?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Progressive familial intrahepatic cholestasis (PFIC) is an ultra-rare disease with a considerable burden on pediatric patients and their caregivers, impacting quality of life (QoL). The mortality rates highlight a significant need for efficacious treatments. Real-world data on associated costs and QoL are needed to gauge the potential impact of new pharmacological treatments.
Methods: Clinical and socio-economic burden of PFIC on patients/caregivers, health systems, and society will be assessed. Patient/caregiver- and physician-level retrospective cross-sectional data will be collected from the US, UK, France, and Germany, for PFIC types 1, 2, 3.
A representative sample of physicians will provide clinical and resource utilization information using an electronic Case Report Form (eCRF). Patient/caregiver surveys will collect socio-economic and QoL data, enabling assessment of PFIC impact on QoL. Mean costs (direct medical/non-medical, indirect) will be calculated.
The study materials were reviewed by medical professionals and patient representatives and received ethical approval from the University of Chester.
Discussion: The study aims to reveal the unmet medical need, disease burden, resource utilization, and costs of PFIC, to raise awareness with policymakers and healthcare professionals, and provide support for the patient/caregiver community. As novel PFIC therapies recently emerged, this study will yield quantifiable data for health technology assessments.
1. Introduction
1.1. Disease background
Progressive familial intrahepatic cholestasis (PFIC) is a rare liver disorder that develops as a result of a recessive genetic defect in bile secretion. Cholestasis manifests as variable jaundice and extensive pruritus that present during infancy or early childhood [Citation1]. The development of significant liver fibrosis and end-stage liver disease (ESLD) before reaching adulthood are not uncommon [Citation1]. Death or liver transplantation may occur from infancy to adolescence, depending on the severity of the disease [Citation2].
The exact prevalence of PFIC is unknown but estimated between 1 per 50,000 to 1 per 100,000 births [Citation2]; its prevalence is not limited to any ethnicity or gender [Citation1]. Diagnosis of the disease is usually performed through a combination of clinical, radiological, histological, and genetic approaches [Citation3].
A few types of genetically distinct progressive cholestatic liver diseases are grouped in the PFIC spectrum [Citation4]. These include the three main types of PFIC (often referred to as types 1, 2, and 3), as well as less common types, caused by TJP2, FXR, and MYO5B gene deficiencies [Citation5,Citation6].
The types addressed in this study are the most prevalent ones in the patient population: PFIC 1, 2, and 3. Type 1 (PFIC1), caused by mutations in the ATP8B1 (or FIC1) gene, typically presents in infancy. With ATP8B1 highly expressed in the small intestine, children with PFIC1 commonly also have chronic diarrhea [Citation1]. Type 2 (PFIC2) is caused by a malfunctioning copy of the gene ABCB11 (encoding the BSEP export pump), while Type 3 (PFIC3) is due to mutations in gene ABCB4, also designated as MDR3 [Citation1].
Presenting in infancy, PFIC1 manifests with a moderately severe intensity: children tend to have severe pruritus, an enlarged liver (hepatomegaly), and sometimes manifest extrahepatic symptoms, including watery diarrhea, pancreatitis, deafness, short stature [Citation2]. Children with PFIC1 may experience ESLD within the first decade of life [Citation2]. Also typically diagnosed in early infancy, PFIC2 is the most severe type, showing rapid disease progression with severe itching, hepatomegaly, and a risk for hepatocellular carcinoma and cholesterol stones; extrahepatic manifestations are not typical with PFIC2 [Citation2]. PFIC3 typically presents later with moderate pruritus, a risk of liver tumors and cholesterol stones, and progression to ESLD possible within the first or second decade of life [Citation2]. Overall approximately 67% of individuals with PFIC have type 1 or 2, while 33% are diagnosed with PFIC3 [Citation1].
In addition to the most characteristic manifestations discussed above, individuals with PFIC may exhibit a variety of other signs and symptoms including rickets, osteopenia, and fractures, fat-soluble vitamin deficiencies, splenomegaly, growth impairment, and developmental delay [Citation7].
1.2. Treatment context
There are no specific approved drugs for the treatment of PFIC. Due to cholestasis, dietary intake is supplemented with medium-chain triglycerides and fat-soluble vitamins [Citation3]. Due to severe pruritus, topical moisturizers and antihistaminic agents are commonly tried, but with minimal benefit [Citation3]. Ursodeoxycholic acid (UDCA) may offer some clinical improvements and potentially delay a liver transplant, without major side effects [Citation8,Citation9]. Rifampicin, bile sequestrants, opiate antagonists, and sometimes nasobiliary drainage are utilized due to the severe pruritus typical of PFIC [Citation10,Citation11].
Children with PFIC may also be good candidates for partial biliary diversion (PBD) surgery, which aids in the successful elimination of bile acids from the patient’s body [Citation12]. PBD surgeries can reduce debilitating pruritus symptoms and may improve hepatic laboratory test results, but are only successful in some patients with PFIC [Citation2,Citation9]. Further, despite PBD, manifestations such as diarrhea or steatosis may still occur [Citation2].
New research around PFIC treatments has focused on improving bile acid transport (BAT) inhibitors (or sequestrants). Despite their role in the development of cholestatic pruritus being currently unclear, depleting a patient’s bile acid pool is considered a good step in tackling this debilitating symptom [Citation11]. The ileal bile acid transporter (IBAT) inhibitor, also known as apical sodium-dependent bile acid transporter (ASBT) inhibitor, was put through a number of trials for liver diseases including PFIC patients or others with similar manifestations [Citation11].
The first IBAT inhibitor investigational drug product (GSK2330672) trial showed that pruritus subsided and laboratory test results improved in patients for which other treatments were not effective [Citation13]. Another type of IBAT inhibitor (SHP625) trial appeared to improve test results even further [Citation14]. Recent trials for IBAT products focused on the A4250 treatment; in this case, a rapid and substantial improvement in pruritus symptoms was reported and patients and caregivers also reported an improved sleep quality [Citation15].
1.3. Economic burden and impact on health-related quality of life (HRQoL)
The pruritus associated with PFIC, compounded by any extrahepatic manifestations, severely impact the quality of life of individuals affected. Many dimensions of a patient’s life can be impacted by PFIC; reports of patient experiences reveal many pediatric patients scratch their skin constantly, to a point of cutaneous mutilation and scarring [Citation16]. The pruritus proves most cumbersome with regard to the patients’ sleep patterns and daily life activities; it is also likely that the burden of illness extends to the HRQoL of caregivers. However, no study has yet been found to inform about the burden of illness on PFIC caregivers.
While the literature is scarce regarding the HRQoL of PFIC patients or their caregivers, no economic data are available for the specific impact of PFIC, recent cost-effectiveness analyses (CEA) relied on information of fibrosis progression and complications from other liver conditions.
2. Study aims and rationale
PFIC is an ultra-rare disease with a significant disease burden on pediatric patients and their caregivers, impacting quality of life. The high mortality rates in this population highlight that there is a significant unmet need for more efficacious treatments. However, without real-world data on the associated costs and QoL, it is difficult to gauge the potential impact and gains that new pharmacological treatments could offer.
The overall aim of the PICTURE study is to quantify the overall clinical, economic, and humanistic burden on PFIC patients, on their caregivers, and ultimately on society across the United States of America, France, Germany, and the United Kingdom. An additional goal of this study is to support the clinical implementation of new therapies emerging in the PFIC treatment landscape.
The clinical, cost, and HRQoL estimates that will be provided by this analysis may be used to justify the prioritization of future research in PFIC disease. The PICTURE study authors have decided to submit this study protocol for publication in order to raise awareness of PFIC among policymakers and to encourage other researchers to carry out cost and HRQoL studies of PFIC. This study protocol presents detailed information about the different steps involved in designing the PICTURE study and may thereby inform the design of future cost studies of PFIC.
3. Patients and methods
3.1. Study design
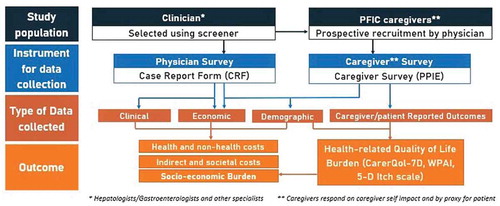
The PICTURE study is a descriptive, retrospective, and cross-sectional burden of illness study involving physicians and caregivers providing past and present clinical, economic, and quality of life data from France, Germany, United Kingdom, and the USA.
Physicians will provide information on PFIC patients via an electronic Case Report Form (eCRF). They will recruit patients with their respective caregivers, as they attend a clinical appointment, regardless of the reason for their visit. They will collect relevant clinical and economic retrospective data at the time of consultation. Estimates of healthcare utilization and costs will be calculated for a 12-month period. The online format of the CRFs should ensure that numeric responses are kept within reasonable boundaries, and that infeasible responses (such as the ability to provide two mutually exclusive responses) are kept to a minimum.
In addition, the caregivers of these PFIC patients will be prospectively recruited by physicians and will provide information – on a voluntary basis and via online specific self-completion questionnaires – of the impact of the disease in their lives, including estimations for indirect and non-health direct costs. While the caregiver questionnaire is focused on the caregiver experience, these will also be able to provide specific information on the impact of the disease on the quality of life and daily activities of the PFIC patients (typically their children).
The nature and structure of outcomes that will be reported can be observed in .
3.2. Setting and study representativeness
As this is an observational and descriptive study that aims to describe costs, clinical, and quality of life variables for the PFIC population, it does not allow for hypothesis testing; therefore, a formal calculation of sample size and statistical power is not applicable. The study could yield an estimated 225 eCRF and 135 caregiver forms for data collection. However, these estimates are to be taken cautiously given the disease ultra-orphan status. Feasibility of these numbers will be regularly monitored and updated in case this is deemed necessary. Data collection is currently under completion and data analysis has not started yet.
The PICTURE study involves the establishment of an ad-hoc steering committee called the Expert Review Group (ERG), consisting of specialized clinicians and allied health professionals, academics, and patient advocacy representatives (Children’s Liver Disease Foundation (CLDF) and PFIC Advocacy and Resource Network). The role of the ERG in this study is to ensure quality standards are maintained and to provide overall study oversight. The ERG provides feedback at different stages of the project, including recommendations on the study design, approval of the protocol and all the study materials and publication and communication of findings.
3.3. Participants
The study aims to recruit a number of physicians and interview an appropriate group to enable return of study-specific eCRFs and caregiver-specific surveys. Physicians are recruited via a real-world panel approach from a representative sample of physicians that manage PFIC patients for each country. The physician sample will not be limited by any restrictions other than the eligibility criteria listed below – deemed sufficient to capture relevant data as per the study scope.
The following criteria must be met by all participating physicians:
The physician’s primary specialty must be one of the following ones: hepatologist, gastroenterologist, pediatrician, general practitioner, internal medicine or medical genetics.
Recruited physicians must be the main point of contact for these patients (i.e. they must have the lead role in managing and coordinating care for these patients).
Physicians must have at least 2 years of experience and must have managed at least 1 PFIC patient (of subtypes 1, 2 or 3) in total.
Physicians must agree to comply with the study protocol and the documentation procedure.
In order to minimize the risk of selection bias, physicians were encouraged to recruit the next eligible patients and caregivers with whom they consulted, irrespective of their reason for consultation.
The study population includes:
Adult caregivers/guardians of patients (of all ages and genders) with genetic diagnosis of PFIC subtypes 1, 2 or 3 for at least 12 months.
Patients (of all ages) with genetic diagnosis of PFIC subtypes 1, 2, or 3 for at least 12 months, with a consistent caregiver/guardian.
Caregivers must be willing and able to complete the study questionnaire and give informed consent (and assent) as appropriate on their behalf and on the patient’s behalf if they are below 18. Patients must give informed consent (and assent) as appropriate if they are 18 or older. Patients (and their caregivers) would be excluded if they were currently participating in clinical trials or within the 12 months period before the consultation date.
The work the physicians undertake as part of this study will be outside of the clinical consultation and undertaken in their own time. The incentive remuneration system was decided by country and specialty, and is based on the principle of fair market value. Patient’s caregivers/companion will be also offered remuneration to complete their questionnaire.
Finally, all study materials (protocol, profiling questions, eCRFs, patient ‘Invitation to Participate’ information sheet and caregiver questionnaire) were developed in English (UK) and translated into native languages using a third-party translation service.
3.4. Main variables & data sources study materials
3.4.1. Electronic case report forms (eCRFs)
For each patient seen during the clinical consultation, physicians will complete an eCRF. Eligible recruited physicians will be invited to prospectively enroll at least 1 PFIC (and up to 10) patient of types 1, 2, or 3 and his/her caregiver/companion seen during clinical consultation. The enrollment period will last for three to 4 months from the start of the fieldwork (September 2020).
Physicians will retrospectively extract real-world information from the patients’ health medical records. The types of data collected from the physician completed CRF will be clinical, economic, and demographic. The primary aim will be to document direct health PFIC-related resource use over the 12 months prior to the date of clinical consultation, but data such as diagnosis, disease history, clinical events, and symptomatology will be abstracted from diagnosis where possible, in order to provide an accurate understanding of the disease and clinical pathway from a longitudinal point of view. Time to completion is expected to be on average 25–30 minutes per physician.
3.4.2. Caregiver self-completion questionnaires
After clinical consultation, eligible caregivers who accepted to be enrolled in the study will be invited to complete a corresponding pen and paper or online questionnaires, in the clinic or at any other place of their convenience. For the pen & paper version, the questionnaire will be returned to the physician on the same day as clinical consultation or after the clinical consultation in a sealed envelope. For the online version, the physicians will be notified when participants complete the surveys.
The caregivers will provide information about their socioeconomic and humanistic burden of PFIC, as well as data on direct and indirect costs associated with PFIC, lifestyle changes, and the impact of PFIC in the child’s education, including the measurement of pruritus on the patients.
The caregiver questionnaires include the following validated Patient/Caregiver Reported Outcomes (PRO) questionnaires: CarerQol-7D, Work Productivity and Activity Impairment (WPAI) for caregivers, and the 5-D Itch scale for patients (via parent proxy completion) [Citation17–19]. For the 5-D Itch scale, the caregiver will be responsible for completing the questionnaire, at least for all patients <18 years old, owing to this tool being originally worded for adult populations, and also owing to the disabling nature of the disease and its impact on patients. If the patient is 18 years old or older, they will be optionally allowed to complete the pruritus 5-D Itch scale on their own, if they wish to do so. Time to completion is expected to be on average 20 minutes per caregiver.
identifies the main items from both eCRF and caregiver questionnaire which need to be considered when calculating the costs of PFIC from a societal perspective.
Table 1. Main resource use and cost categories in the PICTURE study questionnaires (CRF and Caregiver)
3.5. Statistical methods
Once collected, all anonymized eCRF will be encrypted and matched with corresponding and anonymized caregiver questionnaires, and all matched data will be analyzed. With the collation of physician-level and caregiver-level data, the following process will be used in order to calculate the overall annual cost of PFIC across France, Germany, UK, and the US:
The equation below is applied to identify the total cost at the individual level (or cost per patient) commonly used in bottom-up approach to cost of illness studies [Citation20]:
In this equation, P denotes the price of one unit of a specific resource in the previous 12 months to the patient’s consultation date, while Q is the quantity of the resource used. This formula will yield the total cost (TC) for an individual denoted with the subscript i- for a specific resource use item denoted with the subscript j-. TC can be used as a variable for summary statistics. This equation can be applied to all resource use items where unit costs and resource use items are available.
To calculate the mean total cost (MTC), the following equation is applied:
Here, n represents the specific country sample size. The inclusion of this variable ensures that the results reported from this study will be specific to each included country (France, Germany, UK and US), facilitating comparisons between the different results.
Total costs are broken down into major cost categories as depicted in , with direct costs coming from the physician information (eCRFs) and indirect and direct non-health coming from the caregiver questionnaires. However, sample sizes for both questionnaires are expected to differ, as the caregiver questionnaire is of a voluntary nature and will compose a proportion or subsample of the eCRF sample.
Given the descriptive nature of the study, the study outcomes will be analyzed using exploratory statistics, where characteristics will be reported as relative frequencies for categorical data, as a mean or median (with measures of dispersion) for continuous data, assuming normality of the data. A comparison of HRQoL (CarerQol-7D and 5D-Itch) and work productivity (WPAI) measures between disease types and country will be made by comparing means and standard deviation. Standard statistical software packages, such as Excel, STATA®16 and R will be utilized to deliver outputs. Where appropriate, univariate comparisons will be tested for significance.
Resource use and cost data are commonly positively skewed with a small number of people consuming a disproportionate amount of resources. In this case bootstrapping techniques can be applied to standard parametric statistics, which does not require the assumption of normality.
Collected data will be audited for completeness, accuracy, and clarity. Data clean-up and cross-checking will be performed prior to data analysis by the statisticians and health economists in charge. The frequency and percentage of missing data will be quantified for all variables. The pattern of missing data across the sample will be evaluated. If considered missing at random, case wise deletion or imputation may be implemented using different methods (overall mean, subgroup mean, regression on non-missing values for imputation, etc.). Decisions on imputation techniques will be discussed internally prior to implementation and the process of data imputation will be reported transparently.
3.6. Sourcing and applying costs
In order to calculate aggregated economic healthcare outcomes, a dataset of unit costs will be created for the resource use items captured in the study questionnaires for each country. From this dataset, costing profiles will be developed. These costs will be collated via access to public tariff information and public data sources.
Unit costs will be assembled for each non-drug resource use item included in the survey, including, but not limited to the costs of medical consultations, hospitalizations, surgery, and professional care. For the medication costs, and because only molecule level information is collected (e.g., no branded medication), the lowest available list price will be used for total costs computation within the different country-specific sources. Estimates of the cost of working days lost will be undertaken using the human capital method, i.e., using relevant daily wage rates.
Although all PFIC-related resource use will be quantified, it is proposed to restrict PFIC drug costing to relevant supportive treatment only. These costs will be collated using national datasets and through discussions with ERG members.
3.7. Ethical approval & data collection
The study protocol was approved by the Research Ethics Sub-Committee of the Faculty of Health and Social care within the University of Chester. The approval stipulated that the study was to be carried out in correspondence with regional and relevant guidelines. Caregivers and adult patients are required to sign an informed consent form in order to participate in the study.
4. Discussion
The authors are aware of the complexity involved in estimating and attaining a truthful and comprehensive representation of the burden of an ultra-orphan, genetic and pediatric disease such as PFIC.
In order to minimize bias and provide accurate estimates of burden for patients and their caregivers, the study population should be representative and generalizable of the real-world PFIC population. For this, the researchers applied broad inclusion and exclusion criteria aimed at capturing a representative sample of the PFIC population in the real-world. Study population will only exclude patients that are not subtypes 1, 2, or 3 (out of scope in the present study) and that are currently or have previously participated in PFIC-related clinical trials in the last 12 months before inclusion in this study (due to the bias this may entail in the resource consumption and patient health outcomes).
However, despite recruiting and sampling of participating physicians and patients which will aim to be representative, the voluntary nature of participation on this study implies that there is a risk of selection bias in physicians and caregivers (families). Additionally, the caregiver information will come from a subsample of the main CRF sample as we have pointed in the statistical methods section above, and the questionnaires are self-completed by caregivers and are non-compulsory.
The possibility of recall bias for participants is low, given that physicians will directly look at their patients records to provide CRF data. However, this may happen for caregivers in certain questions, though the number of questions where recalling past data is needed will be kept to a minimum to reduce this bias and to prevent survey fatigue.
The cost analysis within this study takes a prevalence-based approach, which implies the estimation of costs within a given time interval (a year) rather than an incidence-based approach that would quantify lifetime costs from onset to death, which was not feasible due to practical and economic constraints.
The clinical, costs and quality of life estimates from this study will be used to raise awareness of this disease with policymakers and healthcare professionals and provide wider support for the medical and patient/caregiver community. The hypothesis is that results can justify investment in PFIC to better understand disease and treatment pathways, which may lead to improved diagnosis and therapeutics and in turn reduce the socioeconomic impact of the disease. Given that novel therapies for PFIC have recently emerged and provided positive trial outcomes [Citation21], the results of this study are highly likely to provide support, in the form of quantifiable unmet need data, to any health technology assessments undertaken.
This initiative will be, to the best of the authors’ understanding, the first and only international multicenter study to assess the burden of the disease in a cohort of PFIC patients from a societal perspective. The reader should note that differences in the health system organization and financing can influence the estimates, which justify the need for country-specific analysis that can be used to inform national health policies.
Abbreviations
| AFP | = | Alpha fetoprotein |
| ALT | = | Alanine aminotransferase |
| AST | = | Aspartate aminotransferase |
| BAT | = | Biliary acid transporter |
| BOI | = | Burden of illness |
| BSEP | = | Bile Salt Exporter Pump |
| CarerQol | = | Care-related Quality of Life instrument |
| CBC | = | Complete blood count |
| CEA | = | Cost-effectiveness analysis |
| eCRF | = | electronic Case Report Form |
| EM | = | Electron microscopy |
| ERG | = | Expert Review Group |
| ESLD | = | End-Stage Liver Disease |
| GGT | = | Gamma glutamyl transpeptidase |
| HRQoL | = | Health-Related Quality of Life |
| MRCP | = | Magnetic resonance cholangiopancreatography |
| MRE | = | Magnetic resonance elastography |
| MRI | = | Magnetic resonance imaging |
| MTC | = | Mean total cost |
| PBD | = | Partial biliary diversion |
| PFIC | = | Progressive Familial Intrahepatic Cholestasis |
| PPIE | = | Patient, Public Involvement and Engagement |
| PRO | = | Patient reported outcomes |
| PT | = | Prothrombin |
| QoL | = | Quality of life |
| TC | = | Total cost |
| TSH | = | Thyroid stimulating hormone |
| UDCA | = | Ursodeoxycholic Acid |
| WPAI | = | Work Productivity and Activity Impairment |
Declaration of interest
Leonardo Ruiz-Casas, Claudia Mighiu and Sonia O’Hara are salaried employees of HCD Economics, the healthcare consultancy firm conducting the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
Leonardo Ruiz-Casas provided contribution to study design and governance. Sonia O’Hara and Claudia Mighiu were responsible for write-up and ongoing critical review of the article.
KOLs: Professor Alan Finnegan provided feedback in preparation for the ethical submission of the study protocol and materials. Alison Taylor, Professor Jorn M Schattenberg, Doctor Anil Dhawan, Doctor Karen F Murray, Jose Willemse, Emily Ventura, Melanie Karakaidos and Harpreet Brrang were responsible for ongoing study review and feedback regarding design, data collection, analysis and critical review of the manuscript.
Supplemental Material
Download MS Word (29.9 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Davit-Spraul A, Gonzales E, Baussan C,et al. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 4(1):1 (2009).
- Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. Elsevier; Vol.4 p. 25–36 (2014).
- Gunaydin M, Bozkurter Cıl AT. Progressive familial intrahepatic cholestasis: diagnosis, management, and treatment. Hepatic Med Evid Res. Volume 10:95–104 (2018).
- Bull LN, Thompson RJ. Progressive Familial Intrahepatic Cholestasis. Clinics in liver disease. W B Saunders; Vol.22, p. 657–669 (2018).
- Gomez-Ospina N, Potter CJ, Xiao R,et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 7(1):1–8 (2016).
- Sambrotta M, Strautnieks S, Papouli E,et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 46(4):326–328 (2014).
- Baker A, Kerkar N, Todorova L,et al. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 43(1):20–36 (2019).
- Jacquemin E, Hermans D, Myara A,et al. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 25(3):519–523 (1997).
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 36(SUPPL.1):S26–35 (2012).
- Yasuda I, Iwashita T, Ohnishi T,et al. ENDOSCOPIC NASOBILIARY DRAINAGE: CURRENT INDICATIONS AND EVALUATION OF THE PRODUCTS. Digestive Endoscopy 18(s1):S92–5 (2006).
- Al-Dury S, Marschall HU. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Frontiers in Pharmacology. Frontiers Media S A. 9: 931 (2018).
- Bustorff-Silva J, Neto LS, Olímpio H,et al. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis-a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg. 42(8):1337–1340 (2007).
- Hegade VS, Kendrick SFW, Dobbins RL,et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 389(10074):1114–1123 (2017).
- Mayo MJ, Pockros P, Jones D,et al. Clarity: A phase 2, randomized, double-blind, placebo-controlled study of lopixibat chloride (Formerly Lum001), a novel apical sodium-dependent bile acid transporter inhibitor, in the treatment of primary biliary cirrhosis associated with itching. J Hepatol. 64(2):S 197 (2016).
- Al-Dury S, Wahlström A, Wahlin S,et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci Rep. 8(1):6658 (2018).
- Lee WS, Chai PF, Looi LM. Progressive familial intrahepatic Cholestasis in Malaysian patients – A report of five cases. Med J Malaysia. 64(3): 216 (2009)
- Brouwer WBF, Van Exel NJA, Van Gorp B,et al. The CarerQol instrument: A new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res. 15(6):1005–1021 (2006).
- Elman S, Hynan LS, Gabriel V,et al. The 5-D itch scale: A new measure of pruritus. Br J Dermatol. 162(3):587–593 (2010).
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 4(5):353–365 (1993).
- Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. Korean Association for the Study of the Liver; 20:327–337 (2014).
- Albireo Phase 3 Trial Meets Both Primary Endpoints for Odevixibat in PFIC:: Albireo Pharma official website (September 2020). [cited 2020 Nov 11]. Available from: http://ir.albireopharma.com/newsreleases/news-release-details/albireo-phase-3-trial-meets-both-primary-endpoints-odevixibat