ABSTRACT
Introduction
Vestibular rehabilitation (VR) is now a subject of active studies and has been shown to be effective for multiple vestibular disorders, peripheral or central. VR is a physical therapy that helps train the central nervous system to compensate for vestibular dysfunction. There is moderate to strong evidence that VR is safe and effective for the management of peripheral vestibular dysfunction. Nonetheless, the studies on how VR works on central vestibular dysfunction remains scanty.
Areas covered
This article addressed the rehabilitation strategies and possible mechanisms, including how central vestibular function might improve upon rehabilitation. In addition, it provides some examples concerning the effect of VR on central vestibular dysfunction.
Expert opinion
VR works on the vestibular system through repetition of specific physical exercises that activate central neuroplastic mechanisms to achieve adaptive compensation of the impaired functions. VR has become a mainstay in the management of patients with dizziness and balance dysfunction. Individualized VR programs are a safe and effective treatment option for a large percentage of patients with central vestibular disease reporting imbalance and dizziness. Exploration of various treatment strategies and possible mechanisms will help develop the best and personalized VR treatment for patients with central vestibular dysfunction.
1. Introduction
The International Classification of Vestibular Disorders (ICVD) proposed three specific syndromes comprising the bulk of all vestibular presentations, including acute vestibular syndrome, episodic vestibular syndrome and chronic vestibular syndrome [Citation1]. These syndromes can occur in both peripheral and central vestibular disorders. From 2017 to 2020, the central vestibular diseases have been on the rise by the year and significantly surpassed their peripheral counterparts () [Citation2,Citation3]. The central vestibular system participates in the perception of head and body motion, generation of eye motion driven by vestibular and visual signals, balance and posture. The central version tends to be more persistent, disabling, and debilitating than that resulting from peripheral vestibulopathy. The treatment of central vestibular disease mainly includes comprehensive management, such as medical, surgical and physical therapy ().Though medications are indispensable for some diseases, they tend to cause serious side effects, are costly, and currently surgical are believed to be traumatically [Citation4,Citation5]. Upon physical therapy such as VR, patients with central vestibular dysfunction reportedly showed improvement in balance, dizziness, and quality of life [Citation6].
Figure 1. Trends in the incidence of various vestibular disorders in recent years. From 2017 to 2020 [Citation2,Citation3], the central vestibular diseases have been on the rise by the year and significantly surpassed their peripheral counterparts. In order to more vividly express the changes of diseases, we make a new comparison graph according to the two data from different years. BPPV, benign paroxysmal positional vertigo.
![Figure 1. Trends in the incidence of various vestibular disorders in recent years. From 2017 to 2020 [Citation2,Citation3], the central vestibular diseases have been on the rise by the year and significantly surpassed their peripheral counterparts. In order to more vividly express the changes of diseases, we make a new comparison graph according to the two data from different years. BPPV, benign paroxysmal positional vertigo.](/cms/asset/0777fa72-c037-48ea-ba26-80b3e3f9239a/iern_a_2106129_f0001_oc.jpg)
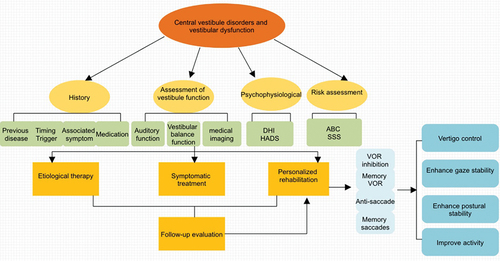
Figure 2. Diagnosis and treatment process of vestibular rehabilitation in central vestibular disorders and vestibular dysfunction. The diagnosis of central vestibular diseases mainly included history, assessment of vestibular function, psychophysiological, risk assessment and so on. Etiological therapy, symptomatic treatment, personalized rehabilitation especially VOR suppression, Memory VOR, Anti-Saccade, Memory saccades play the leading role. VOR, Vestibulo-Ocular Reflex; DHI, Dizziness Handicap Inventory; Hads, Hospital Anxiety Depression Scales; ABC, Activity-specific Balance Confidence Scale; SSS, Somatic Self-rating Scale.
VR dates back to the 1940s when it was first introduced by Cawthone and Cooksey to treat patients with peripheral vestibular disorders [Citation7,Citation8]. The Cochrane Collaboration concluded that there is moderate to strong evidence that VR is safe and effective for the management of peripheral vestibular dysfunction [Citation9]. In 2016, the American Physical Therapy Association issued the ‘Clinical Practice Guidelines for Vestibular Rehabilitation of Peripheral Vestibular Hypofunction’ [Citation10]. Modern research has extended the application of VR to patients with dizziness and vertigo of different origins. Thanks to recent advances in clinical and laboratory research, our understanding of the central vestibular dysfunction has been substantially enhanced over the past years. Prior studies indicated that VR could also have a positive effect on dizziness and vertigo of central origin. In 2020, Tramontano et al. conducted a systematic review of individualized VR for central vestibular diseases and reviewed several prospective randomized controlled trial studies, showing that individualized VR was efficacious for the treatment of central diseases [Citation11]. VR has increasingly become a generally- accepted non-drug, noninvasive and physical treatment choice, and has been gradually used as a routine treatment [Citation12–20].
However, studies on how VR works on central vestibular disease has been scanty [Citation21,Citation22]. This review, for the first time, explore the mechanism underlying vestibulo-ocular reflex (VOR), especially VOR suppression, VOR memory, anti-saccade, and memory-guided saccade works on the central vestibular disease. In this review, we also we focused on several disorders resulting in central vestibular dysfunction, i.e. vestibular migraine (VM), persistent postural-perceptual dizziness (PPPD), multiple sclerosis (MS), cerebellar stroke to discuss the application and efficacy of VR. This review may lay a foundation for further studies on the mechanism by which rehabilitation works on central vestibular disorders and help develop better-targeted and more effective VR treatment against central vestibular diseases.
2. Mechanisms and principles of vestibular rehabilitation
VR is mechanistically based on the plasticity and functional compensation of the central nervous system and the vestibular system. By means of compensation of the cortex, brainstem, and cerebellar pathway, the stability of eye movement and posture is attained. The cerebellum plays a pivotal part in the adjustment of plasticity between the pompons and the medial vestibular nucleus [Citation23]. The cerebral cortex possesses tremendous plasticity. Expanding cortical function or re-organizing the conduction pathway can maintain the vestibular function [Citation24].
VR exercises are designed to neurophysiologically reduce the disabling symptoms on the strength of neuroplasticity, including adaptation [Citation25–27], habituation, and substitution [Citation28,Citation29], which accelerate the vestibular compensation [Citation13,Citation15,Citation30–34]. Physiologically, habituation results from decreased excitatory postsynaptic potential caused by presynaptic membrane calcium channel blockers, while adaptation is the results of the tissue remodeling due to the up-regulated expression of corresponding genes and proteins [Citation35]. In the literature on vestibular disorders, adaptation referred to long-term changes in the neuronal response to head movements, with the goal of reducing symptoms and normalizing gaze and postural stability [Citation10]. Synaptic inhibition or membrane hyperpolarization of medial vestibular nucleus neurons elicit a persisting elevation in intrinsic excitability through a process known as ‘firing rate potentiation,’ which might be utilized in vivo to mediate behavioral plasticity [Citation36,Citation37]. Substitution is a process by which a sensory system compensates for the deficiency of another sensory system [Citation38,Citation39]. Such substitution takes place via vision and proprioception to replace or enhance the lack of vestibular inputs, via the central compensation, i.e. sensory integration, to improve the postural and gaze stability [Citation40,Citation41]. VR exercises must challenge balance mechanisms holistically and target motor, sensory (vision/vestibular/somatosensory), and cognitive aspects, it must be goal – orientated and context-specific [Citation42,Citation43]. Postural stability is accomplished by the central processing of sensory inputs consisting of visual, vestibular, auditory and proprioceptive information. The vestibular system, whose job is to integrate this information, establishes novel neural networks to replace the lost afferent inputs [Citation44]. These changes are basis of the central compensation, which occurs due to neuronal and neurochemical activity caused by sensory conflicts [Citation45,Citation46].
2.1. Mechanism of rehabilitation for central vestibular dysfunction
The peripheral vestibular receptors take the sensory signals via VOR pathway. Afterward, the signals go, along the vestibular nerve, to the vestibular nerve nucleus, and then to the oculomotor nucleus via the medial longitudinal fascicle, eventually arriving at the extraocular muscles nuclei. Any problems in the pathway can affect the field of vision. The VOR can be viewed as an adaptive control system that maintains compensatory eye movements during head motion, thereby allowing people to have a clear vision in the fovea retina. VOR Suppression and VOR memory are commonly used for rehabilitation of central vestibular hyperfunction. Anti-saccade, and memory-guided saccade are commonly used for rehabilitation of central fixation dysfunction [Citation23].
2.1.1. Vestibulo-ocular reflex suppression
VOR suppression occurs when a normal person is moving his or her head, and, at the same time, is tracking a visual target that is going with the head movement. During VOR suppression, the eyeballs are virtually still and does not have abnormal movements. Since the VOR response is canceled by the simultaneously moving visual target, the VOR suppression is also dubbed VOR cancellation [Citation47,Citation48].
VOR suppression is used for the rehabilitation of central vestibular hyperfunction because the VOR pathway is a part of the neural reflex of the brainstem, and the pathway is controlled by the cerebellar and cortical centers. This control mainly takes on the form of inhibition. Central vestibular diseases damage the superior center of VOR, and its inhibitory effect is weakened. As a result, VOR is hyperactive (Tracking a visual target that moves synchronously with the head movement while the head is moving, the eyeballs cannot remain still and nystagmus results, which means that the VOR response during head movement cannot be canceled by the synchronized eye movement, resulting in the failure of VOR suppression). It is common in central damage. Consequently, the vestibular eye reflex gain increases, and a series of symptoms develop. Therefore, the central vestibular hyperfunction requires VOR suppression training to maintain a constant eye and head speed, regain the VOR and lower the VOR hyperactivity. Therefore, clinically, when performing VR for central vestibular dysfunction, VOR suppression is predominantly used. Briefly, fixation of a target while rotating the head and moving the target at the same angular velocity (Each session is repeated 5 times).
The prerequisite for VOR suppression: First, the vestibule functions properly. If the vestibular function is totally removed, there will be neither VOR nor VOR suppression. Second, the visual tracking works properly. Patients with tracking dysfunction tend to have impaired VOR fixation suppression. In peripheral vestibular diseases, such as unilateral vestibular dysfunction, the VOR gain drops, and the visual image slides on the retina to produce signals that stimulate the vestibular system, the vestibular center pools the inputs to increase the VOR gain.
2.1.2. Vestibulo-ocular reflex memory
The memory visual target serves to utilize the cognitive function of the central nervous system, such as cerebral cortex, the cerebellum, and brainstem, to initiate the outgoing copy signal and send the copy signal to the relevant structure via the perceptual internal template, and then by adjusting the central nervous system [Citation49,Citation50]. Training sessions in VOR memory include: the patient stares at the central target, then closes eyes, slowly turns head to one side, the eyes are glued to the imaginary target in memory, open eyes after 5 seconds to see if there is a gap between the actual point and the imaginary one, and then turn head back to normal position. The session is repeated 5 times.
2.2. Mechanism of rehabilitation for central fixation dysfunction
2.2.1. Anti-saccade and memory-guided saccade
A saccade is defined as a rapid eye movement whose purpose is to fixate the gaze on a visual target. The purpose of a saccade is to fixate the fovea on new targets of interest or to make corrective ‘catch up’ movement when a target escapes the more slowly tracking smooth-pursuit system [Citation51]. Anatomically, Saccade pathways involve several regions of the cerebral cortex, the cerebellum, and brainstem. It is in the brainstem-cerebellum eye movement center, which is a reflex eye movement pathway, or the exogenous eye movement pathway. It is a conduction pathway under the visual cortex and does not necessarily go through the visual cortex. During a saccade, retina sensory signals, via retinal ganglion cells and optic nerve afferent pathways, reach the lateral geniculate body, and go upward to the superior colliculus system. Then, they, make their way, via the superior colliculus, then paramedian pontine reticular formation (PPRF) (vertical) and rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) (horizontal), respectively, to the eye motor nucleus. An extremely rapid and precise eyeball conjugate movement can be either reflexive or random [Citation52]. Saccades are easy to execute, but anti-saccades require suppression mechanisms to prevent the automatic execution of a visually driven saccade toward the stimulus; hence anti-saccades impose a higher demand on attentional and cognitive resources [Citation53,Citation54]. Anti-saccade, when inhibiting a reflex saccade, initiates a random saccade at the same time. The superior colliculus is inhibited to reduce the excitability of the central superior colliculus to PPRF (vertical) and riMLF (horizontal), thereby suppressing reflex saccades [Citation52]. The cortical initiation center of the random saccade is located in the cortical center of each lobe of the brain [Citation55], which starts the random saccade by sending excitatory impulses to the brainstem reflex saccade center (PPRF and riMLF).
The anti-saccade task, first developed by Peter Hallett [Citation56], has been used extensively for investigating mechanisms of voluntary saccade control. Anti-saccade suddenly appears in the field of vision at the mirror position of the target. It was initially developed as a way to dissociate between the stimulus location and the goal of the saccade [Citation57]. Memory-guided saccade is based on the position of the target that appeared in memory. When an action is memory-guided, its control must access a stored representation of the target, and this stored representation cannot be provided by the visuomotor mechanisms in the dorsal pathway [Citation58]. Both are the endogenous higher cortex eye movement reflex and include cortical-subcortical eye movements. Such reflexes involve the center and structure alone the random eye movement pathways, with the will, the higher cortex and the cognitive processing process being implicated in them. The difference between the two saccades lies in that the visual target moves in different manners. In anti-saccade, the examiner raises the target with both hands, and randomly indicates one of the two visual targets. With patient’s head staying static, and the eyes quickly scan in the opposite direction of the indicated target. With memory-guided saccade, the examiner holds two vision targets with both hands. At a certain angle, the patient stares at the two vision targets on the left and the right. After allowing for adaptation for some time, the patient closes eyes and imagines the eye movement when eyes are open and move eyes to the left and right. The session is repeated 5 times.
3. Efficacy and pathologies of vestibular rehabilitation in central vestibular dysfunction
3.1. Vestibular migraine
3.1.1. Introduction of vestibular migraine
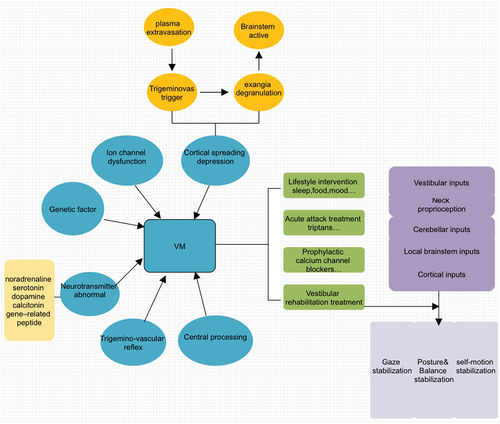
Vestibular migraine (VM) is now a distinct disease entity as proposed by both the Barany Society and the International Headache Society [Citation59,Citation60]. VM patients may suffer from peripheral or central vestibular dysfunction between episodes [Citation61,Citation62]. The pathophysiology of VM is not fully known, and it is generally believed to involve trigemino-vascular reflex, cortical spreading depression, ion channel dysfunction, genetic factors and neurotransmitter abnormality () [Citation63]. Meanwhile, the growing base of knowledge concerning migraine generally points to both central and peripheral mechanisms. In the cerebral cortex, evidence suggests that visual auras are due to cortical spreading depression, a transient reversible wave of depolarization triggered by the activation of cortical pyramidal cells [Citation64]. The diagnosis of vestibular migraine is based on recurrent vestibular symptoms, a history of migraine, a temporal association between vestibular symptoms and migraine ones and elimination of other causes of vestibular symptoms. It mimics virtually all forms of dizziness in terms of symptoms, duration, and pattern of attacks and tends to be misdiagnosed [Citation65,Citation66]. The prevalence of VM is 2.7% in population studies and 10% in outpatient clinics [Citation67–69]. For patients with VM, it is also important to consider comorbidities such as Meniere’s disease, BPPV, anxiety and depression, which significantly affect the quality of life. Subjects with VM also pose a heavy burden on the health-care system [Citation68].
Figure 3. The treatment mechanism of vestibular migraine. Pathogenesis of vestibular migraine (VM) is still poorly understood and researchers fail to agree regarding whether its origin is predominantly central or peripheral. The factors involved in the pathogenesis of VM do not work separately but are intricately interwoven. Treatment options for VM patients include reducing or removing triggering factors, medications, physical rehabilitation therapies, and treatment of comorbidities.
3.1.2. Efficacy of vestibular rehabilitation for vestibular migraine
Treatment options for VM patients include reducing or removing triggering factors, medications, physical therapies, and treatment of comorbidities () [Citation70–72]. Acute medical treatment can quickly and persistently relieve pain and reduce the recurrence of headache. Prophylactic medications can lower the frequency of attacks, mitigate the severity of attacks, and minimize functional impairment [Citation73–75]. However, adverse reactions caused by some medicines include ataxia, impaired concentration, and fatigue. Moreover, vestibular suppressants should be tapered and discontinued, as they can stop or slow vestibular compensation [Citation71]. Preliminary studies showed that VR helped to ease tension-type headache, relive anxiety and depression, and improve physical performance measures and self-perceived abilities [Citation76–78]. Furthermore, VR exercises may also work on VM, either independently or in combination with medications [Citation79,Citation80].
3.1.3. Vestibular rehabilitation program and mechanism in vestibular migraine
Pathogenesis of VM is still poorly understood and researchers fail to agree regarding whether its origin is predominantly central or peripheral. The factors involved in the pathogenesis of VM do not work separately but are intricately interwoven [Citation81,Citation82]. Abnormal sensory modulation or integration within the thalamo-cortical network could result in dizziness and spatial disorientation, which may lead to a ‘higher level’ dysfunction of the multisensory integration function of spatial orientation. Activities such as ballet dancing and yoga can enhance spatial perception and physical coordination [Citation64]. Cortical spreading depression hypothesis assumes that, during aura migraine, various factors stimulate the cerebral cortex and then the inhibitory cortical electrical activity spreads from the stimulation site to the surrounding regions. Vestibular connections can be divided into downward-projecting vestibulospinal tracts and upward projections [i.e. to the ocular motor nuclei that organize the VOR] [Citation83]. When it diffuses to the vestibular cortex (the parietal lobe and insular lobe), the activity is inhibited, and the inhibitory effect on the brainstem vestibular nucleus is weakened, thereby affecting the processing of vestibular signals and causing vestibular symptoms, or leading to transient vestibulo-ocular dysfunction or vestibular hypersensitivity associated with migraine [Citation84].
VM patients reportedly had a greater VOR time constant [Citation85,Citation86]. In these patients, the enhanced suppression of the VOR in patients suffering from dizziness/vertigo was likely to be a cerebellar adaptation to suppress the hypersensitive vestibular system [Citation81,Citation82]. Decreased cerebellar inhibition on VOR may be the neuropathological basis of the vestibular hypersensitivity associated with migraine [Citation87]. VR may involve training patients to bring on the symptoms to ‘desensitize’ the vestibular system, teaching them to achieve coordination between eye and head movements, helping them attain better balance and engage in ambulation [Citation88,Citation89].
Some studies postulated that an increase in sensitivity to visual-vestibular mismatch is culpable for the development of symptoms. Patients with VM have a dramatically greater sensitivity to complex motion than normal subjects and migraineurs without vertigo [Citation90,Citation91]. This hypothesis may explain the motion sensitivity and vestibular sensitivity to active visual surroundings in VM patients [Citation92]. Future VR strategies such as VOR suppression should designed to ‘desensitize’ the symptoms of VM.
3.2. Persistent postural-perceptual dizziness
3.2.1. Introduction of persistent postural-perceptual dizziness
Persistent postural-perceptual dizziness (PPPD) is characterized by persistent dizziness, unsteadiness, and non-spinning vertigo lasting for three months or longer. The Barany Society has developed a diagnostic framework and outlined the putative pathophysiologic mechanisms [Citation93,Citation94]. The term PPPD is new, but the condition is not. Its diagnostic criteria were based on a consensus of experts who integrated phobic postural vertigo (PPV), space-motion discomfort (SMD), visual vertigo (VV), and chronic subjective dizziness [Citation95–98]. Victims often feel that the symptoms worsen in an upright posture, in movement or when exposed to complex visual stimulation, or during active or passive head movements [Citation99]. It can occur at any age group and the most frequently in the age group of about 40 years, with a female predominance [Citation100].
3.2.2. Persistent postural-perceptual dizziness and psychiatric condition
PPPD is categorized as a chronic functional vestibular dysfunction and not an organic or a psychiatric condition [Citation93]. The fact that VR and cognitive behavioral therapy (CBT) are similar in some respects may be a further expression of the linkage between vestibular dysfunction and anxiety disorders [Citation101]. Many vertigo diseases, especially PPPD, are related to mental-psychological conditions. A recent study has observed that PPPD patients registered higher depression scores were registered than the victims of other vestibular disorders. The same pattern was also observed in the VM group and other central vestibular dysfunction too [Citation102]. So it is worth noting the importance of multidisciplinary therapy associated with psychiatrical and psychological management.
3.2.3. Efficacy of vestibular rehabilitation for persistent postural-perceptual dizziness
PPPD treatment mainly includes VR treatment in combination with CBT, supplemented with medications () [Citation103–105]. A retrospective study conducted by Thompson et al. in 2015 showed that VR based on acclimatization training was effective against PPPD, showing that the head/body movement-related symptoms improved more than environmentally/visually-induced dizziness symptoms did [Citation106]. In 2019, a prospective study in Egypt customized personalized home VR training showed that it helped to reduce dizziness symptoms and improve the quality of life for PPPD patients [Citation107]. At present, it is believed that the combination of guided therapy by physical therapists and customized family training is more beneficial to the rehabilitation of PPPD [Citation106].
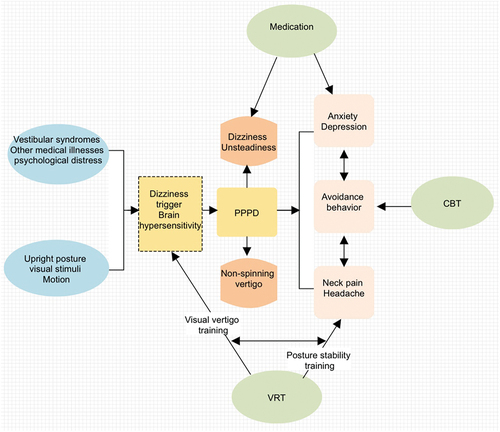
Figure 4. The brief introduction of PPPD. Affected individuals of PPPD feel worst when upright, exposed to moving or complex visual stimuli, and during active or passive head motion. These situations may not be equally provocative. Ingredients of treatment include (a) A clear positive diagnosis and explanation that you can work with; (b) Recognition during assessment of all the various component symptoms that may or may not be going along with your PPPD; (c) Physiotherapy/Desensitization of movement such as VRT. PPPD, persistent postural-perceptual dizziness; VRT, vestibular rehabilitation treatment; CBT, cognitive-behavioral therapy.
3.2.4. Vestibular rehabilitation program for persistent postural-perceptual dizziness and mechanism
Habituation and visual/optokinetic motion desensitization is the principal VR exercise in PPPD. First, from general physical exercise to specific acclimatization exercise that triggers dizziness, then the visual/optokinetic motion desensitization was performed by looking at moving or complex images, and further, multitasking gait training was carried out to gradually establish a normal gait. The VR treatment of PPPD overlaps with CBT and the two methods were detailed in following session. If the either of the treatments doesn’t work well, medications can be initiated.
The pathophysiological processes underlying PPPD are not fully understood. Mounting evidence suggests that it might arise from changes in postural control, multi-sensory data processing or cortical incorporation of spatial orientation and threat evaluation [Citation108–117]. PPPD per se does not lead to abnormalities in vestibular or oculomotor reflexes, such as the vestibulo-ocular reflex or smooth pursuit, but individuals with PPPD may have peripheral or central vestibular deficits related to the triggering or co-existing conditions [Citation118]. Presence of vestibular dysfunction may result in a compensatory increase in the sensitivity to visual or proprioceptive balance cues, the chronic hypersensitivity to motion stimuli and visual complexity, the core symptom of PPPD, means that it requires VOR Suppression or VOR memory as habituation/desensitization approach, rather than a compensation approach.
3.3. Multiple sclerosis
3.3.1. Introduction of multiple sclerosis
Multiple Sclerosis (MS) is a chronic autoimmune disease of the central nervous system that involves the white and gray matters, in which inflammation, demyelination and axonal loss take place at much more early stages of the condition [Citation119,Citation120]. It is a complex disease characterized by a wide array of symptoms, including fatigue, vision problems, abnormal sensations, muscle spasms, stiffness and weakness, trouble walking and difficulty with balance symptoms. Among them postural intolerance reportedly affect about 49%-59% of MS patients [Citation121–123], and balance dysfunction was found in 75–82% of mild to moderate cases [Citation124]. MS symptoms result from lesions in the brain, spinal cord, or optic nerves that can affect balance, gait and lead to fall [Citation125]. A total of 2.8 million people are estimated to live with MS worldwide (35.9 per 100,000 population) and females are twice as likely to have MS as males [Citation126]. Independent of prevalence, the incidence of MS seems to be on the rise around the globe [Citation127]. Compared with the general population, MS patients tend to have a higher death rate and a shorter life expectancy by about 10 years [Citation128].
3.3.2. Efficacy of vestibular rehabilitation for multiple sclerosis
In view of MS is a complicated, chronic and progressive condition that assumes various forms, varying efficacy drugs aims to reduce relapses and delay disease progression [Citation129]. For example, methylprednisolone, IFN-beta, rituximab, fingolimod, teriflunomide and so on, are effective disease-modifying drug therapies for MS [Citation130]. What is more, non-pharmacological multidisciplinary care can, in some cases, improve patient outcomes. Some studies showed that VR, as an exercise-based program, primarily designed to reduce vertigo and dizziness, gaze instability, and/or imbalance and falls, was effective for MS patients [Citation131]. One meta-analysis supports the notion that VR is more effective than no intervention in attaining improvement in balance in subjects with MS [Citation132]. In addition, Tramontano el al. found that VR plus the cerebellar intermittent theta burst stimulation (c-iTBS), as a novel rehabilitative strategy could increase cerebellar activity, and improve gait and balance ability in 20 hospitalized MS patients [Citation133]. Incidentally, one case report found that applying immersive virtual reality (VRi) vestibular training protocol to the MS population could improve dizziness, balance, gait, impact of fatigue and quality of life [Citation134].
3.3.3. vestibular rehabilitation program for multiple sclerosis and mechanisms
Mechanistically, several areas along the peripheral and central vestibular neural pathways may be involved in the pathogenesis of MS. These regions include the eighth nerve, the vestibular nuclei, the oculomotor tracts, the medial longitudinal fasciculus and the cerebellum [Citation135]. Furthermore, a deficit in the integration of these sensory cues along the subcortical and/or cortical areas has also been observed to be linked to impaired balance performance [Citation136,Citation137]. Impaired balance in MS is associated with lesions in the brainstem and cerebellum [Citation138,Citation139]. Irrespective of the location of the lesion, either in the cerebellum or in brainstem, VR strategies such as anti-saccade or memory-guided saccade may improve balance, dizziness, fatigue symptoms and health-related quality of life.
Interventions specifically designed to improve balance should yield better results than interventions to strengthen lower limbs or aerobic exercises [Citation140]. A randomized controlled study found that fixation exercises, static and dynamic balance exercises, acclimatization, walking in various postures, and walking training that change visual inputs could effectively improve vestibular function in MS patients [Citation141]. Furthermore, evidence suggests that unsteady gaze fixation in people with MS enhances intention tremor during visuo-motor tasks requiring eye-hand coordination [Citation138]. What is more, a sensory integration balance training performed under different sensory conditions may improve balance more in MS patients [Citation142–144]. Intensive multidisciplinary rehabilitation is recommended for all MS patients and the treatment should start as early as possible to maximize functional recovery [Citation145–147].
3.4. Cerebellar stroke
3.4.1. Introduction of cerebellar stroke
Cerebellar stroke resulting from either hemorrhage or infarction may present with vertigo, ataxia, and unique eye movements [Citation83]. Acute cerebellar infarction usually manifests as solitary vertigo, possibly not accompanied by other nervous system signs, and might be easily missed or misdiagnosed as peripheral vertigo. The condition should be diagnosed in time to avoid serious post-infarction edema [Citation148]. The incidence of cerebellar infarction is relatively low, making up about 2% to 3% of acute cerebral infarction. Most symptoms are nonspecific. Dizziness or vertigo is the most common complaint of patients suffering from cerebellar infarction. Patients with cerebellar infarction tend to have ataxia and balance dysfunction, and their principal manifestations include staggering gait, slow movement, difficulty in walking straight and maintaining body balance, all seriously affecting quality of life [Citation149,Citation150]. Because cerebellar infarction usually has no specific clinical symptoms, in many cases, it mimics peripheral vestibular vertigo, and the emergency brain CT examination only identified 26% of acute cerebral infarction [Citation151].
3.4.2. Vestibular rehabilitation for cerebellar stroke and mechanisms
The cerebellar flocculus is involved in the inhibitory regulation of excitatory and inhibitory branches of VOR pathway. The cerebellum is believed to play an important role in the recovery of static symptoms after unilateral labyrinthectomy, and the entire cerebellum-related pathways are involved in the initial stage of vestibular compensation [Citation152]. A complete cerebellum is required for calibration by long-term vestibular ocular reflex in post-injury animals. The cerebellum plays an important role in the integration of sensory and motor signals, coordination of motor and cognition, and motor learning.
At present, no clinically effective drugs are available for the treatment of the cerebellar lesions. A recent study reported that the fixation suppression was impaired in about 50% of patients with cerebellar infarctions [Citation153]. Rehabilitation during the acute phase of cerebral infarction can effectively improve the quality of life. Therefore it is crucial that appropriate rehabilitation strategy should be used for patients with cerebellar infarction in the acute phase [Citation154,Citation155]. Some scholars found that balance exercises can improve the motor coordination by remodeling nerve synapses and activating astrocytes to improve the patient’s balance. However, early post-stroke multisensorial training, under visual deprivation with somatosensorial and vestibular stimulation, could be more effective than a traditional approach based on neurodevelopmental concepts [Citation156,Citation157].
4. Conclusion
The aim of this review is to summarize the efficacy and mechanism of VRT in central vestibular dysfunction and to provide a foundation for future studies. VOR suppression, VOR memory, anti-saccade, and memory-guided saccade deeply analysis for the rehabilitation of central vestibular diseases. Individualized VR programs are a safe and effective treatment option for a large number of patients suffering from imbalance and dizziness due to central vestibular disease.
5. Expert opinion
Vertigo, dizziness, poor balance and abnormalities in the control of eye movements are symptoms that may be associated with vestibular problems. Such symptoms are common in people with central vestibular diseases, can lead to falls, injury, and a restriction in outdoor mobility, and, subsequently, may affect social functions and quality of life.
5.1. New insights: where are we going?
VR is the golden-standard care for patients with vestibular problems. VR involves progressive exercises, including eye, head, and body movements in sitting, standing, and walking. Given the complexity of symptoms in people with central vestibular issues, it may be that more effective personal exercises delivered via proficient in mechanism. Therefore, this research is important for in-depth exploration of the mechanism of central vestibular diseases. On the basis of the study, future research should be directed at formulating personalized VR tailored to different diseases.
Factors affecting VR, including rehabilitation assessment procedures [Citation158], strategies and dosage, and patients’ involvement [Citation159]. With the advent of new diagnostic tools, it is possible to assess the function of each component of the vestibular apparatus. Incorporating emerging technologies, such as virtual reality, Internet/mobile phone software application and sensory enhanced biofeedback into VR can provide the best and personalized VRT for patients with central vestibular dysfunction [Citation160–163]. At present, understanding of the optimal intervention time, training type, frequency and intensity of VR still warrants more high-quality and large-scale RCTs. Moreover, VR protocols have proven to be effective in improving vestibular compensation in clinical practice. Nonetheless, the underlying neurobiological mechanisms remain unknown.
It is necessary to improve clinical practice guidelines (CPGs) to help physical therapists better treat patients with central vestibular dysfunction to optimize rehabilitation outcomes. I hope our review can provide a starting point for the formulation of future CPGs, since CPGs could allow clinicians to know who, what, how and when to treat, to ensure that exercise interventions are currently the best choice available.
Article highlights
Vestibular rehabilitation (VR) has increasingly become an extensively accepted non-drug, non-invasive and physical treatment choice, and has been gradually used as a routine treatment.
Vestibulo-ocular reflex (VOR) suppression and VOR memory are commonly used for rehabilitation of central vestibular hyperfunction. Anti-saccade, and memory-guided saccade are commonly used for rehabilitation of central fixation dysfunction.
This study gives some examples that VR is efficacious for the treatment for the central vestibular dysfunction, such as vestibular migraine (VM), multiple sclerosis (MS), persistent postural-perceptual dizziness (PPPD), cerebellar stroke and explains their mechanisms.
The study paved the way to the further exploration on the rehabilitative mechanisms in central vestibular diseases and to the future development of more-targeted and effective VR alternatives for the central vestibular dysfunction.
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bisdorff AR, Staab JP, Newman-Toker DE. Overview of the international classification of vestibular disorders. Neurol clin. 2015;33(vii):541–550.
- Brandt T, Dieterich M. The dizzy patient: don’t forget disorders of the central vestibular system. Nat Rev Neurol. 2017;13:352–362.
- Strupp M, Dlugaiczyk J, Ertl-Wagner BB, et al. Vestibular disorders. Dtsch Arztebl Int. 2020;117:300–310.
- Rainer S, Heiko R, and Thomas B, et al. Treatment of dizziness: an interdisciplinary update. Swiss Med Wkly. 2017;147:w14566.
- Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013:Cd010610.
- Pamela MD, Janene MH, and Susan LW. Vestibular rehabilitation: advances in peripheral and central vestibular disorders. Curr Opin Neurol. 2018;32:137–144.
- Cawthorne T. Vestibular Injuries. Proc Royal Soc Med. 1946, 39:270–273.
- Cooksey FS. Rehabilitation in vestibular injuries. Proce Royal Soc Med. 1946, 39:273–278.
- McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;1:Cd005397.
- Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American physical therapy association neurology section. J Neurol Phys Ther. 2016;40:124–155.
- Tramontano M, Russo V, Spitoni GF, et al. Efficacy of vestibular rehabilitation in patients with neurologic disorders: a systematic review. Arch Phys Med Rehabil. 2021;102:1379–1389.
- Gans RE. Vestibular rehabilitation: critical decision analysis. Seminars in Hearing . 2002;23:149–160.
- Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184–196.
- Whitney SL, Sparto PJ, Furman JM. Vestibular rehabilitation and factors that can affect outcome. Semin Neurol. 2020;40:165–172.
- Dunlap PM, Holmberg JM, Whitney SL. Vestibular rehabilitation: advances in peripheral and central vestibular disorders. Curr Opin Neurol. 2019;32:137–144.
- Crane BT, Schubert MC. An adaptive vestibular rehabilitation technique. Laryngoscope. 2018;128:713–718.
- Whitney SL, Alghwiri AA, Alghadir A. An overview of vestibular rehabilitation. Handb Clin Neurol. 2016;137:187–205.
- Alghadir AH, Iqbal ZA, Whitney SL. An update on vestibular physical therapy. J Chin Med Assoc. 2013;76:1–8.
- Kundakci B, Sultana A, Taylor AJ, et al. The effectiveness of exercise-based vestibular rehabilitation in adult patients with chronic dizziness: a systematic review. F1000Res. 2018;7:276.
- Ricci NA, Aratani MC, Doná F, et al. A systematic review about the effects of the vestibular rehabilitation in middle-age and older adults. Revista brasileira de fisioterapia (Sao Carlos (Sao Paulo, Brazil)). 2010;14:361–371.
- Wrisley DM, Sparto PJ, Whitney SL, et al. Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther. 2000;30:755–766.
- Shepard NT, Telian SA, Smith-Wheelock M, et al. Vestibular and balance rehabilitation therapy. Ann Otol Rhinol Laryngol. 1993;102:198–205.
- Jang DC, Shim HG, Kim SJ. Intrinsic plasticity of cerebellar purkinje cells contributes to motor memory consolidation. J Neurosci. 2020;40:4145–4157.
- Lacour M. Betahistine treatment in managing vertigo and improving vestibular compensation: clarification. J vestibul res equilib orientat. 2013;23:139–151.
- Alrwaily M, Whitney SL. Vestibular rehabilitation of older adults with dizziness. Otolaryngol Clin North Am. 2011;44:473–496, x.
- Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist’s best friend. J Neurol. 2016;263(1):S54–64.
- Tjernström F, Zur O, Jahn K. Current concepts and future approaches to vestibular rehabilitation. J Neurol. 2016;263(1):S65–70.
- Pavlou M, Lingeswaran A, Davies RA, et al. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251:983–995.
- Hall CD, Cox LC. The role of vestibular rehabilitation in the balance disorder patient. Otolaryngol Clin North Am. 2009;42:161–169, xi.
- Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol. 2012;3:25.
- Lambert FM, Malinvaud D, Gratacap M, et al. Restricted neural plasticity in vestibulospinal pathways after unilateral labyrinthectomy as the origin for scoliotic deformations. J Neurosci. 2013;33:6845–6856.
- Brandt T. Management of vestibular disorders. J Neurol. 2000;247:491–499.
- Curthoys IS. Vestibular compensation and substitution. Curr Opin Neurol. 2000;13:27–30.
- Lacour M. Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opin. 2006;22:1651–1659.
- Schubert MC, Migliaccio AA. New advances regarding adaptation of the vestibulo-ocular reflex. J Neurophysiol. 2019;122:644–658.
- Nelson AB, Krispel CM, Sekirnjak C, et al. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620.
- Nelson AB, Faulstich M, Moghadam S, et al. BK channels are required for multisensory plasticity in the oculomotor system. Neuron. 2017;93:211–220.
- Patten C, Horak FB, Krebs DE. Head and body center of gravity control strategies: adaptations following vestibular rehabilitation. Acta Otolaryngol. 2003;123:32–40.
- Schubert MC, Das V, Tusa RJ, et al. Cervico-ocular reflex in normal subjects and patients with unilateral vestibular hypofunction. Otol Neurotol. 2004;25:65–71.
- Jafarzadeh S, Pourbakht A, Bahrami E, et al. Effect of early vestibular rehabilitation on vertigo and unsteadiness in patients with acute and sub-acute head trauma. Iran J Otorhinolaryngol. 2018;30:85–90.
- Herdman SJ, Hall CD, Schubert MC, et al. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngology Head Neck Surg. 2007;133:383–389.
- Shumway-Cook A, Silver IF, LeMier M, et al. Effectiveness of a community-based multifactorial intervention on falls and fall risk factors in community-living older adults: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62:1420–1427.
- Levack WM, Taylor K, Siegert RJ, et al. Is goal planning in rehabilitation effective? A systematic review. Clin Rehabil. 2006;20:739–755.
- Duffau H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. 2006;13:885–897.
- Horak FB. Postural compensation for vestibular loss and implications for rehabilitation. Restor Neurol Neurosci. 2010;28(1):57–68.
- Telian SA, Shepard NT. Update on vestibular rehabilitation therapy. Otolaryngol Clin North Am. 1996;29:359–371.
- Helm MR. Vestibulo-ocular reflex abnormalities in patients with migraine. Headache. 2005;45:332–336.
- Harno H, Hirvonen T, Kaunisto MA, et al. Subclinical vestibulocerebellar dysfunction in migraine with and without aura. Neurology. 2003;61:1748–1752.
- Choi JY, Kim JS. Modulation of central nystagmus by vision, proprioception, and efference copy signals: a systematic evaluation. J Neurol. 2016;263:735–742.
- Genzel D, Firzlaff U, Wiegrebe L, et al. Dependence of auditory spatial updating on vestibular, proprioceptive, and efference copy signals. J Neurophysiol. 2016;116:765–775.
- Konrad HR. Clinical application of saccade-reflex testing in man. Laryngoscope. 1991;101:1293–1302.
- Ramat S, Leigh RJ, Zee DS, et al. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35.
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228.
- Ettinger U, Ffytche DH, Kumari V, et al. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cerebral cortex (New York, NY: 1991). 2008;18:1148–1159.
- Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006;5:255–280.
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296.
- Coe BC, and Munoz DP. Mechanisms of saccade suppression revealed in the anti-saccade task. Philos Trans R Soc London, Ser B. 2017;372:20160192.
- Massendari D, Lisi M, Collins T, et al. Memory-guided saccades show effect of a perceptual illusion whereas visually guided saccades do not. J Neurophysiol. 2018;119:62–72.
- Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestibul Res-Equil. 2012;22:167–172.
- Furman JM, Balaban CD. Vestibular migraine. Ann N Y Acad Sci. 2015;1343:90–96.
- von Brevern M, Zeise D, Neuhauser H, et al. Acute migrainous vertigo: clinical and oculographic findings. Brain. 2005;128:365–374.
- Furman JM, Marcus DA. Migraine and motion sensitivity. Continuum (Minneap Minn). 2012;18:1102–1117.
- Krishnan PS, Carey JP. Vestibular migraine: clinical aspects and pathophysiology. Otolaryngol Clin North Am. 2022;55(3):531–547.
- Huang TC, Wang SJ, Kheradmand A. Vestibular migraine: an update on current understanding and future directions. Cephalalgia. 2020;40:107–121.
- Yan M, Guo X, Liu W, et al. Temporal patterns of vertigo and migraine in vestibular migraine. Front Neurosci. 2020;14:341.
- Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol. 2016;263(1):S82–89.
- Cho SJ, Kim BK, Kim BS, et al. Vestibular migraine in multicenter neurology clinics according to the appendix criteria in the third beta edition of the international classification of headache disorders. Cephalalgia. 2016;36:454–462.
- Formeister EJ, Rizk HG, Kohn MA, et al. The epidemiology of vestibular migraine: a population-based survey study. Otol Neurotol. 2018;39(8):1037–1044.
- Van Ombergen A, Van Rompaey V, Van de Heyning P, et al. Vestibular migraine in an otolaryngology clinic: prevalence, associated symptoms, and prophylactic medication effectiveness. Otol Neurotol. 2015;36:133–138.
- Lauritsen CG, Marmura MJ. Current treatment options: vestibular migraine. Curr Treat Options Neurol. 2017;19:38.
- Bisdorff Arjtai ND. Management of vestibular migraine. Ther Adv Neurol Disord . 2011;4:183–191.
- von Brevern M, Lempert T. Vestibular migraine: treatment and prognosis. Semin Neurol. 2020;40:83–86.
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349.
- Baier B, Winkenwerder E, Dieterich M. “Vestibular migraine”: effects of prophylactic therapy with various drugs. A retrospective study. J Neurol. 2009;256:436–442.
- Salviz M, Yuce T, Acar H, et al. Propranolol and venlafaxine for vestibular migraine prophylaxis: a randomized controlled trial. Laryngoscope. 2016;126:169–174.
- Whitney SL, Wrisley DM, Brown KE, et al. Physical therapy for migraine-related vestibulopathy and vestibular dysfunction with history of migraine. Laryngoscope. 2000;110:1528–1534.
- Sugaya N, Arai M, Goto F. Is the headache in patients with vestibular migraine attenuated by vestibular rehabilitation? Front Neurol. 2017;8:124.
- Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol. 2013;260:3039–3048.
- Lee YY, Yang YP, Huang PI, et al. Exercise suppresses COX-2 pro-inflammatory pathway in vestibular migraine. Brain Res Bull. 2015;116:98–105.
- Alghadir AH, Anwer S. Effects of Vestibular rehabilitation in the management of a vestibular migraine: a review. Front Neurol. 2018;9:440.
- Balaban CD. Migraine, vertigo and migrainous vertigo: links between vestibular and pain mechanisms. J vestibul res equilib orientat. 2011;21:315–321.
- Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706–715.
- Ranalli P. An overview of central vertigo disorders. Adv Otorhinolaryngol. 2019;82:127–133.
- Furman JM, Balaban CD, Jacob RG, et al. Migraine-anxiety related dizziness (MARD): a new disorder? J Neurol Neurosurg Psychiatry. 2005;76:1–8.
- Jeong SH, Oh SY, Kim HJ, et al. Vestibular dysfunction in migraine: effects of associated vertigo and motion sickness. J Neurol. 2010;257:905–912.
- Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49:1378–1386.
- Lempert T, von Brevern M. Vestibular Migraine. Neurol Clin. 2019;37:695–706.
- van Vugt VA, van der Horst HE, Payne RA, et al. Chronic vertigo: treat with exercise, not drugs. BMJ (Clinical research ed). 2017;358:j3727.
- Treleaven J, Jull G, Grip H. Head eye co-ordination and gaze stability in subjects with persistent whiplash associated disorders. Manual ther. 2011;16:252–257.
- Lewis RF, Priesol AJ, Nicoucar K, et al. Dynamic tilt thresholds are reduced in vestibular migraine. J vestibul res equilib orientat. 2011;21:323–330.
- King S, Wang J, Priesol AJ, et al. Central integration of canal and otolith signals is abnormal in vestibular migraine. Front Neurol. 2014;5:233.
- Russo A, Marcelli V, Esposito F, et al. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014;82:2120–2126.
- Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the bárány society. J vestibul res equilib orientat. 2017;27:191–208.
- The L. ICD-11. Lancet (London England). 2019;393:2275
- Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psychiatry. 1995;59:472–476.
- Staab JP, Ruckenstein MJ, Amsterdam JD. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope. 2004;114:1637–1641.
- Dieterich M, Staab JP, Brandt T. Functional (psychogenic) dizziness. Handb Clin Neurol. 2016;139:447–468.
- Jacob RG, Lilienfeld SO, and Furman JMR, et al. Panic disorder with vestibular dysfunction: further clinical observations and description of space and motion phobic stimuli. J anxiety disord . 1989;3:117–130.
- Popkirov S, Staab JP, Stone J. Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness. Pract Neurol. 2018;18:5–13.
- Staab JP. Persistent postural-perceptual dizziness. Semin Neurol. 2020;40:130–137.
- Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9–26.
- Molnár A, Maihoub S, Mavrogeni P, et al. Depression scores and quality of life of vertiginous patients, suffering from different vestibular disorders. Europ archiv oto-rhino-laryngol. 2022. DOI:10.1007/s00405-022-07366-y.
- Popkirov S, Stone J, Holle-Lee D. Treatment of persistent postural-perceptual dizziness (PPPD) and related disorders. Curr Treat Options Neurol. 2018;20:50.
- Dieterich M, Staab JP. Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness. Curr Opin Neurol. 2017;30:107–113.
- Morisod B, Mermod M, Maire R. Posturographic pattern of patients with chronic subjective dizziness before and after vestibular rehabilitation. J Vestibul Res-Equil. 2017;27:305–311.
- Thompson KJ, Goetting JC, Staab JP, et al. Retrospective review and telephone follow-up to evaluate a physical therapy protocol for treating persistent postural-perceptual dizziness: a pilot study. J vestibul res equilib orientat. 2015;25:97–103; quiz 103–104.
- Nada EH, Ibraheem OA, Hassaan MR. Vestibular rehabilitation therapy outcomes in patients with persistent postural-perceptual dizziness. Ann Otol Rhinol Laryngol. 2019;128:323–329.
- Staab JP, Rohe DE, Eggers SD, et al. Anxious, introverted personality traits in patients with chronic subjective dizziness. J Psychosom Res. 2014;76:80–83.
- Chiarella G, Petrolo C, Riccelli R, et al. Chronic subjective dizziness: analysis of underlying personality factors. J vestibul res equilib orientat. 2016;26:403–408.
- Söhsten E, Bittar RS, Staab JP. Posturographic profile of patients with persistent postural-perceptual dizziness on the sensory organization test. J vestibul res equilib orientat. 2016;26:319–326.
- Adamec I, Juren Meaški S, Krbot Skorić M, et al. Persistent postural-perceptual dizziness: clinical and neurophysiological study. J Clin Neurosci. 2020;72:26–30.
- Salvatore N, Iole I, and Roberta R, et al. Reduced cortical folding in multi-modal vestibular regions in persistent postural perceptual dizziness. Brain Imaging Behav. 2019;13:798–809.
- Riccelli R, Passamonti L, Toschi N, et al. Altered insular and occipital responses to simulated vertical self-motion in patients with persistent postural-perceptual dizziness. Front Neurol. 2017;8:529.
- Jeffrey PS. Persistent postural-perceptual dizziness. Semin Neurol. 2020;40:130–137.
- Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry. 2015;86:1113–1119.
- Lee JO, Lee ES, Kim JS, et al. Altered brain function in persistent postural perceptual dizziness: a study on resting state functional connectivity. Hum Brain Mapp. 2018;39:3340–3353.
- Li K, Si L, Cui B, et al. Altered intra- and inter-network functional connectivity in patients with persistent postural-perceptual dizziness. NeuroImage Clin. 2020;26:102216.
- Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngology Head Neck Surg. 2007;133:170–176.
- Lucchinetti CF. Update on the international project on pathologic correlates in MS. Multiple sclerosis (Houndmills, Basingstoke, England). 2005;11:99–100
- Owens B. Multiple sclerosis. Nature. 2016;540:S1.
- Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019;26:27–40.
- Zeigelboim BS, Arruda WO, Mangabeira-Albernaz PL, et al. Vestibular findings in relapsing, remitting multiple sclerosis: a study of thirty patients. Int Tinnitus J. 2008;14:139–145.
- Marrie RA, Cutter GR, Tyry T. Substantial burden of dizziness in multiple sclerosis. Mult Scler Relat Disord. 2013;2:21–28.
- Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2009;15:59–67
- Cameron MH, Nilsagard Y. Balance, gait, and falls in multiple sclerosis. Handb Clin Neurol. 2018;159:237–250.
- Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Multiple sclerosis (Houndmills, Basingstoke, England). 2020;26:1816–1821.
- Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–273.
- Thormann A, Sørensen PS, Koch-Henriksen N, et al. Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology. 2017;89:1668–1675.
- He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–316.
- Yamout B, Sahraian M, Bohlega S, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. 2020;37:101459.
- Sosnoff JJ, Finlayson M, McAuley E, et al. Home-based exercise program and fall-risk reduction in older adults with multiple sclerosis: phase 1 randomized controlled trial. Clin Rehabil. 2014;28:254–263.
- García-Muñoz C, Cortés-Vega MD, and Heredia-Rizo AM, et al. Effectiveness of vestibular training for balance and dizziness rehabilitation in people with multiple sclerosis: a systematic review and meta-analysis. J Clin Med. 2020;9: 590 .
- Tramontano M, Grasso MG, Soldi S, et al. Cerebellar intermittent theta-burst stimulation combined with vestibular rehabilitation improves gait and balance in patients with multiple sclerosis: a preliminary double-blind randomized controlled trial. Cerebellum (London, England). 2020;19:897–901.
- García-Muñoz C, Cortés-Vega MD, Hernández-Rodríguez JC, et al. Immersive virtual reality and vestibular rehabilitation in multiple sclerosis: case report. JMIR Serious Games. 2022;10:e31020.
- Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989–1002.
- Doty RL, MacGillivray MR, Talab H, et al. Balance in multiple sclerosis: relationship to central brain regions. Exp Brain Res. 2018;236:2739–2750.
- Fling BW, Dutta GG, Schlueter H, et al. Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Front Hum Neurosci. 2014;8:814.
- Prosperini L, Kouleridou A, Petsas N, et al. The relationship between infratentorial lesions, balance deficit and accidental falls in multiple sclerosis. J Neurol Sci. 2011;304:55–60.
- Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture. 2013;38(1):37–42.
- Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis: a randomized study. Neurology. 2004;63:2034–2038.
- Hebert JR, Corboy JR, Vollmer T, et al. Efficacy of balance and eye-movement exercises for persons with multiple sclerosis (BEEMS). Neurology. 2018;90:e797–e807.
- Gandolfi M, Munari D, Geroin C, et al. Sensory integration balance training in patients with multiple sclerosis: a randomized, controlled trial. Multiple sclerosis (Houndmills, Basingstoke, England). 2015;21:1453–1462.
- Zhang S, Xu W, Zhu Y, et al. Impaired multisensory integration predisposes the elderly people to fall: a systematic review. Front Neurosci. 2020;14:411.
- Cornelio P, Velasco C, Obrist M. Multisensory integration as per technological advances: a review. Front Neurosci. 2021;15:652611.
- Pompa A, Morone G, Iosa M, et al. Does robot-assisted gait training improve ambulation in highly disabled multiple sclerosis people? A pilot randomized control trial. Multiple sclerosis (Houndmills, Basingstoke, England). 2017;23:696–703.
- Brichetto G, Piccardo E, Pedullà L, et al. Tailored balance exercises on people with multiple sclerosis: a pilot randomized, controlled study. 2015;21. Basingstoke England: Multiple sclerosis (Houndmills 1055–1063.
- Hebert JR, Corboy JR, Manago MM, et al. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91:1166–1183.
- Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–964.
- Kim WS, Jung SH, Oh MK, et al. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med. 2014;46:418–423.
- Maihoub S, Molnár A, Tamás L, et al. The diagnosis of central vestibular disorders based on the complementary examination of the vestibulospinal reflex. J Otol. 2022;17:1–4.
- Kerber KA, Brown DL, Lisabeth LD, et al. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37:2484–2487.
- Molnár A, Maihoub S, Gáborján A, et al. Intratympanic gentamycine for Ménière’s disease: is there a selective vestibulotoxic effect? European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-rhino-laryngological societies (EUFOS): affiliated with the German society for oto-rhino-laryngology. Head Neck Surg. 2020;277:1949–1954.
- Kim HA, Yi HA, Lee H. Failure of fixation suppression of spontaneous nystagmus in cerebellar infarction: frequency, pattern, and a possible structure. Cerebellum (London, England). 2016;15:182–189.
- Mizutani K, Sonoda S, Hayashi N, et al. Analysis of protein expression profile in the cerebellum of cerebral infarction rats after treadmill training. Am J Phys Med Rehabil. 2010;89:107–114.
- Bruun M, Højgaard JL, Kondziella D. Acute vertigo of neurological origin. Ugeskr Laeger. 2013;175:2709–2711.
- Bonan IV, Colle FM, Guichard JP, et al. Reliance on visual information after stroke. Part I: balance on dynamic posturography. Arch Phys Med Rehabil. 2004;85:268–273.
- Yelnik AP, Le Breton F, Colle FM, et al. Rehabilitation of balance after stroke with multisensorial training: a single-blind randomized controlled study. Neurorehabil Neural Repair. 2008;22:468–476.
- Whitney SL, Alghadir AH, Anwer S. Recent evidence about the effectiveness of vestibular rehabilitation. Curr Treat Options Neurol. 2016;18:13.
- Aspinwall LG, Tedeschi RG. The value of positive psychology for health psychology: progress and pitfalls in examining the relation of positive phenomena to health. Ann Behav Med. 2010;39:4–15.
- Khan S, Chang R. Anatomy of the vestibular system: a review. NeuroRehabilitation. 2013;32:437–443.
- Todd CJ, Hubner PP, Hubner P, et al. StableEyes-a portable vestibular rehabilitation device. IEEE Trans Neural Syst Rehabil Eng. 2018;26:1223–1232.
- Saki N, Bayat A, Nikakhlagh S, et al. Vestibular rehabilitation therapy in combination with transcranial direct current stimulation (tDCS) for treatment of chronic vestibular dysfunction in the elderly: a double-blind randomized controlled trial. Braz J Otorhinolaryngol. 2020. DOI:10.1016/j.bjorl.2020.11.004.
- Simon C, Bolton DAE, Kennedy NC, et al. Challenges and opportunities for the future of brain-computer interface in neurorehabilitation. Front Neurosci. 2021;15:699428.
