ABSTRACT
Introduction
Despite recent advances in diagnostic technologies and new drugs becoming available, tuberculosis (TB) remains a major global health burden. If detected early, screened for drug resistance, and fully treated, TB could be easily controlled.
Areas Covered
Here the authors discuss M. tuberculosis culture methods which are considered the definitive confirmation of M. tuberculosis infection, and limited advances made to build on these core elements of TB laboratory diagnosis. Literature searches showed that molecular techniques provide enhanced speed of turnaround, sensitivity, and richness of data. Sequencing of the whole genome, is becoming well established for identification and inference of drug resistance. PubMed® literature searches were conducted (November 2022-March 2024).
Expert Opinion
This section highlights future advances in diagnosis and infection control. Prevention of prolonged hospital admissions and rapid TAT are of the most benefit to the overall patient experience. Host transcriptional blood markers have been used in treatment monitoring studies and, with appropriate evaluation, could be rolled out in a diagnostic setting. Additionally, the MBLA is being incorporated into latest clinical trial designs. Whole genome sequencing has enhanced epidemiological evidence. Artificial intelligence, along with machine learning, have the ability to revolutionize TB diagnosis and susceptibility testing within the next decade.
1. Introduction
Despite recent advances in diagnostic technologies and new drugs becoming available for treatment, tuberculosis (TB) remains a major global health burden. TB has been a leading infectious disease, causing an estimated 1.6 million deaths in 2021, most in low- and middle-income countries (LMICs) [Citation1]. In 2021, of the estimated 10.6 million people who developed TB, only 6.4 million people were diagnosed and notified to national TB programs worldwide, leaving a significant gap of undiagnosed cases. This is, in part, due to the slowness in roll out of innovative technologies but also to a large degree is a result of the complex biology of Mycobacterium tuberculosis (M. tuberculosis) which limits the applicability of solutions found for other bacterial pathogens. If detected early, screened for drug resistance, and fully treated with appropriate short-course regimens, TB could be easily controlled. In this review, we focus on recent advances in laboratory diagnosis of TB, an area in which there has been increased activity with new tools translating into routine diagnostic use.
It is essential to establish the necessary information required to develop a relevant diagnostic strategy when dealing with TB, with four main domains to be taken into consideration: 1) Diagnosis of TB, 2) Susceptibility testing, 3) Treatment monitoring, 4) Infection control implications. This is the practical framework that will follow in this review to provide an overview of current and future approaches to laboratory diagnosis of TB.
2. Diagnosis of TB
Although the focus of this review is laboratory diagnosis, clinical evaluation of the patient is the first step and, in many cases, overrides any subsequent microbiology. Conventional diagnostic protocols have limitations in TB endemic areas such as: equipment failures, delays in reporting results, insufficient infrastructure, unreliable environmental conditions, poor maintenance, and support of diagnostic machinery and high turnover of trained technical staff [Citation2]. As a result, presumptive treatment is the norm in the absence of a positive microbiology result (still pending), based on the clinical suspicion and/or the radiological appearances (in particular for respiratory TB). Sputum smear microscopy for acid fast bacilli, is universally accessible, with few innovations in this area since it was first developed by Ziehl and Neelsen in the early 1880s. The Ziehl-Neelsen stain differentially stains the lipid-rich cell wall and has good specificity for mycobacteria, with sensitivity around 103 colony forming units (CFU)/mL of sputum or 60–70% of culture-positive samples [Citation3]. Use of the fluorescent auramine-phenol stain increases sensitivity but at the cost of specificity [Citation3]. At least 5000 to 10,000 bacilli per mL are needed for the detection of bacteria in stained smears, but the fluorochrome technique is up to 10-fold more sensitive than carbol fuchsin methods [Citation4,Citation5]. Whilst microscopy remains widely used, the Ziehl-Neelsen cannot differentiate between live and dead bacilli (as well as all the molecular methods currently in clinical use) which renders such staining methods with low sensitivity and a significant limitation [Citation6,Citation7].
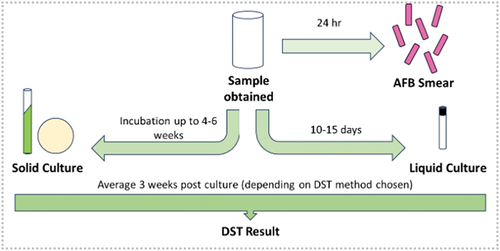
Culture is typically considered the definitive confirmation of M. tuberculosis infection. Solid media can be used for isolating M. tuberculosis from clinical specimens, these include Lowenstein-Jenson (LJ), Ogawa, Middlebrook 7H10 and Middlebrook 7H11 [Citation8]. Choice of solid media varies and is largely chosen based on personal or laboratory preference, as there is no universal consensus on which solid media is the most reliable. LJ and Ogawa both utilize homogenized whole eggs and malachite green, which can inhibit the growth of many contaminants, whilst 7H10 and 7H11 (agar-based media) avoid the inconvenience of using drug-free eggs. Middlebrook 7H10 and 7H11 have the added advantage of detecting growth faster and being able to culture fastidious Mycobacteria [Citation8]. Although solid media are still used for maintenance and archiving, automated liquid culture in systems, such as the use of the BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960 (Becton Dickinson) is most commonly used for primary diagnosis. A negative correlation between time to detection (TTD) in the BACTEC MGIT 960 and CFU/ml exists, such that a decrease in TTD correlates to an increase in bacterial load [Citation9]. These systems bring increased sensitivity and specificity when compared to solid culture and, importantly, have a faster time to positivity (typically 10–15 days in the MGIT compared to 6 weeks for LJ for a patient in the UK) and hence become a rapid alternative to colony counting [Citation9]. In contrast to microscopy, 10 to 100 organisms are needed for a positive culture [Citation10]. The workflow of TB diagnosis, in the UK was recently described by Satta et al and is summarized in [Citation11].
There have been limited advances in building on these core elements of TB laboratory diagnosis. A much-studied area of the biology of M. tuberculosis is the presence of bacterial sub-populations in differing physiological states, and it is thought that the presence of dormant bacilli contributes to reduced sensitivity in culture and an increased difficulty in eliminating such non-replicating cells [Citation12]. The work of Mukamolova and colleagues (2010) has identified a family of secreted bacterial proteins, resuscitation promoting factors (rpfs) that act as ‘wake up molecules’ and switch populations from dormancy to growth [Citation13]. These molecules have been mooted as growth enhancers in liquid culture and preliminary data suggest they may be useful as potential antigens for diagnostic assays, although this approach has not yet been translated to practice [Citation14,Citation15]. Whilst rpfs may increase the number of bacilli, an alternative approach uses mycobacteriophage to amplify the signal from the organisms present and thus increases sensitivity of the test [Citation16].
The lack of innovation in microscopy and culture may be a result of the focus on the introduction of molecular techniques providing enhanced speed of turnaround, sensitivity, and data. Since the first use of the polymerase chain reaction (PCR) as a diagnostic for TB in the late 1980s, robust, quality-assured commercial assays have gained traction in the 21st Century, particularly associated with the Cepheid GeneXpert System which gained early World Health Organization (WHO) approval and subsequently there have been incremental improvements. The Cepheid Xpert MTB/RIF Ultra increases sensitivity, when compared to the Xpert MTB/RIF, from 114 to 16 CFU/mL. More recently, in 2020, WHO endorsed the MolBio Truenat MTB system (Molbio Diagnostics, India), an alternative point of care platform to the Xpert systems, utilizing real-time micro-PCR for initial TB diagnosis with the Truenat MTB-RIF Dx assays also able to detect rifampicin resistance [Citation6]. In 2021, Penn-Nicholson et al conducted a multicenter diagnostic accuracy study, with 19 healthcare centers and seven reference laboratories, to compare the diagnostic accuracy with Xpert MTB/RIF. Truenat tests showed a sensitivity of 84% (95% CI 62–95%) and high specificity. Conclusions were that Truenat assays have a similar performance to Xpert MTB/RIF [Citation17]. The Truenat systems were developed for near patient and low resource settings and, moving away from PCR, isothermal systems such as TB LAMP (Eiken) [Citation18,Citation19]. Despite the fact that these assays confirm the presence of M. tuberculosis or the M. tuberculosis complex (MTBC), increased species-specific data can be obtained using line probe assays (LPAs), such as the Bruker Genotype portfolio of tests e.g. GenoType MTBC [Citation20]. LPAs are PCR single stranded probe reverse hybridization assays and approved by WHO in 2008 for the diagnosis of TB, due to high efficiency. A 2022 study by Lin et al, concluded that LPAs are more suitable as a complementary diagnostic assay due to the low sensitivity in extra pulmonary TB patients, high cost and requirement for trained personnel and infrastructure. To note, further studies are required for the use of LPA in the diagnosis of Pre-XDR TB [Citation21]. See , for a summary of the molecular tests used in the diagnosis of TB.
Table 1. Advantages and disadvantages of molecular tests, used in the diagnosis/detection of drug resistance for TB.
All the above tests require a sample and, as with all pathology, the test is only as good as the sample, reliant on both quality and appropriateness. Sputum remains the most frequently collected sample, although this presents challenges as a sample matrix; it is chemically complex inhibiting nucleic acid extraction and amplification, and encouraging clumping of bacteria. Patients on treatment quickly stop producing sputum, and children often do not produce it at all [Citation26]. An approach that is gaining increased traction is to look for a host response that indicates the presence of a TB infection. In this respect, the interferon-gamma release assays (IGRA), such as T-Spot (Oxford Immunotec) and Quantiferon (Qiagen) are of limited use for acute infection but have a roll in detecting latent TB. Diagnosing pleural TB is particularly challenging due to the need for invasive sampling and limited sensitivity and specificity when using pleural fluid. The interferon-gamma ultrasensitive rapid immunosuspension assay (IRISA-TB) developed by Antrum (Antrum Biotech, South Africa) is a low-cost immunoassay, measuring unstimulated IFN-γ to diagnose active TB with same day diagnosis [Citation27].
There has been considerable research on the use of host transcriptional blood markers to detect acute infection, pioneered by the O’Garra group at the Crick Institute (London, UK) there have been many combinations of markers produced. The first strong evidence of the use of transcriptional markers in a clinical setting comes with the Cepheid 3-gene host response assay that was demonstrated to distinguish TB from other respiratory diseases. This assay is now undergoing clinical evaluation and is being considered in treatment monitoring studies [Citation28].
3. Susceptibility testing for TB
The universal programmatic approach to the treatment of TB provides a framework for the management of the individual; however, it is necessary to establish if their bacilli are susceptible or resistant to the regimen proposed.
Many countries globally perform drug susceptibility testing (DST) using solid media. The method is cost-effective and reliable, however extremely time consuming due to the slow growing nature of M. tuberculosis, with results generally unavailable within 2 months after sampling [Citation29,Citation30]. With the WHO endorsed roll out of the Becton Dickinson (BD) MGIT, a liquid culture system, it became possible to establish the drug susceptibility phenotype of the cultured organisms, both quickly and accurately. Whilst other automated Mycobacteria liquid culture systems are available, such as VersaTREK™ Myco Media (Thermo Scientific), they do not integrate a drug susceptibility component.
The MGIT tube contains a fluorescent compound implanted in silicone at the bottom of each tube. Initially, the tubes contain dissolved oxygen and as micro-organisms inside the tube respire, they consume the oxygen which in turn enables the fluorescence of the compound i.e., a positive MGIT tube [Citation31]. With established antimicrobial break points, using the BD MGIT DST system, strains can be classified as susceptible, intermediate, or resistant to the first line treatment drugs as well as some key second line treatments (e.g., moxifloxacin). Principles of the MGIT DST procedure are based on M. tuberculosis growth in a drug-containing tube compared to a drug-free tube (growth control). The BD BACTEC MGIT machine analyses the growth in the drug containing tube, comparing to the growth control, to determine the susceptibility results. Results, which are obtained between 4 and 13 days or 4–21 days in the case of Pyrazinamide, are consequently reported as either susceptible or resistant [Citation31].
Where breakpoints for the epidemiologic cut off (ECOFF) values have not been established (in particular for new drugs), it becomes necessary to determine a minimum inhibitory concentration (MIC) and whilst this can be done using the MGIT, a more advantageous method is to use broth microdilution. Broth microdilution is more cost effective, compared to MGIT methods, and enables up to 12 drugs to be tested (depending on plate arrangement and drug concentrations) in one 96 well plate which simplifies laboratory workflow [Citation32]. Commercially available broth microdilution plates, such as the Trek Sensititre MYCOTB MIC plate (MYCOTB, Trek Diagnostic Systems), contain lyophilized first and second-line antibiotics at defined concentrations. Plate interpretation is possible at 10 days, which although is a similar timeframe to MGIT DST, does not require BD specific machinery, software, and kits [Citation33].
The Comprehensive Resistance Prediction for Tuberculosis (CRyPTIC) database, led by the University of Oxford, for M. tuberculosis aims to provide better whole genome sequencing (WGS) assembly to identify more M. tuberculosis variants and use improved statistical methods which can detect relationships between variants and DST [Citation34]. Due to initiatives, such as the CRyPTIC consortium, in which the relationship between genotype and phenotype has been more fully defined, it has become increasingly possible to define drug susceptibility based on genotype alone. A variety of tools are available to achieve this, again the Bruker GenoType portfolio has been shown to be both robust and flexible for incorporation in diagnostic pathways. Cepheid have extended the range of GeneXpert with the Xpert MTB/XDR cartridge to include amplification and analysis of six further drug susceptibilities (isoniazid, fluoroquinolones, amikacin, kanamycin, capreomycin, and ethionamide) [Citation35].
Along with the Xpert/Truenat assays (described previously) which incorporate susceptibility testing elements, there exists the WHO recommended: Cobas® MTB/RIF/INH using the Cobas® 6800 platform (Roche Diagnostics), Real-Time MTB RIF/INH Resistance (RT MTB RIF/INH) (Abbott Molecular) and the BD MAX MDR-TB using the BD MAX platform (Becton Dickinson), all of which are automated nucleic acid amplification tests [Citation23]. A recent study by David et al, showed the performance of all three assays when using spiked sputum, in comparison to the Xpert MTB/RIF had increased sensitivity. Both Cobas and BD MAX assays have the advantage of the entire testing process only requiring a single platform with the BD MAX reported to be easy to use as all reagents required for testing are incorporated onto the testing strip. In terms of output, the reports generated for the Cobas and BD MAX, do not provide any details on the actual resistance mutations detected. The RT MTB RIF/INH assay has the longest processing time, between all three assays, however the shortest hands-on time [Citation23]. See , for a summary of the molecular tests used in the diagnosis/detection of drug resistance for TB.
Another approach has been developed by GenoScreen Services, the DEEPLEX Myc-TB system that uses targeted next generation sequencing (tNGS) of multiplexed targets to provide single nucleotide polymorphism (SNP) analysis for susceptibility genes [Citation36]. The difficulty with all these approaches is that they are predicated on a sub-set of genes that are known to be associated with susceptibility and also prevalent enough to warrant including in a targeted approach. WGS, on the other hand, provides the opportunity to undertake targeted analysis of known or frequent genes associated with resistance but then allows further analysis to identify low frequency or previously unknown associations.
Sequencing of the whole genome from an isolate is well established and some laboratories around the world have already sequenced TB as part of routine diagnostic practice [Citation37]. The majority of these laboratories utilize Illumina short read sequencing technology due to its high accuracy and high throughput capabilities. Whilst Illumina is well established for TB sequencing, there are some disadvantages. It can be expensive to set up, with the flagship MiSeq sequencer starting at around $130,000 and short read lengths can make it difficult to align complex repeat regions, of which the mycobacteria have significant GC rich stretches [Citation38,Citation39]. The general advantages and disadvantages of different sequencing platforms have been extensively reviewed [Citation40–43].
Long read sequencing technologies can help to overcome genome alignment issues. The Oxford Nanopore Technologies (ONT) MinION system provides a portable and flexible approach to sequencing and the starter pack costs around $1000 [Citation44], making it considerably cheaper than a MiSeq, although users do need to invest in a high specification computer to run the device [Citation45]. This overall low set up cost makes the MinION a competitive prospect for LMICs, where TB diagnosis can make up a larger percent of communicable infections than high income countries (HICs). However, the preparation of DNA for ONT sequencing can be problematic, as the required extraction yields may be hard to achieve routinely for the preferred native DNA library preparation kits (from experience ideally 200 ng, but it can be as low as 50 ng). Illumina sequencing relies upon bridge amplification and therefore can utilize lower input concentrations. In our experience (unpublished data), whilst the input concentration is lower, the PCR-based ONT library preparation kit does not provide good enough genome coverage, and as a result, important sites of interest (e.g. drug resistance SNPs) might be missed. We have also found inhibition of mycobacterial sequencing compared to other bacterial species when using ONT transposase-based rapid sequencing kits. We hypothesize that proteins, likely from the mycolic acid layer of the mycobacterial cell wall, are co-precipitated alongside the DNA in the commonly used cetyltrimethylammonium bromide (CTAB) extraction method hindering transposase attachment to the DNA [Citation46].
Sequencing from cultured isolates can take weeks due to the slow growth rate of mycobacteria and consequent time required to achieve sufficient DNA for sequencing protocols. When the time to result is of high importance or when patient drug regimen decision-making is concerned, this can be problematic, although sequencing from isolates is no slower than culture and DST as an alternative. To achieve a faster turnaround time (TAT), WGS direct from clinical samples has been attempted but has proven challenging to deliver in practice. This would save on the delays involved with culture and may be available in settings were culture is not possible, allowing more laboratories to provide WGS-based drug susceptibility analysis [Citation47,Citation48]. Whilst there are published successes for WGS direct from sputum, these have not been followed by roll out into clinical practice [Citation49,Citation50]. This reflects the complexity of both sputum as matrix and mycobacteria as targets for nucleic acid extraction and subsequent processing [Citation47,Citation48]. Low concentrations of mycobacterial DNA within the clinical sample are reflected in difficulty achieving the necessary minimum input DNA quantity for sequencing. Although the amplification-based sequencing platforms, such as Illumina, are likely to be advantageous, this approach has also been performed using ONT [Citation51]. Please note our list is not exhaustive and other technologies have become available, including targeted next-generation sequencing (tNGS) and metagenomic next-generation sequencing (mNGS). Further details on these new technologies can be found in Arouja et al (2023) and Chen et al (2020) [Citation52,Citation53].
The lack of suitably trained bioinformaticians to analyze sequencing data, especially in LMIC countries, can cause bottlenecks in TAT [Citation54]. A number of groups have been established to try and reduce this knowledge gap, such as the Human Heredity and Health in Africa, CABANA in Latin America and APBioNet in Asia Pacific [Citation55–57]. There are a range of user-friendly, mycobacteria-specific bioinformatics tools available for processing and analyzing TB sequencing data. These tools identify resistance SNPs and lineage, making them useful for incorporating into routine clinical diagnosis. These include PhyResSE, Mykrobe, TGS-TB, and TBProfiler [Citation58–61]. The tools vary in which sequencing platforms they are compatible with and what level of bioinformatic skills is required, but some comparative studies have been done, and it should be noted that when choosing an analysis tool, ensuring that the resistance SNP database is kept up to date is important for monitoring drug resistance. It is also important to mention that all those analysis tools are for research use only and they may need to satisfy the strict criteria of laboratory accreditation if used for diagnostics purposes. Identification of emerging or unclassified SNPs would also require a more complex analysis pipeline [Citation62,Citation63].
With all the different technologies being rolled out, the most impactful (on laboratory workflow and patient outcomes) and cost-effective (in terms of value for money) diagnostic pathway should be considered in each clinical setting. Factors such as the ease of test, TAT, which drug-resistant patterns and accuracy of test should all be considered. Other details to think about include the limit of detection for MTB for each test as well as hetero-resistance and co-resistance with other infections [Citation64,Citation65].
Despite this great progress, WGS is not yet ready for universal roll out and PCR remains an essential tool. The great advantage of PCR techniques is that samples can be processed directly to determine the presence of MTBC as well as certain drug resistance patterns within hours. When drug resistance is suspected, PCR tests such as Xpert MTB/XDR could provide a rapid local diagnostic pathway in PCR positive samples pending culture and WGS, hence allowing appropriate WHO regimens being started earlier for isoniazid or multi-drug resistant TB. However, sensitivities for newer drugs, such as bedaquiline, linezolid, and delamanid are not included in the pre-set Xpert MTB/XDR cartridge. This is where other platforms such as Deeplex Myc-TB could play a role with a 48-hour TAT and prediction of 15 drug resistance patterns [Citation64,Citation65].
The key to getting a full picture of susceptibility in TB will be a combination of both phenotype and genotype. Here, it might be worth considering the situation for one of the new drugs being incorporated into the recently mandated, Bedaquiline-Pretomanid-Linezolid (BPaL) regimen for hard-to-treat TB; Pretomanid. In our recent paper, Bateson et al (2022), we describe the establishment of an epidemiologic cutoff value (ECOFF) for Pretomanid. Members of the MTBC were considered and normal distributions established for Group A and D MTBC members, however when M. tuberculosis sensu stricto was considered a biphasic distribution was observed [Citation66]. Genotypic analysis enabled dissection of this anomaly and it became clear that Lineages 2, 3, 4 & 7 formed one distribution, whereas Lineage 1 formed a second distribution with a higher MIC [Citation66]. Lineage 1 is important as it is the Indo-Oceanic lineage and is associated with South Asia, which has a 43% incidence of TB burden and in consequence will have reduced susceptibility to Pretomanid [Citation66,Citation67].
4. Treatment monitoring
With increasingly complex TB to manage and introduction of new drug regimens, the need to monitor treatment and predict outcome is becoming more important. One established indicator of severity is cavitation, and this is associated with bacterial load [Citation68]. Although not all individuals continue to produce sputum after commencement of therapy, sputum bacterial load has conventionally been measured by counting CFU on plates. The use of automated liquid culture has made this more accessible with the measurement of time to positivity (TTP). Direct measurement of bacterial load is cumbersome, even with the MGIT, since it requires culture there is a lag of days to weeks between sampling and read out and which is dependent on the presenting bacterial load in the sample. Sputum decontamination with sodium hydroxide and anti-microbials added to liquid and solid culture media, to prevent overgrowth with other microorganisms, kill a proportion of the M. tuberculosis present. In addition, even with the addition of antimicrobial samples are commonly indeterminate due to the growth of other rapidly growing microorganisms. With this in mind, we have developed the Molecular Bacterial Load Assay (MBLA) [Citation69]. The MBLA enumerates M. tuberculosis by using quantitative amplification of M. tuberculosis 16S rRNA and accounts for qPCR inhibition and RNA loss during sample processing by using a novel 1957bp internal reference standard [Citation69]. The assay finds serial sampling during the treatment of drug-sensitive TB to follow a bi-phasic decline in bacterial load, which is also seen when measuring using liquid culture [Citation69]. The MBLA also correlates very closely when directly compared to serial sputum colony counts (SSCC) on solid agar [Citation70]. In our original study, we demonstrated increased pre-treatment bacterial load had an increased odds ratio for relapse following treatment completion [Citation69]. The MBLA has been used in management of hard-to-treat disease, for example management of a pediatric case of co-infection with HIV [Citation71]. Variations of the original method have been tested by others [Citation72]. Other RNA-based methods include the isothermal amplification method, simultaneous amplification, and testing TB (SAT-TB) (China), which detects mycobacterial 16S rRNA from sputum and resulting cDNA is detected by fluorescent probes. SAT-TB shows promise in treatment monitoring due to high specificity; however, the assay should be modified to have internal control in order to be able to detect enzymatic inhibitors [Citation73].
One of the considerations for assays measuring M. tuberculosis is the requirement for handling at Biosafety Level 3 (BSL-3). Such facilities are commonly unavailable in LMIC. This complicates both diagnosis and measurement of treatment response in places of greatest need. In addition, any culture-based method increases the handling risk of a sample. Ideally, a treatment monitoring method would require minimal facilities and operator input. A system such as measurement of rpoB DNA using the GeneXpert MTB/RIF or MTB/Rif Ultra (Cepheid) would be useful. However, M. tuberculosis DNA has been found to not correlate well with M. tuberculosis treatment response when measured in longitudinal sputum samples [Citation74,Citation75]. In its published form, the MBLA is a research tool, but we have recently modified it to be incorporated into the diagnostic clinical microbiology laboratory. The new version of the assay is called Rapid Enumeration and Diagnostic for Tuberculosis (READ-TB) and has significant advantages over the MBLA with use of a novel nontoxic reagent for the preservation of sputum RNA. Unlike Guanidine thiocyanate (GTC), this preservative simultaneously kills all sputum microorganisms, including M. tuberculosis within 30 min (unpublished data). This removal of preservation using toxic Guanidine thiocyanate allows the sample to be handled directly at BSL-2 if the preservative is added at the clinic. In addition, RNA is stable for at least 2 weeks when temperatures do not exceed 25°C and −70°C is no-longer required for long-term stability. We have shown that RNA is stable in the preservative for at least 1 year (unpublished data). The assay is also semi-automated and removes most of the toxic extraction chemicals, removing the challenge of safe disposal in LMIC. We expect that READ-TB will be widely adopted by those wanting to rapidly determine M. tuberculosis number and that this version will be fully capable of informing treatment strategy and incorporation into dynamic clinical trial designs. In addition, we have shown that our novel preservative is fully compatible with GeneXpert cartridges, a major advantage for laboratories without BSL-3 facilities (unpublished data).
Alternative approaches to markers of bacterial load include the Lipoarabinomannan (LAM) assay, previously endorsed by WHO for diagnosis of TB from urine samples in selected populations (specifically HIV positive). LAM, a glycolipid found in the M. tuberculosis cell wall, is an immunogenic virulence factor which is released from cells during TB infection. The detection of antigens via urine has the advantage of fewer infection control concerns, easier sample collection, processing and storage when compared to sputum samples [Citation76]. This assay has been modified and improved and is currently under evaluation as a monitoring tool. Building on RNA as a target molecule, Walter and colleagues (2021) have proposed the use of precursor rRNA as a biomarker. The principle is based on M. tuberculosis rRNA synthesis quantification, in the form of an RS ratio, being able to act as a marker for drug potency [Citation77]. Whilst this assay has great potential, in its current form using digital PCR, it does not yet meet the requirements for a clinical trial setting. Other novel biomarkers are extensively discussed in Nogueira et al (2022) [Citation78].
Recently, the WHO has developed ‘target product profiles containing minimum and optimum targets for key characteristics for tests for TB treatment monitoring and optimization’ [Citation79]. The documents list of high importance features includes high sensitivity/specificity, ≤2 h’ time to result (no more than one day), sample taken to be minimally invasive/easily obtainable, test can be located in a health facility without a laboratory and the test should be affordable to LMIC which allows scale-up. Tests which incorporate these target profiles will promote the development of TB treatment monitoring [Citation79].
5. Infection control implications
The use of new molecular and sequencing technologies has the potential to revolutionize long-standing infection prevention and control (IPC) practices (i.e., the need for two to three negative smears before discontinuing isolation or the dogma of 2 weeks of treatment rule) and it has already transformed the management of outbreaks by providing much higher and needed definition [Citation11]. However, trials and guidelines supporting this are still pending or missing, hence we have further explored the IPC implications under the Expert opinion section in this manuscript.
6. Conclusion
At present, significant challenges in TB diagnosis remain. One of the core difficulties remains diagnosis in children (under 10 years old), who develop disease symptoms different to adolescent or adult patients, with additional complexities in the collection of respiratory samples. TB in children also tends to be paucibacillary, which results in a lower diagnostic yield for microbiological assays [Citation80]. A key to progressing the diagnosis of pediatric TB will be a robust approach to evaluating diagnostic accuracy, and the recent RaPaed-TB study has established a platform for such evaluations [Citation26]. TB diagnosis also has a limited number of commercially available point of care (POC) tests, which are simple to use. Whilst none of the currently available POC tests meet WHO criteria, the use of the LAM assay has promising evidence to support implementation [Citation81]. Latent TB infection is currently diagnosed by the tuberculin skin test (TST) and the IGRA. However, neither tests are useful in discriminating between active and latent TB and are unable to predict whether an individual will go on to develop active TB [Citation82]. Further advances are required to evaluate the effectiveness of treating latent TB in relation to the risk of progressing to active disease.
Whilst the landscape for diagnosis of TB is improving and new modalities are emerging, progress has been incremental, and we need to speed up. This will be helped by an improved understanding of the biology of TB and the pathogenesis of disease, which will help us use established tools better and have the ability to develop new tools.
7. Expert opinion: diagnostic and infection control implications
From a microbiological perspective, we have previously explained that thousands of bacilli are needed for a smear to become positive, whilst the next Xpert MTB Ultra has demonstrated a much lower limit of detection of 16 CFU/ml, making it a much more sensitive and specific diagnostic tool that could also be applied for infection control measures, at least in case where a negative result is needed [Citation18]. We appreciate the potential cost implications, but these could be easily written off when considering the cost of prolonged hospital admission in negative pressure (or other types of isolation) rooms, the rapid TAT compared to traditional microscopy and the overall patient’s experience. Conversely, the highest sensitivity of the test (including the ‘trace’ reading in Xpert) would likely detect the TB DNA for longer periods compared to simple smears, and it would not be useful for those patients with prolonged smear positivity despite treatment. Advances in the use of host transcriptional blood markers to detect acute infection, in a clinical setting, comes with the Cepheid 3-gene host response assay which is able to distinguish TB from other respiratory diseases. Whilst the short-term use is aimed at treatment monitoring studies, such assays have the potential to be rolled out in a diagnostic setting, with thorough clinical evaluation and validation. Transcriptional marker assays also eliminate the challenges associated with processing sputum samples.
For patients with multi drug resistant TB, three negative culture samples (and not simple smear) are strongly suggested before discontinuing isolation precautions [Citation83,Citation84]. For these patients, we can assess the risk of transmission by considering the bacterial load and mRNA expression as confirmation of actively replicating versus dead bacteria. MBLA is already being incorporated into the latest dynamic clinical trial designs. As READ-TB has significant advantages over MBLA, we expect that it will be widely adopted.
Finally, WGS has also been applied to enhance the epidemiological evidence, identifying previously undetected linkages as well as the emergence of drug resistance which may influence enhanced interventions such as isolation [Citation85]. Several studies have demonstrated the utility of WGS in public health interventions and in the detection of outbreaks and transmission events, confirming its higher resolution when compared with MIRU-VNTR typing, IS6110 restriction fragment length polymorphism (RFLP) typing, and spoligotyping methods [Citation86–88]. Further improvements can be made to WGS technologies by incorporating artificial intelligence (AI) methods.
AI methods such as machine learning models and deep learning algorithms, can encode sequences into numerical values, enabling use in drug susceptibility classification [Citation89,Citation90]. AI approaches are very much in their infancy and as drug-resistant mechanisms and drug–target interaction knowledge is still being built, machine learning algorithms may differ in the way they model and result output [Citation91]. We believe the next 10 years will have extensive AI advances in both TB diagnosis and susceptibility testing, with WGS approaches no longer being the most informative computer-based technology.
Article highlights
Recent focus has been on the introduction of molecular techniques for TB providing enhanced speed of turn around, sensitivity, and data.
The most impactful (on laboratory workflow and patient outcomes) and cost-effective (in terms of value for money) diagnostic pathway should be considered in each clinical setting.
Molecular tests, which can be used in the diagnosis and detection of drug resistance for TB, should be evaluated in each clinical setting with the most advantageous test chosen.
Sequencing of the whole genome from an isolate is well established and some laboratories around the world already sequence TB as part of routine diagnostic practice.
With increasingly complex TB to manage and introduce new drug regimens, the need to monitor treatment and predict outcome is becoming more important.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- World Health Organisation. Global Tuberculosis report 2022. Geneva; 2022 [cited 2024 Mar 21]. Available from: https://apps.who.int/bookorders
- England K, Masini T, Fajardo E. Detecting tuberculosis: rapid tools but slow progress. Public Health Action. 2019 Nov 2;9(3):80–83. doi: 10.5588/pha.19.0013
- Shingadia D, and Burgner D. Mycobacterial Infections. In: Taussig LM, Landau LI, editors. Pediatric Respiratory Medicine. 2nd ed. Philadelphia, USA:Mosby; 2008. doi: 10.1016/B978-032304048-8.50043-8
- Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6(9):570–581. doi: 10.1016/S1473-3099(06)70578-3
- Rasool G, Khan AM, Mohy-Ud-Din R, et al. Detection of mycobacterium tuberculosis in afb smear-negative sputum specimens through MTB culture and GeneXpert® MTB/RIF assay. Int J Immunopathol Pharmacol. 2019;33:205873841982717. doi: 10.1177/2058738419827174
- World Health Organization. Module 3: Diagnosis. WHO consolidated guidelines on tuberculosis Rapid diagnostics for tuberculosis detection. 3rd ed. 2021.
- Maitra A, Solanki P, Sadouki Z, et al. Improving the drug development pipeline for mycobacteria: modelling antibiotic exposure in the hollow fibre infection model. Antibiotics. 2021 Dec 1;10(12):1515. doi: 10.3390/antibiotics10121515
- Joloba ML, Johnson JL, Feng PJI, et al. What is the most reliable solid culture medium for tuberculosis treatment trials? Tuberculosis. 2014;94(3):311–316. doi: 10.1016/j.tube.2014.03.002
- Pheiffer C, Carroll † NM, Beyers N, et al. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis. 2008;12(7):792–798.
- Morgan MA, Horstmeier CD, Deyoung DR, et al. Comparison of a radiometric method (BACTEC) and conventional culture media for recovery of mycobacteria from smear-negative specimens. J Clin Microbiol. 1983;18(2):384–388. doi: 10.1128/jcm.18.2.384-388.1983
- Satta G, Lipman M, Smith GP, et al. Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect. 2018;24:604–609. doi: 10.1016/j.cmi.2017.10.030
- Louie A, Duncanson B, Myrick J, et al. Activity of moxifloxacin against mycobacterium tuberculosis in acid phase and nonreplicative-persister phenotype phase in a hollow-fiber infection model. Antimicrob Agents Chemother. 2018;62(12). doi: 10.1128/AAC.01470-18
- Mukamolova GV, Turapov O, Malkin J, et al. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med. 2010 Jan 15;181(2):174–180. doi: 10.1164/rccm.200905-0661OC
- Rosser A, Stover C, Pareek M, et al. Resuscitation-promoting factors are important determinants of the pathophysiology in Mycobacterium tuberculosis infection. Crit Rev Microbiol. 2017;43:621–630. doi: 10.1080/1040841X.2017.1283485
- Van Loon W, Gomez MP, Jobe D, et al. Use of resuscitation promoting factors to screen for tuberculosis infection in household-exposed children in the Gambia. BMC Infect Dis. 2020 Jul 2;20(1). doi: 10.1186/s12879-020-05194-1
- Shield CG, Swift BMC, McHugh TD, et al. Application of bacteriophages for mycobacterial infections, from diagnosis to treatment. Microorganisms. 2021 Nov 1;9(11):2366. doi: 10.3390/microorganisms9112366
- Penn-Nicholson A, Gomathi SN, Ugarte-Gil C, et al. A prospective multicentre diagnostic accuracy study for the truenat tuberculosis assays. Eur Respir J. 2021 Nov 1;58(5):2100526. doi: 10.1183/13993003.00526-2021
- Biswas S, Uddin MKM, Paul KK, et al. Xpert MTB/RIF Ultra assay for the detection of Mycobacterium tuberculosis in people with negative conventional Xpert MTB/RIF but chest imaging suggestive of tuberculosis in Dhaka, Bangladesh. Inter J Infect Dis. 2022 Jan 1;114:244–251. doi: 10.1016/j.ijid.2021.11.010
- Singh UB, Singh M, Sharma S, et al. Expedited diagnosis of pediatric tuberculosis using Truenat MTB-Rif Dx and GeneXpert MTB/RIF. Sci Rep [Internet]. 2023 Apr 28;13(1):6976. Available from: https://www.nature.com/articles/s41598-023-32810-2
- Bruker. Tuberculosis rapid diagnostics of tuberculosis and its resistances your benefits with the TB product series from Hain Lifescience • Fast and reliable results • Step-wise diagnostics • Cost efficiency • CE-marked Innovation with Integrity PCR/MYCOBACTERIA IVD hain lifescience-a bruker company. 2023.
- Lin M, Chen YW, Li YR, et al. Systematic evaluation of line probe assays for the diagnosis of tuberculosis and drug-resistant tuberculosis. Clinica Chimica Acta. 2022 Aug 1;533:183–218. doi: 10.1016/j.cca.2022.06.020
- Vaezipour N, Fritschi N, Brasier N, et al. Towards accurate point-of-care tests for tuberculosis in children. Pathogens. 2022 Mar 1;11(3):327. doi: 10.3390/pathogens11030327
- David A, de Vos M, Scott L, et al. Feasibility, ease-of-use, and operational characteristics of world health organization–recommended moderate-complexity automated nucleic acid amplification tests for the detection of tuberculosis and resistance to rifampicin and isoniazid. J Mol Diagn. 2023 Jan 1;25(1):46–56. doi: 10.1016/j.jmoldx.2022.10.001
- WHO. Information sheet: practical considerations for implementation of the nipro genoscholar PZA-TB II assay. 2021.
- MacLean E, Kohli M, Weber SF, et al. Advances in molecular diagnosis of tuberculosis. J Clin Microbiol. 2020;58(10). doi: 10.1128/JCM.01582-19
- Olbrich L, Nliwasa M, Sabi I, et al. Rapid and accurate diagnosis of pediatric tuberculosis disease: a diagnostic accuracy study for pediatric Tuberculosis. Pediatr Infect Dis J. 2023 May 16;42(5):353–360. doi: 10.1097/INF.0000000000003853
- Meldau R, Randall P, Pooran A, et al. Same-day tools, including xpert ultra and IRISA-TB, for rapid diagnosis of pleural tuberculosis: A prospective observational study. J Clin Microbiol. 2019;57(9). doi: 10.1128/JCM.00614-19
- Sutherland JS, Van Der Spuy G, Gindeh A, et al. Diagnostic accuracy of the cepheid 3-gene host response fingerstick blood test in a prospective, multi-site study: interim results. Clin Infect Dis. 2022 Jun 15;74(12):2136–2141. doi: 10.1093/cid/ciab839
- Amini S, Hoffner S, Allahyar Torkaman MR, et al. Direct drug susceptibility testing of Mycobacterium tuberculosis using the proportional method: a multicenter study. J Glob Antimicrob Resist. 2019 Jun 1;17:242–244. doi: 10.1016/j.jgar.2018.12.022
- Yusoof KA, García JI, Schami A, et al. Tuberculosis phenotypic and genotypic drug susceptibility testing and immunodiagnostics: a review. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.870768
- Becton D. BACTECTM MGITTM 960 SIRE kits for the antimycobacterial susceptibility testing of mycobacterium tuberculosis. 2019.
- World Health Organisation. Optimized broth microdilution plate methodology for drug susceptibility testing of mycobacterium tuberculosis complex [Internet]. 2022 [cited 2024 Mar 21]. Available from: http://apps.who.int/bookorders
- Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing mycobacterium tuberculosis susceptibility to first-and second-Line drugs. Antimicrob Agents Chemother. 2014 Jan;58(1):11–18. doi: 10.1128/AAC.01209-13
- Solanki P, Lipman M, McHugh TD, et al. Whole genome sequencing and prediction of antimicrobial susceptibilities in non-tuberculous mycobacteria. Front Microbiol. 2022 Nov 29;13:13. doi: 10.3389/fmicb.2022.1044515
- Pillay S, Steingart KR, Davies GR, et al. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst Rev. 2022;2022. doi: 10.1002/14651858.CD014841.pub2
- Jouet A, Gaudin C, Badalato N, et al. Deep amplicon sequencing for culture-free prediction of susceptibility or resistance to 13 anti-tuberculous drugs. Eur Respir J. 2021 Mar 1;57(3):2002338. doi: 10.1183/13993003.02338-2020
- Papaventsis D, Casali N, Kontsevaya I, et al. Whole genome sequencing of Mycobacterium tuberculosis for detection of drug resistance: a systematic review. Clin Microbiol Infect. 2017;23:61–68. doi: 10.1016/j.cmi.2016.09.008
- Quail MA, Smith M, Coupland P, et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012 Jul 24;13(1):341. doi: 10.1186/1471-2164-13-341
- Cechova M. Probably correct: Rescuing repeats with short and long reads. Genes (Basel). 2021;12(1):1–13. doi: 10.3390/genes12010048
- Quainoo S, Coolen JPM, van Hijum SAFT, et al. Whole-genome sequencing of bacterial pathogens: The future of nosocomial outbreak analysis. Clin Microbiol Rev. 2017;30(4):1015–1063. doi: 10.1128/CMR.00016-17
- Kumar KR, Cowley MJ, Davis RL. Next-generation sequencing and emerging technologies. Semin Thromb Hemost. 2019;45(7):661–673. doi: 10.1055/s-0039-1688446
- Liu L, Li Y, Li S, et al. Comparison of next-generation sequencing systems. J Biomed And Biotech. 2012;2012. doi: 10.1155/2012/251364
- Slatko BE, Gardner AF, Ausubel FM. Overview of next-generation sequencing technologies. Curr Protoc Mol Biol. 2018 Apr 1;122(1). doi: 10.1002/cpmb.59
- Oxford Nanopore Technologies. MinION Starter Pack. 2023.
- Oxford Nanopore Technologies. MinION Mk1B it requirements Overview [Internet]. [cited 2023 Jun 30]. Available from: https://www.techpowerup.com/gpu-specs/
- Kent L, McHugh TD, Billington O, et al. Demonstration of Homology between IS6110 of Mycobacterium tuberculosis and DNAs of Other Mycobacterium spp. J Clin Microbiol. 1995;33(9):2290–2293. doi: 10.1128/jcm.33.9.2290-2293.1995
- Goig GA, Cancino-Muñoz I, Torres-Puente M, et al. Articles whole-genome sequencing of mycobacterium tuberculosis directly from clinical samples for high-resolution genomic epidemiology and drug resistance surveillance: an observational study. Lancet Microbe. 2020;1(4):e175–e183. doi: 10.1016/S2666-5247(20)30060-4
- Nilgiriwala K, Rabodoarivelo MS, Hall MB, et al. Genomic sequencing from sputum for tuberculosis disease diagnosis, lineage determination, and drug susceptibility prediction. J Clin Microbiol. 2023 Mar 23;61(3). doi: 10.1128/jcm.01578-22
- Brown AC, Bryant JM, Einer-Jensen K, et al. Rapid whole-genome sequencing of mycobacterium tuberculosis isolates directly from clinical samples. J Clin Microbiol. 2015 Jul 1;53(7):2230–2237. doi: 10.1128/JCM.00486-15
- Nimmo C, Doyle R, Burgess C, et al. Rapid identification of a Mycobacterium tuberculosis full genetic drug resistance profile through whole genome sequencing directly from sputum. Inter J Infect Dis. 2017 Sep 1;62:44–46. doi: 10.1016/j.ijid.2017.07.007
- George S, Xu Y, Sanderson N, et al. MinION nanopore sequencing of multiple displacement amplified mycobacteria DNA 2 Direct from Sputum 3 4 5 short title: nanopore sequencing of mycobacteria DNA amplified direct from Sputum. doi: 10.1101/490417
- de Araujo L, Cabibbe AM, Mhuulu L, et al. Implementation of targeted next-generation sequencing for the diagnosis of drug-resistant tuberculosis in low-resource settings: a programmatic model, challenges, and initial outcomes. Front Public Health. 2023;11:11. doi: 10.3389/fpubh.2023.1204064
- Chen P, He Y, Sun W. Comparison of metagenomic next-generation sequencing technology, culture and GeneXpert MTB/RIF assay in the diagnosis of tuberculosis. J Thorac Dis. 2020 Aug 1;12(8):4014–4024. doi: 10.21037/jtd-20-1232
- Ebenezer TE, Muigai AWT, Nouala S, et al. Africa: sequence 100,000 species to safeguard biodiversity Setting the agenda in research. Nature Picture Library. 2022;603(7901):388–392. doi: 10.1038/d41586-022-00712-4
- H3Africa Human Heredity & Health in Africa [Internet]. 2023 [cited 2023 Jun 30]. Available from: https://h3africa.org/
- Akintayo Akintola A, Tunde Aborode A, Aborode AT. Africa needs more bioinformaticians for population studies. Nature. 2022;605(7911):619–619. doi: 10.1038/d41586-022-01378-8
- CABANA Partners. CABANA. Capacity building for bioinformatics in Latin America [Internet]. 2019 [cited 2023 Jun 30]. Available from: https://www.cabana.online/contact
- EMBL-EBi. Mykrobe [Internet]. 2020 [cited 2023 Jun 30]. Available from: https://www.mykrobe.com/
- Sekizuka T, Yamashita A, Murase Y, et al. TGS-TB: Total genotyping solution for Mycobacterium tuberculosis using short-read whole-genome sequencing. PLOS ONE. 2015 Nov 1;10(11):e0142951. doi: 10.1371/journal.pone.0142951
- Phelan JE, O’Sullivan DM, Machado D, et al. Integrating informatics tools and portable sequencing technology for rapid detection of resistance to anti-tuberculous drugs. Genome Med. 2019 Jun 24;11(1). doi: 10.1186/s13073-019-0650-x
- Feuerriegel S, Schleusener V, Beckert P, et al. PhyResSE: A web tool delineating Mycobacterium tuberculosis antibiotic resistance and lineage from whole-genome sequencing data. J Clin Microbiol. 2015 Jun 1;53(6):1908–1914. doi: 10.1128/JCM.00025-15
- Quagliaro P, Dziri S, Magdoud El Alaoui F, et al. Performances of bioinformatics tools for the analysis of sequencing data of Mycobacterium tuberculosis complex strains. Tuberculosis. 2023 Mar 1;139:102324. doi: 10.1016/j.tube.2023.102324
- Satta G, Atzeni A, McHugh TD. Mycobacterium tuberculosis and whole genome sequencing: a practical guide and online tools available for the clinical microbiologist. Clin Microbiol Infect. Elsevier B.V.; 2017;23:69–72. doi: 10.1016/j.cmi.2016.09.005
- Park M, Lalvani A, Satta G, et al. Evaluating the clinical impact of routine whole genome sequencing in tuberculosis treatment decisions and the issue of isoniazid mono-resistance. BMC Infect Dis. 2022 Dec 1;22(1). doi: 10.1186/s12879-022-07329-y
- Olaru ID, Patel H, Kranzer K, et al. Turnaround time of whole genome sequencing for mycobacterial identification and drug susceptibility testing in routine practice. Clin Microbiol Infect. 2018 Jun 1;24(6):.e659.5–.e659.7. doi: 10.1016/j.cmi.2017.10.001
- Bateson A, Ortiz Canseco J, McHugh TD, et al. Ancient and recent differences in the intrinsic susceptibility of Mycobacterium tuberculosis complex to pretomanid. J Antimicrob Chemother. 2022 May 29;77(6):1685–1693. doi: 10.1093/jac/dkac070
- World Health Organisation. Tuberculosis in South-East Asia Region. 2023 [cited2024 Jun 03]. https://www.who.int/southeastasia/health-topics/tuberculosis
- Perrin FMR, Woodward N, Phillips PPJ, et al. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14(12):1596–1602.
- Honeyborne I, McHugh TD, Phillips PPJ, et al. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol. 2011 Nov;49(11):3905–3911. doi: 10.1128/JCM.00547-11
- Honeyborne I, Mtafya B, Phillips PPJ, et al. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol. 2014;52(8):3064–3067. doi: 10.1128/JCM.01128-14
- Evangelopoulos D, Whittaker E, Honeyborne I, et al. Pediatric tuberculosis-human immunodeficiency virus co-infection in the United Kingdom highlights the need for better therapy monitoring tools: a case report. J Med Case Rep. 2017 Feb 26;11(1). doi: 10.1186/s13256-017-1222-6
- Sabiiti W, Azam K, Farmer ECW, et al. Tuberculosis bacillary load, an early marker of disease severity: the utility of tuberculosis molecular bacterial load assay. Thorax. 2020;75(7):606–608. doi: 10.1136/thoraxjnl-2019-214238
- Cui Z, Wang Y, Fang L, et al. Novel real-time simultaneous amplification and testing method to accurately and rapidly detect Mycobacterium tuberculosis complex. J Clin Microbiol. 2012 Mar;50(3):646–650. doi: 10.1128/JCM.05853-11
- Friedrich SO, Rachow A, Saathoff E, et al. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med. 2013 Aug;1(6):462–470. doi: 10.1016/S2213-2600(13)70119-X
- Kayigire XA, Friedrich SO, Venter A, et al. Direct comparison of Xpert MTB/RIF assay with liquid and solid mycobacterial culture for quantification of early bactericidal activity. J Clin Microbiol. 2013 Jun;51(6):1894–1898. doi: 10.1128/JCM.03290-12
- Minion J, Leung E, Talbot E, et al. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur Respir J. 2011;38(6):1398–1405. doi: 10.1183/09031936.00025711
- Walter ND, Born SEM, Robertson GT, et al. Mycobacterium tuberculosis precursor rRNA as a measure of treatment-shortening activity of drugs and regimens. Nat Commun. 2021 Dec 1;12(1). doi: 10.1038/s41467-021-22833-6
- Nogueira BMF, Krishnan S, Barreto‐Duarte B, et al. Diagnostic biomarkers for active tuberculosis: progress and challenges. EMBO Mol Med. 2022 Dec 7;14(12). doi: 10.15252/emmm.202114088
- Gupta-Wright A, den Boon S, MacLean E, et al. Target product profiles: tests for tuberculosis treatment monitoring and optimization. Bull World Health Organ. 2023 Nov 1;101(11):730–737. doi: 10.2471/BLT.23.290901
- Gunasekera KS, Vonasek B, Oliwa J, et al. Diagnostic challenges in childhood pulmonary tuberculosis—optimizing the clinical approach. Pathogens. MDPI; 2022;11(4):382. doi: 10.3390/pathogens11040382
- García-Basteiro AL, DiNardo A, Saavedra B, et al. Point of care diagnostics for tuberculosis. Revista Portuguesa de Pneumologia (English Edition). Elsevier Doyma; 2018;24(2):73–85. doi: 10.1016/j.rppnen.2017.12.002
- Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, et al. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. Frontiers Media S.A.; 2020;11. doi: 10.3389/fimmu.2020.02006
- Schwartzman K, Menzies D. Letters Correspondance Getting in line [Internet]. 2000 [cited 2024 Mar 21]. Available from: www.cma.ca/cmaj
- NICE. Tuberculosis NICE guideline Your responsibility [Internet]. 2016 [cited 2024 Mar 21]. Available from: www.nice.org.uk/guidance/ng33
- Nikolayevskyy V, Niemann S, Anthony R, et al. Role and value of whole genome sequencing in studying tuberculosis transmission. Clin Microbiol Infect. Elsevier B.V.; 2019;25:1377–1382. doi: 10.1016/j.cmi.2019.03.022
- Roetzer A, Diel R, Kohl TA, et al. Whole genome sequencing versus traditional genotyping for investigation of a mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLOS Med. 2013;10(2):e1001387. doi: 10.1371/journal.pmed.1001387
- Estée Török M, Reuter S, Bryant J, et al. Rapid whole-genome sequencing for investigation of a suspected tuberculosis outbreak. J Clin Microbiol. 2013 Feb;51(2):611–614. doi: 10.1128/JCM.02279-12
- Stucki D, Ballif M, Bodmer T, et al. Tracking a tuberculosis outbreak over 21 years: Strain-specific single-nucleotide polymorphism typing combined with targeted whole-genome sequencing. J Infect Dis. Oxford University Press; 2015;211(8):1306–1316. doi: 10.1093/infdis/jiu601
- Liu Y, Qu HQ, Mentch FD, et al. Application of deep learning algorithm on whole genome sequencing data uncovers structural variants associated with multiple mental disorders in African American patients. Mol Psychiatry. 2022 Mar 1;27(3):1469–1478. doi: 10.1038/s41380-021-01418-1
- Ren Y, Chakraborty T, Doijad S, et al. Prediction of antimicrobial resistance based on whole-genome sequencing and machine learning. Bioinformatics. 2022 Jan 15;38(2):325–334. doi: 10.1093/bioinformatics/btab681
- Sharma A, Machado E, Lima KVB, et al. Tuberculosis drug resistance profiling based on machine learning: a literature review. Brazil J Infect Dis. 2022;26(1):102332. doi: 10.1016/j.bjid.2022.102332