutics15020688
- Gringhuis SI, den Dunnen J, Litjens M, et al. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009 Oct;10(10):1081–1088. doi: 10.1038/ni.1778
- Takamiya R, Ohtsubo K, Takamatsu S, et al. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology. 2013 Feb;23(2):178–187. doi: 10.1093/glycob/cws139
- Kushchayev SV, Sankar T, Eggink LL, et al. Monocyte galactose/N-acetylgalactosamine-specific C-type lectin receptor stimulant immunotherapy of an experimental glioma. Part 1: stimulatory effects on blood monocytes and monocyte-derived cells of the brain. Cancer Manag Res. 2012;4:309–323. doi: 10.2147/CMAR.S33248
- Pan Y, Yu Y, Wang X, et al. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084