ABSTRACT
Introduction
This study aims to systematically review current evidence on ablative margins and correlation to local tumor progression (LTP) after thermal ablation of hepatocellular carcinoma (HCC) and colorectal liver metastases (CRLM).
Methods
A systematic search was performed in PubMed (MEDLINE) and Web of Science to identify all studies that reported on ablative margins (AM) and related LTP rates. Studies were assessed for risk of bias and synthesized separately per tumor type. Where possible, results were pooled to calculate risk differences (RD) as function of AM.
Results
In total, 2910 articles were identified of which 43 articles were eligible for final analysis. There was high variability in AM measurement methodology across studies in terms of measurement technique, imaging modalities, and timing. Most common margin stratification was < 5 mm and > 5 mm, for which data were available in 25/43 studies (58%). Of these, all studies favored AM > 5 mm to reduce the risk of LTP, with absolute RD of 16% points for HCC and 47% points for CRLM as compared to AM < 5 mm.
Conclusions
Current evidence supports AM > 5 mm to reduce the risk of LTP after thermal ablation of HCC and CRLM. However, standardization of AM measurement and reporting is critical to allow future meta-analyses and improved identification of optimal threshold value for clinical use.
1. Introduction
Liver tumors constitute a substantial and growing proportion of all malignancies worldwide. Hepatocellular carcinoma (HCC) is the most common primary liver cancer with a global incidence of 0.9 million cases in 2020 [Citation1]. Secondary liver metastases comprise approximately 70% of all liver tumors, with colorectal cancer (CRC) being the most common primary origin [Citation2]. Up to 35% of CRC patients will either be diagnosed synchronously or develop colorectal liver metastases (CRLM) during follow-up [Citation3–7]. Survival rates for patients diagnosed with HCC or CRLM have increased over the past decades, but the prognosis for these patient groups remains poor with a median 5-year survival rate of 20–25% [Citation8–10].
Preferential curative treatments for HCC and CRLM are liver transplantation and surgical tumor resection in combination with (adjuvant) chemotherapy, as these have been regarded to provide best long-term survival rates [Citation11]. Although the number of liver resections increased substantially in recent years, less than half of all liver tumors are deemed resectable [Citation12].
In recent years, thermal ablation has been established as a widely adopted alternative to surgical resection in early-stage HCC and low-volume irresectable CRLM. Compared to surgery, thermal ablation has been associated with shorter hospital stay, shorter time to recovery and a lower rate of complications [Citation13–17] and has been shown to offer comparable 1-, 3- and 5-year overall survival rates [Citation16–20]. Previous less reliable methods of percutaneous treatments of liver tumors, like percutaneous ethanol injection, have been phased out in recent years [Citation18]. Additionally, ablation techniques and knowledge have advanced, allowing for more patients to be treated curatively or as bridging to liver resection or transplantation. This can consequently be attributed as one of the reasons for improved survival of patients with liver tumors [Citation19].
Despite these advantages, local tumor recurrence after thermal ablation tend to be higher, with reported rates between 2.9% and 21.7% and even ranging up to 50% in some smaller studies [Citation20–27]. Multiple predictors for local recurrence after ablation have been suggested, including tumor size, number of tumors, location, and ablation technique. In recent years, the ablative margin (AM) or safety margin, defined as the minimal margin distance that is achieved between the area of tissue necrosis and tumor boundary, has been investigated as a prognostic factor. Current standard practice is to target complete ablation of the tumor including a surrounding margin of at least 5 mm for HCC and 10 mm for CRLM to achieve technical success [Citation28]. However, there is currently no evidence-based consensus on the optimal threshold to ensure complete ablation and different techniques have been used to assess the AM in clinical practice.
Therefore, the aim of this study is to systematically review currently available data on correlation between ablative margin thresholds and local recurrence rates after percutaneous thermal ablation for HCC and CRLM. Additionally, this review provides an overview of concurrent methods that have been studied for ablative margin measurement.
2. Methods
2.1. Literature search
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct and report this systematic literature review [Citation29]. No protocol for this study has been prospectively registered. The search strategy consisted of three elements: liver tumors, image guidance, and percutaneous ablation, and was performed on PubMed and Web of Science. The search strategy was developed in collaboration with an information specialist of the medical library at our institution. The following search terms and synonyms were used: liver tumors, ablation, magnetic resonance imaging, computer tomography, ultrasound, image guided, and guidance. The complete search queries for each search index are available in the Appendix. The date of publication was restricted to between January 2012 up until December 2022, as methods to assess the ablative margin using volumetric analysis and image registration techniques have changed significantly over the past decade.
2.2. Article selection
After removal of duplicates using Mendeley (Mendeley v1.19.8, Mendeley Ltd.) according to the method described by Kwon et al., remaining articles were imported in a browser-based review tool (The Systematic Review Accelerator) to facilitate the screening process [Citation30,Citation31]. Articles were scanned on title and abstract for inclusion criteria: (1) thermal ablation using RFA or MWA in a patient cohort, (2) ablative margins, and (3) local tumor progression (LTP). Articles were excluded if they described (1) combinations of thermal ablation with other treatments, (2) laparoscopic or other non-percutaneous approaches, (3) case studies, (4) animal studies, and (5) the use of nuclear imaging. Furthermore, articles had to be written in English and not be categorized as review, meta-analysis, commentary, or conference proceedings.
Two readers (K.V. and S.A.) independently reviewed a subgroup of 100 articles for the inclusion and exclusion criteria. Inclusions were compared between the two readers and conflicts were discussed and resolved. After agreement was reached for all articles, the first reader (K.V.) continued including articles and adjusted his inclusion based on the agreements reached with the second reader (S.A.). After inclusion, full-text assessment was performed by K.V. Articles were further excluded if no details were given on ablative margin measurements, LTP rates or ablative treatments other than RFA or MWA were used.
2.3. Quality assessment
Risk of bias assessment was performed using the Newcastle-Ottawa Scale (NOS) for nonrandomized cohort studies [Citation32]. Studies could receive stars for subquestions of each category (selection, comparability, and outcome) with a maximum of nine stars. For comparability, studies received one or two stars if the study controlled for tumor size and any additional factor. Overall risk of bias was rated low for studies with 7–9 stars, moderate for 4–6 stars, and high for studies with less than 4 stars.
2.4. Data extraction
From each included article the following data was collected: study design, patient population and characteristics, age, tumor type and size, imaging techniques before, during and after thermal ablation procedure, ablation techniques, image registration techniques, LTP rate, ablative margin thresholds, patients per ablative margin threshold, technical parameters for ablative margin measurement methodology, and significant factors influencing LTP. In studies that did not directly provide numbers on cohort sizes, cohort sizes between different margin thresholds were calculated from indirectly provided data, such as survival graph data and tables.
2.5. Data synthesis and analysis
Data was synthesized and stratified for analysis using Microsoft Office Excel (Microsoft Corp., Redmond, WA, USA). Where possible, data were pooled for subgroups of patients with ablative margins less than 5 mm (<5 mm) and greater than 5 mm (>5 mm), since this was the most common subgrouping available in the included articles. In studies giving multiple thresholds, threshold groups were merged in order to fit the margin stratification for pooled analysis. Absolute risk differences (RD) were calculated for the risk of developing LTP between patients with AM <5 mm and patients with AM >5 mm. There was no common way to categorize AM of 5 mm; therefore, this margin was not assigned to either one of the subgroups, but the categorization of the individual studies was followed. Analysis of the data was performed in RStudio (RStudio Team, PBC, Boston, MA, USA).
3. Results
3.1. Literature search
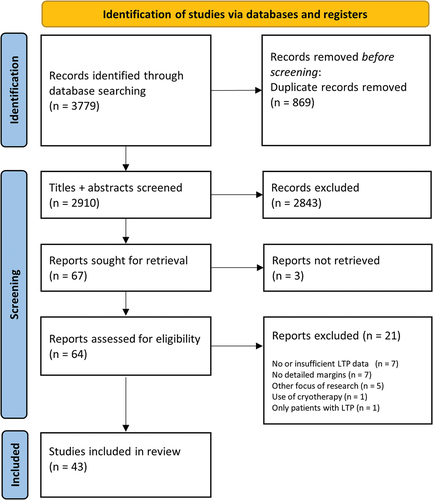
From the research repositories PubMed and Web of Science, 3779 articles were identified. Totally, 869 duplicates were removed from the list. Of the remaining 2910 unique articles, the title and abstracts were read and screened. Based on the pre-determined in- and exclusion criteria, 2843 articles were further excluded. For three of the remaining 67 articles, the full-text articles could not be retrieved and were further excluded. Of the 64 articles included for full-text reading, another 21 were excluded for not adhering to in- and exclusion criteria. A total of 43 articles were selected for final inclusion in this review. A flowchart of the literature search results is available in .
3.2. Study characteristics
Detailed characteristics of the studies included in this review are available in . The majority of the articles originated from Asia (n = 28), with China (n = 14) and Japan (n = 11) as the most common countries of origin of the articles. The remainder of the articles came from The Netherlands (n = 4), the USA (n = 4), South Korea (n = 4), France (n = 2), Austria (n = 2), Romania (n = 1), and Italy (n = 1).
Table 1. Characteristics of articles included in this review. NR = not reported; PS = prospective; RS = retrospective; RFA = radiofrequency ablation; MWA = microwave ablation; LTP = local tumor progression; HCC = hepatocellular carcinoma; CRLM = colorectal liver metastases; AM = ablative margin; 3D-QAM = three-dimensional quantitative ablative margin; NLR = neutrophil-lymphocyte ratio, 3D-CEUS-FI = three-dimensional contrast enhanced ultrasound fusion imaging.
None of the studies were randomized trials. In total, 18 of the 43 articles (42%) were set up as prospective research. The remaining 25 articles used retrospectively selected data. One study used multicenter data, and all other articles were single center investigations.
3.3. Quality assessment
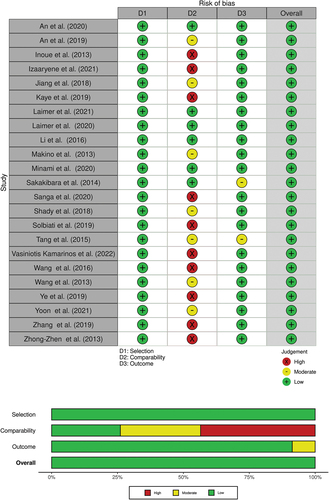
Risk of bias assessment for studies included in meta-analyses is shown in . Almost all studies scored low risk of bias on selection (23/23) and outcome (22/23) domains. Only in comparability domainwere high risk (10/23) and moderate risk of bias (7/23) noted. Main reason for worse scores on the comparability domain was lack of control for confounders. All studies scored ≥ 7 out of 9 stars and overall risk of bias was rated as low.
3.4. Ablative margin and local tumor progression
3.4.1. HCC
Of the 43 articles, 31 (72%) investigated the relation between AM and local recurrence for HCC. Detailed characteristics of each study are available in . Five of the 31 articles (16%) did not employ a registration of pre- and post-ablation image volumes and relied on visual methods to assess the ablation margins; with three articles using side-by-side visualization of pre- and post-ablation images to assess ablation margin, the other two articles using contrast agents to visualize residual tumor and ablative margins on post-ablation imaging.
Table 2. Ablative margin and measurement characteristics of studies investigating hepatocellular carcinoma. AM = ablative margin; LTP = local tumor progression; CT = computer tomography; CECT = contrast enhanced CT; MRI = magnetic resonance imaging; CEMRI = contrast enhanced MRI; US = ultrasound; CEUS = contrast enhanced US. *Results from rigid registered analysis is given; **Results from non-rigid registered analysis is given.
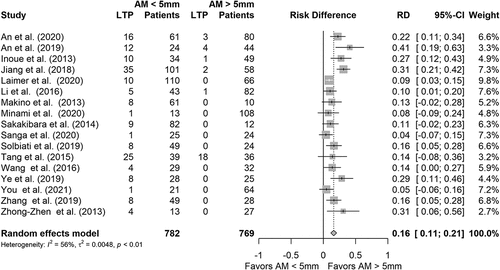
Various methods of AM categorization were applied in the studies. Most studies (17/31; 55%) categorized the lesions according to the AM reaching >5 mm or not. Five articles (16%) graded the ablative margins based on the continuity of the ablative zone around the tumor; AM(+) having an uninterrupted ablative rim around the tumor, in AM(0) the tumor is completely encapsulated but ablative rim is partially discontinued, AM(-) tumor protrudes from ablation zone. Two studies investigated surface coverage of the tumor by a 5 mm margin, with both studies showing a larger coverage of the margin reducing the risk of LTP [Citation40,Citation62]. The other articles used unique categorization of the ablative margins. Where possible, results were pooled to analyze the effect of AM <5 mm vs. >5 mm. Seventeen articles could be included, resulting in a pooled population of 782 lesions with AM <5 mm, and 769 lesions with AM >5 mm. All studies favored AM >5 mm to reduce the risk of LTP. Pooled analysis shows a reduction in the absolute risk for an HCC treated with AM >5 mm of developing LTP by 16% points (95% CI: 0.11–0.21), compared to AM <5 mm ().
3.4.2. CRLM
Nine studies (22%) assessed the LTP rate for ablative margins reached in the treatment for CRLM, detailed characteristics on AM measurements and LTP rates are available in .
Table 3. Ablative margin and measurement characteristics of studies investigating colorectal liver metastases. AM = ablative margin; LTP = local tumor progression; CT = computer tomography; CECT = contrast enhanced CT; MRI = magnetic resonance imaging; CEMRI = contrast enhanced MRI. *Results from rigid registered analysis is given.
One study categorized the AM according to reaching AM <5 mm or >5 mm. Four other studies used multiple threshold categorizations. Three studies analyzed the mean or median MAM for lesions with and without LTP, all concluding that lesions with LTP were associated with a mean negative MAM, while the lesions without LTP had completely ablated tumors with a mean positive margin: −6.6 mm vs. 0.5 mm, −2.4 mm vs. 0.0 mm, and −2.6 mm vs. 2.1 mm, respectively [Citation27,Citation37,Citation57].
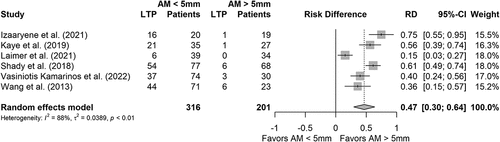
Where possible, results were pooled to analyze the effect of AM <5 mm vs. AM >5 mm () Six studies could be included, with a pooled population of 316 lesions vs. 201 lesions for, respectively, AM <5 mm and AM >5 mm. Again, all studies favored AM >5 mm to reduce the risk of LTP. Pooled analysis shows an absolute RD between CRLM treated with AM <5 mm vs. AM >5 mm of 47% points (95% CI: 0.30–0.64).
3.4.3. Mixed
Three studies reported on mixed populations of both CRLM and HCC, without specifying results per tumor type. Again, these studies show a decrease in LTP with increasing AM. Due to the limited number of studies available, RD was not calculated for these studies.
3.5. Ablative margin assessment
3.5.1. Pre-ablation imaging
Contrast enhanced computed tomography (CECT) scans were acquired prior to thermal ablation in 17 out of 41 (41%) studies. Seven studies (17%) used contrast enhanced magnetic resonance imaging (CEMRI) as pre-treatment imaging. Two studies did not require pre-ablation imaging for AM assessment and used only post-ablation imaging with contrast agents for measuring the AM [Citation54,Citation66]. The other studies used multiple different modalities of pre-ablation imaging for their AM measurements, details are available in , . Time from imaging to treatment session was often not reported (17/41 studies; 41%). Reported periods from imaging to treatment ranged from intraprocedural imaging up to 3 months prior to ablation. Intraprocedural imaging was used in 11 of the 41 studies (27%).
Table 4. Ablative margin and measurement characteristics of studies investigating mixed populations of HCC and CRLM. AM = ablative margin; LTP = local tumor progression; CT = computer tomography; CECT = contrast enhanced CT; MRI = magnetic resonance imaging; CEMRI = contrast enhanced MRI; CEUS = contrast enhanced ultrasound. * sufficient ablative margin is categorized as >5 mm for HCC, > 10 mm for CRLM.
3.5.2. Post-ablation imaging
Post-ablation imaging was most often performed by CECT scans (19/43; 44%), with CEMRI and (3D-) contrast-enhanced ultrasonography (CEUS) following with both six studies (24%). The other studies used various combinations of post-ablation imaging for their AM measurements, details are available in , and . Nineteen studies (44%) performed the AM measurements on imaging acquired intraprocedural or immediate post-ablation. Eleven other articles (26%) acquired their imaging within a week of ablation treatment. In 11 articles (26%), the interval from treatment to image acquisition ranged from 1 month up to 3 months. In the remaining two articles, the interval from treatment to follow-up imaging was not reported. Several studies investigated the agreeability in measured margins between multiple imaging modalities [Citation38,Citation41,Citation47,Citation53,Citation59,Citation71].
3.5.3. Image registration
Multiple methods for image registration were employed in the included studies. The various methods were simplified into two methods: rigid registration vs. non-rigid registration. Nine articles (21%) used a non-rigid registration method to deformably align the pre-and post-ablation image volumes. In 20 studies (47%), a rigid registration was used. Five studies (12%) did not report their method for image registration. Eight studies did not perform a registration of the images, instead assessing the ablative margins on side-by-side visualization of the pre- and post-imaging.
One study compared two different 3D registration methods (rigid with tissue contraction simulation and rigid without tissue contraction simulation) and 2D side-by-side visualization. The study found assessed ablative margin depending heavily on the registration method used, with 30/104 patients reaching >5 mm AM (10% LTP) on 3D registration without tissue contraction versus 70/104 patients (28.6% LTP) when using registration with tissue contraction versus 87/104 patients (36.8% LTP) on 2D side-by-side assessment [Citation68].
3.5.4. 2D vs 3D margin assessment
Margin assessment can be achieved on 2D images, using the coronal, sagittal and axial imaging to measure or rate the ablative margin, or in 3D by segmenting the tumor and ablation zone and calculating distances or volumetric distance maps between the two segmented volumes. Of the 43 articles, 10 studies (23%) measured ablative margins in 3D. One article investigated 3D AM assessment versus 2D assessment and found 3D assessment to be more discriminative with an AUC of 0.893 vs. 0.790, respectively (p = 0.01) [Citation45]. The 3D assessment resulted in fewer patients being categorized as having an AM greater than 5 mm: 27/93 (29%) with 3D measurement vs. 34/93 (37%) with 2D measurement.
3.6. Ablation modality
All studies reported ablation techniques used. Thirty-three out of the 43 (77%) articles employed RFA as their sole technique for thermal ablation. 7 out of 43 studies (16%) used solely MWA, while in three studies (7%) both modalities were used. These three studies further differentiated the groups by the use of either RFA or MWA. One study found patients treated with RFA having a higher chance of not achieving an AM greater than 5 mm, 79% vs. 100% of the patients treated with RFA vs. MWA [Citation63]. This consequently resulted in 1-, 2-, and 3-year LTP rates of 27 vs 7%, 40% vs 15% and 47% vs 32% for RFA vs. MWA. However, in multivariate analysis the ablation modality was not an independent predictor for LTP. Another study found 51% and 42% of patients having an AM greater than 5 mm for RFA and MWA, respectively [Citation60]. This was however not represented in the LTP rate after 1 and 2 years, being 31% and 39% for RFA vs. 25 and 40% for MWA, respectively. The third study found the ablation technique to have no statistically significant impact on LTP, whereas the measured ablative margin did [Citation50].
3.7. Image guidance
Ultrasound (US) for image guidance of the ablation was used in 25 of the 42 included articles (60%). Computed tomography (CT) guidance was employed in 10 out of 42 articles (24%). In four studies (10%), both CT and US were used for image guidance. The remaining three articles did not mention the method of image guidance used during the ablation procedure.
3.8. Intraprocedural assessment
Fifteen studies (35%) performed an intraprocedural assessment of ablation completeness using pre- and post-ablation imaging. Three articles gave data on the effect of intraprocedural ablation verification with an additional study investigating the added value of intraprocedural CEUS image fusion with pre-ablation CECT/CEMRI/CEUS imaging [Citation44,Citation51,Citation59,Citation67]. In this study, the cohort treated with the aid of image fusion achieved a lower rate of insufficient ablations in the first RFA session compared to a control group (88% vs. 60%, p = 0.041). The image fusion cohort also saw a lower LTP rate of 8.3% vs. 12.6%, but these results were not significant (p = 0.98) [Citation67].
4. Discussion
This review provides an overview of the current literature available on ablative margins and its relation to LTP after thermal ablation of HCC and CRLM. Present evidence underlines the current recommendation to aim for an ablative margin of at least 5 mm to significantly reduce the risk of LTP. At the same time, a multitude of methods is currently used for ablative margin measurement. Reported AMs may largely depend on various factors, such as imaging modality, timing of pre- and post-treatment imaging, image co-registration technique, and dimensionality of the measurements.
All studies included in this review found an association between an increased ablative margin and a reduction in risk of LTP. The RD between the pooled patient populations with AM less than 5 mm and greater than 5 mm was 16% points for HCC, while for CRLM, the RD was even larger with 47% points. Considering reported LTP rates in current literature are on the order of 3–50%, with an average of 13.6% in a previous meta-analysis, this indicates local recurrences may to a large degree be attributable to achieving insufficient ablative margins. This corresponds to several studies reporting that upon retrospective analysis of patients previously treated with liver thermal ablation the intended AM of >5 mm was not achieved in large proportions (35% − 92%) [Citation49,Citation53].
However, heterogeneity in pooled analysis for both HCC and CRLM was relatively high, meaning that the found results cannot be completely attributed to the ablative margin but other factors may also be of influence. Although overall risk of bias was low, multiple studies showed moderate or high-risk of bias for comparability due to absence of control for confounders. Studies that did perform multivariate analysis of factors influencing LTP rate all concluded AM to be one of or the only independent predictor of LTP. Other identified predictive factors were tumor diameter, perivascular location and number of tumors or metastases [Citation34,Citation42,Citation44,Citation51,Citation60,Citation63,Citation70]. For both tumor diameter and perivascular location, the effect can be rationalized as both factors make it increasingly more difficult to achieve complete tumor coverage including an adequate circumferential ablative margin. Large tumors will often require multiple overlapping ablations to achieve complete ablation coverage, increasing the risk of not achieving a spherical ablation zone completely encapsulating the tumor. Thermal ablation in perivascular locations is affected by the cooling effect of the blood flow, i.e. the heatsink effect. In these areas, achieved ablative margins are restricted to reaching the vessel wall or fully encircling the vessel with larger vessels often remaining patent. As a result, it is more difficult to achieve complete ablation, which is conceived to be the cause of increased risk of LTP. A recent meta-analyses concluded MWA to provide comparable or better results to RFA in terms of local recurrence rates and tumor control [Citation75–77]. As MWA is known to be less sensitive to the heat sink effect and impedance differences between tissue layers and capable to achieve larger ablation zones, theuse of MWA may improve the ability of achieving sufficient ablative margins [Citation78]. However, none of the included articles found the ablation technique to be a prognostic factor for LTP, suggesting that both techniques can achieve similar control rates as long as sufficient ablative margins are reached. Further analysis is, therefore, needed to determine whether MWA also leads to improved ablative margins and local outcomes at perivascular locations and in larger tumors [Citation78].
Across studies, large variation was observed in methodology for ablative margin measurement in terms of used imaging modality, timing of pre- and post-treatment imaging, image co-registration technique and 2D vs. 3D measurement. In AM assessment, CT and MRI have a theoretical advantage over US, as they can provide volumetric imaging of the tumor and the ablation zone and are not impeded by gas formation hampering full ablation zone assessment. Post-ablation imaging was most often performed with CECT, as it can facilitate treatment of deeper situated tumors and provide direct imaging, enabling intraprocedural confirmation of tumor coverage by the ablation zone. Nevertheless, the use of ionizing radiation has to be considered by the treating physician. While MRI also provides near real-time imaging, the higher costs associated with MRI and the limited compatibility of RFA and MWA equipment can hamper implementation compared to CT guidance. US is a fast and often used tool for procedural guidance, as evidenced by the majority of articles included in this review, mostly limited by the ability of guidance for deeper situated tumors. There have been few studies performed investigating the influence of different imaging modalities on AM assessment. Of the included studies, Kim et al. found MRI to be superior to CECT in differentiating the AM based on subsequent LTP, while Inoue et al., Sanga et al. and Ye et al. found (3D-)CEUS to have good agreeability in the measured AM compared with measurements on CECT or CEMRI scans [Citation41,Citation47,Citation59,Citation71]. As it is currently not known if either of the imaging modalities under- or overestimates the ablative margin, further research in this direction is imperative to find the optimal imaging method of ablative margin measurements in the workflow for thermal ablation.
Time interval between ablation procedure and image acquisition varied between studies. Many studies did not mention the timing of pre-ablation imaging, with two studies including imaging made up to 2 months before the treatment. Any time interval may introduce errors to the AM assessment, since both HCC and CLRM are fast growing tumors that can double in volume within months [Citation79–81]. Similarly, a delay in post-ablation imaging may bias the AM assessment due to morphological changes resulting from tissue responses occurring in the first days to weeks after ablation, followed by shrinkage [Citation82,Citation83]. Nine articles reported to have used both intraprocedural pre- and post-ablation imaging, but due to poor reporting of the interval for pre-ablation imaging this number is uncertain.
Image registration of pre- and post-ablation imaging is paramount to accurately assessing ablative margins. Side-by-side assessment of the ablative margin on pre- and post-ablation imaging has poor intrareader agreeability, with an intraclass correlation coefficient of 0.357 (95% CI: 0.194–0.522) in a study by Schaible et al [Citation84]. This is backed up by studies from Yoon et al., Vasiniotis Kamarinos et al., Shin et al., and a recent article by Zhou et al. All found a relatively large proportion of ablations having an insufficient ablative margin after margin assessment using registered images, compared to initial side-by-side assessment [Citation61,Citation68,Citation72,Citation85]. In many articles conditions for successful registration were given. Oftentimes, registration was considered successful if target registration error between a chosen landmark was less than 3 mm or a similar measurement [Citation52]. When measuring margins of 5 mm, a target registration error of up to 3 mm can have significant impact on the measured AM.
Most studies measured AM in 2D on coronal, axial and sagittal images. 3D measurement based on tumor and ablation volume surfaces potentially allows for a more accurate representation of the true minimal ablative margin in any given direction. The ten studies that performed measurements on 3D segmentations of tumor and ablation zone also were enabled to calculate subpercentages of the tumor that were treated with a certain ablative margin [Citation40,Citation48,Citation57,Citation60,Citation62]. Nearly, all studies categorized AM according to predefined thresholds. In future studies it would be imperative to have studies providing data on continuous ablative margins. This allows for more detailed analysis of all ablative margins to find the optimal threshold to reduce risk of LTP.
Intraprocedural ablation verification is still relatively rarely used, with 16 included articles reporting the verification of ablation zone being part of their ablation work process. Articles and data on the effects of intraprocedural image fusion are even more scarce. Three articles provided numbers on the amount of re-ablations within the first session, after intraoperative imaging showed insufficient ablation margins. Joo et al., Li et al., and Sanga et al. performed additional ablations in 14% and 34% of patients, after intraprocedural AM assessment revealed insufficient ablative margins [Citation44,Citation51,Citation59].
This review has a several limitations. Many different categorizations of ablative margins were used in the included studies, complicating homogenization of the data. A complete meta-analysis of the studies was therefore not possible. No consensus on reporting of ablative margins exists. The most common method of categorization was differentiation between AM <5 mm vs. AM >5 mm. However, this method is relatively crude and methodology of measurements varied greatly between different studies, introducing uncertainty to the found margins and the comparability. Additionally, the margin of 5 mm was not consistently grouped in either the group of AM <5 mm or AM >5 mm between different studies and remains ambiguous. Despite these uncertainties, margins measured to be greater than 5 mm were found to have a decreased risk of LTP. Standardization of AM measurement and reporting is critical to allow future meta-analyses and identification of optimal threshold value for clinical use.
Another limitation of this study is the inclusion of only studies related to HCC or CRLM. While these are the most common hepatic malignancies, nearly all tumors can metastasize to the liver. We can therefore not conclude whether these margins are also applicable to thermal ablation of other hepatic tumors. Some data were not systematically reported in all studies, such as imaging interval prior to treatment and the use of intraprocedural imaging to assess the ablative margin immediately post-ablation. These are all factors that can impact the achieved ablation zone and accuracy of measurements.
For future studies, it is recommended to report the complete work process and timing from pre-operative imaging to ablation treatment to post-operative imaging. Ideally, the imaging should all be performed intraprocedurally, to provide the most accurate and recent imaging to measure tumor, ablation zone and ablative margin. It is also recommended to perform measurements on segmented 3D models of the tumor and ablative zones, to provide more accurate and detailed results and the acquired ablative margins.
5. Conclusion
Current evidence supports the aim of achieving an ablative margin >5 mm to reduce the risk of LTP after percutaneous thermal ablation of HCC and CRLM. However, heterogeneity in AM measurement methods and a lack of continuous data on ablative margin thresholds hampers a detailed analysis of optimal margin for the minimization of LTP. Standardization of ablative margin measurement and reporting is critical to allow future meta-analyses and identification of optimal threshold value for clinical use.
6. Expert opinion
This review underlines the critical importance of achieving sufficient ablative margins for effective liver thermal ablation and summarizes the currently used methods for AM measurement. Even in the presence of substantial heterogeneity in AM measurement methodology, all studies confirm the influence of AM to reduce the risk of LTP. Therefore, to the author’s opinion, assessment of ablative margins should become a mandatory requirement to confirm treatment adequacy and ensure optimal and reliable outcomes of liver thermal ablation in daily practice.
As the importance of ablative margins has been increasingly recognized in recent years, multiple software tools to assess ablative margins are now entering the clinic. However, most of these tools have not been extensively validated in large cohorts and given the large dependency of AM measurement on imaging modality, timing of pre-ablation and post-ablation imaging and image co-registration technique, predictive value for LTP and the optimal threshold value may vary across different software tools and methodologies. The field would greatly benefit from standardization in AM measurement and reporting, allowing improved comparison of results across studies. At minimum, studies should report details on AM measurement methodology, provide appropriate 2 × 2 tables as well as absolute margin measurements and report related LTP rates. Ideally, continuous data of AM measurement studies should become available to facilitate data pooling and allow more accurate determination of an optimal cutoff value for clinical use. In addition, it would be recommendable to establish a large multicenter dataset with known outcome data to validate AM software tools and methodologies against and serve as a benchmark to assess predictive performance before entering the clinic.
Finally, optimal strategies to incorporate margin analysis in the clinical process need to be established. Ideally, we recommend the use of intra-interventional pre- and post-ablation CECT acquired in apnea to yield best accuracy in margin determination and allow for immediate re-ablation if required, thereby preventing the need for repeated treatment. Diagnostic pre-treatment imaging or post-treatment follow-up (e.g. day 1 or 7 post-ablation) imaging or other modalities (e.g. MRI or CEUS) may be useful surrogates when thoroughly validated but are not able to reduce the need for an additional treatment session. Thereby, to the author’s opinion, intra-interventional AM assessment should become the standard. In the coming years, larger prospective studies are needed to evaluate the effects of ablative margin analysis on primary treatment efficacy and safety and firmly establish a clinically adopted methodology leading to optimized outcomes of liver thermal ablation. Ultimately, these strategies and their clinical consequences need to be integrated to come to well-evidenced recommendations on intra-interventional margin evaluation, indications for immediate or repeat re-ablation or intensified follow-up in high-risk patients based on the achieved ablative margin.
Article highlights
Thermal ablation is an established treatment in early stage hepatocellular carcinoma (HCC) and low-volume colorectal liver metastases (CRLM), however reported local recurrence rates remain high.
In recent years, the ablative margin (AM) has been shown a main predictor of local tumor progression (LTP), but there is no consensus on the optimal margin threshold to avoid LTP.
Current evidence shows achieving an AM >5 mm to reduce the risk of LTP with 16% points for HCC and 47% points for CRLM as compared to AM < 5mm.
Included studies however showed high variability in AM measurement methodology in terms of measurement technique, imaging modalities, and timing.
Standardization of AM measurement and reporting is critical to allow future meta-analyses and improved identification of optimal threshold value for clinical use.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- de Ridder J, de Wilt JH, Simmer F, et al. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget. 2016;7(34):55368–55376. doi: 10.18632/oncotarget.10552
- Hugen N, van de Velde CJH, de Wilt JHW, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–657. doi: 10.1093/annonc/mdt591
- Riihimaki M, Thomsen H, Sundquist K, et al. Clinical landscape of cancer metastases. Cancer Med. 2018;7(11):5534–5542. doi:10.1002/cam4.1697
- Engstrand J, Nilsson H, Stromberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78. doi: 10.1186/s12885-017-3925-x
- van der Geest LG, Lam-Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32(5):457–465. doi: 10.1007/s10585-015-9719-0
- Qaderi SM, Galjart B, Verhoef C, et al. Disease recurrence after colorectal cancer surgery in the modern era: a population-based study. Int J Colorectal Dis. 2021;36(11):2399–2410. doi: 10.1007/s00384-021-03914-w
- Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer. 2021;21(1):1157. doi: 10.1186/s12885-021-08904-3
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492
- Brar G, Greten TF, Graubard BI, et al. Hepatocellular carcinoma survival by Etiology: a SEER-Medicare database analysis. Hepatol Commun. 2020;4(10):1541–1551. doi: 10.1002/hep4.1564
- Petrowsky H, Fritsch R, Guckenberger M, et al. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17(12):755–772. doi: 10.1038/s41575-020-0314-8
- de Ridder JA, Lemmens VE, Overbeek LI, et al. Liver resection for metastatic disease; a population-based analysis of trends. Dig Surg. 2016;33(2):104–113. doi: 10.1159/000441802
- Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):98. doi: 10.1186/s12957-019-1632-6
- Yang G, Xiong Y, Sun J, et al. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Int J Surg. 2020;77:85–93. doi: 10.1016/j.ijsu.2020.03.006
- Dijkstra M, Nieuwenhuizen S, Puijk RS, et al. Thermal ablation compared to partial hepatectomy for recurrent colorectal liver metastases: an Amsterdam Colorectal liver met Registry (AmCORE) based study. Cancers (Basel). 2021;13(11):2769. doi: 10.3390/cancers13112769
- van Amerongen MJ, Jenniskens SFM, van den Boezem PB, et al. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford). 2017;19(9):749–756. doi: 10.1016/j.hpb.2017.05.011
- van Amerongen MJ, van der Stok EP, Futterer JJ, et al. Results after simultaneous surgery and RFA liver ablation for patients with colorectal carcinoma and synchronous liver metastases. Eur J Surg Oncol. 2019;45(12):2334–2339. doi: 10.1016/j.ejso.2019.07.016
- Garuti F, Neri A, Avanzato F, et al. The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int. 2021;41(3):585–597. doi: 10.1111/liv.14735
- Reinders MTM, van Meer S, Burgmans MC, et al. Trends in incidence, diagnosis, treatment and survival of hepatocellular carcinoma in a low-incidence country: data from the Netherlands in the period 2009-2016. Eur J Cancer. 2020;137:214–223. doi: 10.1016/j.ejca.2020.07.008
- Takahashi H, Kahramangil B, Berber E. Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series. Surgery. 2018;163(4):709–713. doi: 10.1016/j.surg.2017.10.026
- Kingham TP, Tanoue M, Eaton A, et al. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19(3):834–841. doi: 10.1245/s10434-011-2048-x
- Leung U, Kuk D, D’Angelica MI, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102(1):85–91. doi: 10.1002/bjs.9649
- Schullian P, Laimer G, Johnston E, et al. Technical efficacy and local recurrence after stereotactic radiofrequency ablation of 2653 liver tumors: a 15-year single-center experience with evaluation of prognostic factors. Int J Hyperthermia. 2022;39(1):421–430. doi: 10.1080/02656736.2022.2044522
- Shi Y, Wang Z, Chi J, et al. Long-term results of percutaneous microwave ablation for colorectal liver metastases. HPB (Oxford). 2021;23(1):37–45. doi: 10.1016/j.hpb.2020.04.007
- Jiang B, Luo H, Yan K, et al. Ten-year outcomes of percutaneous radiofrequency ablation for colorectal cancer liver metastases in perivascular vs. Non-perivascular locations: a propensity-score matched study. Front Oncol. 2020;10:553556. doi: 10.3389/fonc.2020.553556
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171. doi:10.1097/01.sla.0000171032.99149.fe
- Sibinga Mulder BG, Hendriks P, Baetens TR, et al. Quantitative margin assessment of radiofrequency ablation of a solitary colorectal hepatic metastasis using MIRADA RTx on CT scans: a feasibility study. BMC Med Imaging. 2019;19(1):71. doi: 10.1186/s12880-019-0360-2
- Wells SA, Hinshaw JL, Lubner MG, et al. Liver ablation: best practice. Radiol Clin North Am. 2015;53(5):933–971. doi: 10.1016/j.rcl.2015.05.012
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71
- Kwon Y, Lemieux M, McTavish J, et al. Identifying and removing duplicate records from systematic review searches. J Med Libr Assoc. 2015;103(4):184–188. doi: 10.3163/1536-5050.103.4.004
- Clark J, Glasziou P, Del Mar C, et al. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90. doi: 10.1016/j.jclinepi.2020.01.008
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2022. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- An C, Jiang Y, Huang Z, et al. Assessment of ablative margin after microwave ablation for hepatocellular carcinoma using deep learning-based deformable image registration. Front Oncol. 2020;10:573316. doi: 10.3389/fonc.2020.573316
- An C, Li X, Liang P, et al. A tumor map generated from three-dimensional visualization of image fusion for the assessment of microwave ablation of hepatocellular carcinoma: a preliminary study. Cancer Manag Res. 2019;11:1569–1578. doi: 10.2147/CMAR.S195354
- Bo XW, Xu HX, Guo LH, et al. Ablative safety margin depicted by fusion imaging with post-treatment contrast-enhanced ultrasound and pre-treatment CECT/CEMRI after radiofrequency ablation for liver cancers. Br J Radiol. 2017;90(1078):20170063. doi: 10.1259/bjr.20170063
- Du H, Tan X, Cheng L, et al. Application and evaluation of a 64-slice CT three-dimensional fusion technique in the determination of the effective ablation margin after radiofrequency ablation of hepatocellular carcinoma. Comput Math Methods Med. 2022;2022:1–12. doi: 10.1155/2022/6898233
- Faber RA, Burghout KST, Bijlstra OD, et al. Three-dimensional quantitative margin assessment in patients with colorectal liver metastases treated with percutaneous thermal ablation using semi-automatic rigid MRI/CECT-CECT co-registration. Eur J Radiol. 2022;156:110552. doi: 10.1016/j.ejrad.2022.110552
- Fukuda K, Mori K, Hasegawa N, et al. Safety margin of radiofrequency ablation for hepatocellular carcinoma: a prospective study using magnetic resonance imaging with superparamagnetic iron oxide. Jpn J Radiol. 2019;37(7):555–563. doi: 10.1007/s11604-019-00843-1
- Hendriks P, Noortman WA, Baetens TR, et al. Quantitative volumetric assessment of ablative margins in hepatocellular carcinoma: predicting local tumor progression using nonrigid registration software. J Oncol. 2019;2019:1–8. doi: 10.1155/2019/4049287
- Hocquelet A, Trillaud H, Frulio N, et al. Three-dimensional measurement of hepatocellular carcinoma ablation zones and margins for predicting local tumor progression. J Vasc Interv Radiol. 2017;27(7):1038–1045.e2. doi: 10.1016/j.jvir.2016.02.031
- Inoue T, Kudo M, Hatanaka K, et al. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology. 2013;84(Suppl 1):51–57. doi: 10.1159/000345890
- Izaaryene J, Drai M, Deniel C, et al. Computed tomography-guided microwave ablation of perivascular liver metastases from colorectal cancer: a study of the ablation zone, feasibility, and safety. Int J Hyperthermia. 2021;38(1):887–899. doi: 10.1080/02656736.2021.1912413
- Jiang C, Liu B, Chen S, et al. Safety margin after radiofrequency ablation of hepatocellular carcinoma: precise assessment with a three-dimensional reconstruction technique using CT imaging. Int J Hyperthermia. 2018;34(8):1135–1141. doi:10.1080/02656736.2017.1411981
- Joo I, Morrow K, Raman S, et al. CT-monitored minimal ablative margin control in single-session microwave ablation of liver tumors: an effective strategy for local tumor control. Eur Radiol. 2022;32(9):6327–6335. doi: 10.1007/s00330-022-08723-5
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29(5):2698–2705. doi: 10.1007/s00330-018-5809-0
- Ke S, Ding X, Qian X, et al. Radiofrequency ablation of hepatocellular carcinoma sized > 3 and ≤ 5 cm: is ablative margin of more than 1 cm justified? World J Gastroenterol. 2014;19(42):7389. doi: 10.3748/wjg.v19.i42.7389
- Kim S, Shin S, Lee B, et al. Imaging evaluation of ablative margin and index tumor immediately after radiofrequency ablation for hepatocellular carcinoma: comparison between multidetector-row CT and MR imaging. Abdom Radiol (NY). 2018;42(10):2527–2537. doi: 10.1007/s00261-017-1146-z
- Laimer G, Jaschke N, Schullian P, et al. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur Radiol. 2021;31(9):6489–6499. doi: 10.1007/s00330-020-07579-x
- Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30(5):2463–2472. doi: 10.1007/s00330-019-06609-7
- Li FY, Li JG, Wu SS, et al. An optimal ablative margin of Small single hepatocellular carcinoma treated with image-guided percutaneous thermal ablation and local recurrence Prediction Base on the ablative margin: A multicenter study. J Hepatocell Carcinoma. 2021;8:1375–1388. doi: 10.2147/JHC.S330746
- Li K, Su ZZ, Xu EJ, et al. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016;16(1):277. doi: 10.1186/s12885-016-2306-1
- Makino Y, Imai Y, Igura T, et al. Utility of computed tomography fusion imaging for the evaluation of the ablative margin of radiofrequency ablation for hepatocellular carcinoma and the correlation to local tumor progression. Hepatol Res. 2013;43(9):950–958. (Ed.^(Eds). doi: 10.1111/hepr.12049
- Makino Y, Imai Y, Igura T, et al. Feasibility of extracted-overlay fusion imaging for intraoperative treatment evaluation of radiofrequency ablation for hepatocellular carcinoma. Liver Cancer. 2016;5(4):269–279. doi: 10.1159/000449338
- Matsuki Y, Matono T, Koda M, et al. Preablation three-dimensional ultrasonography can predict therapeutic effect and local tumor progression after radiofrequency ablation for hepatocellular carcinoma. Eur J Radiol. 2021;133:109358. doi: 10.1016/j.ejrad.2020.109358
- Minami Y, Minami T, Takita M, et al. Radiofrequency ablation for hepatocellular carcinoma: clinical value of ultrasound-ultrasound overlay fusion for optimal ablation and local controllability. Hepatol Res. 2020;50(1):67–74. doi: 10.1111/hepr.13407
- Park J, Lee J, Lee D, et al. Value of nonrigid registration of pre-procedure MR with post-procedure CT after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2018;40(6):873–883. doi: 10.1007/s00270-017-1571-y
- Ruiter SJS, Tinguely P, Paolucci I, et al. 3D quantitative ablation margins for prediction of ablation site recurrence after stereotactic image-guided microwave ablation of colorectal liver metastases: a multicenter study. Front Oncol. 2021;11:757167. doi: 10.3389/fonc.2021.757167
- Sakakibara M, Ohkawa K, Katayama K, et al. Three-dimensional registration of images obtained before and after radiofrequency ablation of hepatocellular carcinoma to assess treatment adequacy. Am J Roentgenol. 2014;202(5):W487–W495. doi: 10.2214/AJR.13.11384
- Sanga K, Numata K, Nihonmatsu H, et al. Use of intra-procedural fusion imaging combining contrast-enhanced ultrasound using a perflubutane-based contrast agent and auto sweep three-dimensional ultrasound for guiding radiofrequency ablation and evaluating its efficacy in patients with hepatocellular carcinoma. Int J Hyperthermia. 2020;37(1):202–211. doi: 10.1080/02656736.2020.1729422
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275 e261. doi: 10.1016/j.jvir.2017.08.021
- Shin S, Lee JM, Kim KW, et al. Postablation assessment using follow-up registration of CT images before and after radiofrequency ablation (RFA): prospective evaluation of midterm therapeutic results of RFA for hepatocellular carcinoma. Am J Roentgenol. 2014;203(1):70–77. doi: 10.2214/AJR.13.11709
- Solbiati M, Muglia R, Goldberg SN, et al. A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia. 2019;36(1):337–343. doi: 10.1080/02656736.2019.1569267
- Sparchez Z, Mocan T, Hajjar NA, et al. Percutaneous ultrasound guided radiofrequency and microwave ablation in the treatment of hepatic metastases. A monocentric initial experience. Med Ultrason. 2019;21(3):217–224. doi: 10.11152/mu-1957
- Takeyama N, Mizobuchi N, Sakaki M, et al. Evaluation of hepatocellular carcinoma ablative margins using fused pre- and post-ablation hepatobiliary phase images. Abdom Radiol (NY). 2019;44(3):923–935. doi: 10.1007/s00261-018-1800-0
- Tang H, Tang Y, Hong J, et al. A measure to assess the ablative margin using 3D-CT image fusion after radiofrequency ablation of hepatocellular carcinoma. HPB (Oxford). 2015;17(4):318–325. doi: 10.1111/hpb.12352
- Tokunaga S, Koda M, Matono T, et al. Assessment of ablative margin by MRI with ferucarbotran in radiofrequency ablation for liver cancer: comparison with enhanced CT. Br J Radiol. 2012;85(1014):745–752. doi: 10.1259/bjr/64518148
- Toshikuni N, Matsue Y, Ozaki K, et al. An image fusion System for Estimating the therapeutic effects of radiofrequency ablation on hepatocellular carcinoma. Radiol Oncol. 2017;51(3):263–269. doi: 10.1515/raon-2017-0028
- Vasiniotis Kamarinos N, Gonen M, Sotirchos V, et al. 3D margin assessment predicts local tumor progression after ablation of colorectal cancer liver metastases. Int J Hyperthermia. 2022;39(1):880–887. doi: 10.1080/02656736.2022.2055795
- Wang X, Li K, Su Z, et al. Assessment of radiofrequency ablation margin by MRI-MRI image fusion in hepatocellular carcinoma. World J Gastroenterol. 2016;21(17):5345. doi: 10.3748/wjg.v21.i17.5345
- Wang XD, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175. doi: 10.1007/s00270-012-0377-1
- Ye J, Huang G, Zhang X, et al. Three-dimensional contrast-enhanced ultrasound fusion imaging predicts local tumor progression by evaluating ablative margin of radiofrequency ablation for hepatocellular carcinoma: a preliminary report. Int J Hyperthermia. 2019;36(1):55–64. doi: 10.1080/02656736.2018.1530460
- Yoon J, Lee J, Klotz E, et al. Prediction of local tumor progression after radiofrequency ablation (RFA) of hepatocellular carcinoma by assessment of ablative margin using pre-RFA MRI and post-RFA CT registration. Korean J Radiol. 2019;19(6):1053–1065. doi: 10.3348/kjr.2018.19.6.1053
- Zhang X, Huang G, Ye J, et al. 3-D contrast-enhanced ultrasound fusion imaging: A New technique to evaluate the ablative margin of radiofrequency ablation for hepatocellular carcinoma. Ultrasound Med Biol. 2019;45(8):1933–1943. doi: 10.1016/j.ultrasmedbio.2019.03.019
- Zhong-Zhen S, Kai L, Rong-Qin Z, et al. A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall der Medizin. 2013;33(7):E250–E255. doi: 10.1055/s-0032-1325466
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):264–272. doi: 10.1080/02656736.2018.1562571
- Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438. doi: 10.2147/OTT.S204340
- Facciorusso A, Abd El Aziz MA, Tartaglia N, et al. Microwave ablation versus radiofrequency ablation for treatment of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Cancers (Basel). 2020;12(12):3796. doi: 10.3390/cancers12123796
- Kim C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Future Oncol. 2018;14(17):1755–1764. doi: 10.2217/fon-2017-0736
- Finlay IG, Meek D, Brunton F, et al. Growth rate of hepatic metastases in colorectal carcinoma. Br J Surg. 1988;75(7):641–644. doi: 10.1002/bjs.1800750707
- Kubota K, Ina H, Okada Y, et al. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48(3):581–586. doi: 10.1023/A:1022505203786
- Taouli B, Goh JS, Lu Y, et al. Growth rate of hepatocellular carcinoma: evaluation with serial computed tomography or magnetic resonance imaging. J Comput Assist Tomogr. 2005;29(4):425–429. doi: 10.1097/01.rct.0000164036.85327.05
- Yu MH, Kim YJ, Park HS, et al. Shrinkage of hepatocellular carcinoma after radiofrequency ablation following transcatheter arterial chemoembolization: analysis of contributing factors. Plos One. 2019;14(2):e0210667. doi: 10.1371/journal.pone.0210667
- Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia. 2014;30(7):419–428. doi: 10.3109/02656736.2014.957250
- Schaible J, Pregler B, Bäumler W, et al. Safety margin assessment after microwave ablation of liver tumors: inter- and intrareader variability. Radiol Oncol. 2021;54(1):57–61. doi: 10.2478/raon-2020-0004
- Zhou H, Yang G, Jing X, et al. Predictive value of ablative margin assessment after microwave ablation for local tumor progression in medium and large hepatocellular carcinoma: computed tomography–computed tomography image fusion method versus side-by-side method. J Comput Assist Tomogr. 2023;47(1):31–37. doi: 10.1097/RCT.0000000000001395
Appendix
Search queries
PubMed
(‘Liver Neoplasms’[Mesh] OR Hepatocellular Carcinoma*[tiab] OR Liver Cell Carcinoma*[tiab] OR Hepatoma*[tiab] OR Colorectal liver metastas*[tiab] OR metastatic liver cancer*[tiab] OR liver tumor[tiab] OR liver tumor[tiab])
AND
(‘Ablation Techniques’[Mesh] OR ablation[tiab])
AND
(image guided [tiab] OR image-guided [tiab] OR magnetic resonance [tiab] OR ‘Magnetic Resonance Imaging’[Mesh] OR ‘Tomography, X-Ray Computed’[Mesh] OR x-ray[tiab] OR x-rays[tiab] OR CECT[tiab] OR CT-guided[tiab] OR ‘ultrasonography, interventional’[MeSH Terms] OR US[tiab] OR ultrasonography[tiab] OR ultrasound[tiab])
Web of Science
(‘Liver Neoplasms’ OR ‘Hepatocellular Carcinoma*’ OR ‘Liver Cell Carcinoma*’ OR Hepatoma* OR ‘Colorectal liver metastas*’ OR ‘metastatic liver cancer*’ OR ‘liver tumor’ OR ‘liver tumor’)
AND
(‘Ablation Techniques’ OR ablation)
AND
(‘image guided’ OR image-guided OR ‘magnetic resonance’ OR ‘Magnetic Resonance Imaging’ OR ‘Tomography, X-Ray Computed’ OR x-ray OR x-rays OR CECT OR CT-guided OR ‘ultrasonography, interventional’ OR US OR ultrasonography OR ultrasound)