Abstract
In vitro enzymes inhibition activities of the crude methanolic extract and various fractions of Colchicum luteum Baker (Liliaceae) including chloroform, ethyl acetate, n-butanol and aqueous were carried out against actylcholinesterase, butyrylcholinesterase, lipoxygenase and urease enzymes. A significant enzyme inhibition activity (89%) is shown by the crude methanolic extract and its fractions against lipoxygenase, while low to significant activity (32–75%) was evident against butyrylcholinesterase. The crude methanolic extract and its various fractions demonstrated low activity (29–61%) against acetylcholinesterase and no activity against urease.
Introduction
Acetylcholinesterase is an extremely active enzyme and cholinesterase inhibitors exert their effects by inhibiting the enzyme so increasing the concentration of endogenous acetylcholine in the vicinity of cholinoreceptors [Citation1]. According to the cholinergic hypothesis, the memory impairment in patients with senile dementia of Alzheimer's type results from a deficiency in cholinergic function in the brain [Citation2]. Hence the most promising therapeutic strategy for activating central cholinergic functions has been the use of cholinomimetic agents. The enzyme acetylcholinesterase (AChE) has long been an attractive target for the rational drug design and discovery of inhibitors. Because of its role in the hydrolysis of the neurotransmitter acetylcholine [Citation3] the inhibition of the enzyme is considered as a promising approach for the treatment of Alzheimer's disease (AD) and for other possible therapeutic applications in the treatment of Parkinson's disease, ageing, myasthenia gravis and glaucoma [Citation4,Citation5,Citation6]. The role of butyrylcholinesterase (BChE) in the normal ageing and diseased brain is still unknown. However, recently it has been found that BChE inhibition may also be an effective tool for the treatment of AD and related dementias [Citation7].
Arachidonate 5-lipoxygenase (5-LO), an actin-binding protein [Citation8], is the key enzyme in the leukotriene (LT) biosynthesis and catalyzes the initial steps in the conversion of arachidonic acid to biologically active leukotrienes (LTs). Leukotrienes are considered as potent mediators of inflammatory and allergic reactions that are released by leukocytes and other 5-LO expressing cells. Arachidonic acid metabolism through the lipoxygenase (LOX) pathway generates various biologically active lipids that play an important role in inflammation [Citation9]. Angiogenesis, the formation of new capillary vessels from pre-existing ones, underpins a number of physiological processes and participates in the development of several pathological conditions such as arthritis and cancer [Citation10]. Lipoxygenases are therefore potential targets for the rational drug design and discovery of inhibitors for the treatment of a variety of disorders including bronchial asthma, inflammation, cancer and autoimmune disease and the search for new lipoxygenase inhibitors appears to be a promising approach for the development of new drugs.
Urease is directly involved in the formation of infectious stones and contributes to the pathogenesis of urolithiasis, pyelonephrities, ammonia and hepatic coma, urinary catheter encrustation and ulcers [Citation11,Citation12]. In agriculture, high urease activity induces plant damage by depriving them of their essential nutrient and ammonia toxicity which increases the pH of the soil [Citation13]. Therefore, strategies based on urease inhibition are now considered as first line therapy for infection caused by urease-producing bacteria.
In herbal medicine, the corms of colchicum luteum Baker are extensively used for the treatment of gout, rheumatism and for diseases of the liver and spleen. [Citation14]. The corms are also used as a blood purifier [Citation15]. Similarly some of the colchicinoid alkaloids have been isolated from this plant species [Citation16] but it still needs to be thoroughly investigated for its beneficial medicinal uses Here, its inhibitory adirty towards the cholinesterases, lipoxygenase and urease is examined.
Methods and materials
Plant material
Colchicum luteum. Baker, was collected as a whole plant from Sherengel, upper Dir, N.W.F.P (Pakistan) during the months Feb-March 2004. The plant material was identified by Dr Jahandar Shah (Plant Taxonomist), Vice Chancellor Malakand University, and verified by Dr Abd-ur-Rashid, Department of Botany, University of Peshawar.
Extraction
The shade dried plant material was chopped into small pieces and finally pulverized into a fine powder. The powdered plant material (10 kg) was soaked in methanol (3 × 10 L), with occasional shaking, at room temperature. After 15 days, the methanol soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator to give a yellowish crude extract (246 g).
Fractionation
The crude methanolic extract (246 g) was suspended in distilled water (500 ml) and partitioned with n-hexane (3 × 500 ml), chloroform (3 × 500 ml), ethyl acetate (3 × 500 ml) and n-butanol (3 × 500 ml) to yield the n- hexane (26 g), chloroform (59 g), ethyl acetate (32 g), n-butanol (35 g) and aqueous (68 g) fractions, respectively. The enzyme inhibition activity assays were performed by using different concentrations of the crude extract and various fractions as per the requirement of the individual assay method.
a) In vitro cholinesterase inhibition assay:
Acetylcholinesterase and butyrylcholinesterase inhibiting activities were measured by slightly modifying the spectrophotometric method previously developed [Citation17]. Electric-eel AChE (type VI-S, Sigma) and horse-serum BChE (Sigma) were used as source of the cholinesterases and acetylthiocholine iodide and butyrylthiocholine chloride (Sigma), respectively, were used as substrates in the reaction. 5,5 - Dithiobis (2-nitrobenzoic acid) (DTNB, Sigma) was used for the measurement of cholinesterase activity. 140 μL of sodium phosphate buffer 100 mM, (pH 8.0), 10 μL of DTNB, 20 μL of the test samples solutions and 20 μL of acetylcholinesterase/ butyrylcholinesterase solution were mixed and incubated for 15 min at 25°C. The reactions were then initiated by the addition of l0 μL acetylthiocholine/butyrylthiocholine, respectively. The hydrolysis of acetylthiocholine and butyrylthiocholine was monitored at a wavelength of 412 nm by the formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine and butyrylthiocholine respectively, The samples and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate.
b) In vitro lipoxygenase inhibition assay:
Lipoxygenase inhibiting activity was conveniently measured by slightly modifying the spectrometric method developed previously [Citation18]. Lipoxygenase (1.13.11.12) type I-B and linoleic acid were purchased from Sigma (St. Loius, MO) and were used without further purification. All other chemicals were of analytical grade. 160 μL of sodium phosphate buffer, 0.1 mM (pH 7.0), 10 μL of the sample solution and 20 μL of lipoxygenase solution were mixed and incubated for 5 min at 25°C. The reaction was initiated by the addition of 10 uL linoleic acid solution (substrate) and the absorption change with the formation of (9Z, 11E)-13S)-13-hydroperoxyoctadeca-9, 11-dienoate was followed for 10 min. The test sample and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate. The IC5o values (concentrations of sample causing 50% reduction in activity relative to the control) were calculated using the EZ-Fit Enzymes kinetics programme.
c) In vitro urease inhibition assay:
Reaction mixture comprising 25 μL of enzyme (Jack bean urease) solution and 55 μL of buffers pH 8.2 (0.01 M K2HPO4.3H2O, 1 mM EDTA and 0.01 M LiC12) containing 100 mM urea were incubated with 5 μL of test compounds (1 mM concentration) at 30°C for 15 min in 96-well plates. Urease activity was determined by measuring ammonia production using the indophenol method as previously described [Citation19]. Briefly 45 μL each of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 μL of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCl) were added to each well. The increasing absorbance at 630 nm was measured after 50 min using a micro plate reader (Molecular Device, USA). All reactions were performed in triplicate in a final volume of 200 μL. The results (change in absorbance per min), were processed by using Soft Max Pro software (Molecular Device, USA). Percentage inhibitions were calculated from 100- (ODtestwell/ODcontrol) × 100. Thiourea was used as a standard urease inhibitor.
Results and Discussion
Despite all the progress in synthetic chemistry and biotechnology, plants are still an indispensable source of medicinal preparations, both preventive and curative and the WHO estimates that approximately 80% of the developing world's populations meet their primary health care needs through traditional medicine [Citation20]. Similarly, like most developing nations, the rural population of Pakistan relies on the indigenous health care system to a great extent [Citation21]. Therefore, the current study was designed to screen, confirm and provide a scientific basis for the use of colchicum luteum Baker in the transitional system of medicine especially to probe potential enzyme inhibition activity. In vitro inhibitory activities of the crude methanolic extract and subsequent fractions of Colchicum luteum Baker were carried out against actylcholinesterase, butyrylcholinesterase, lipoxygenase and urease enzymes.
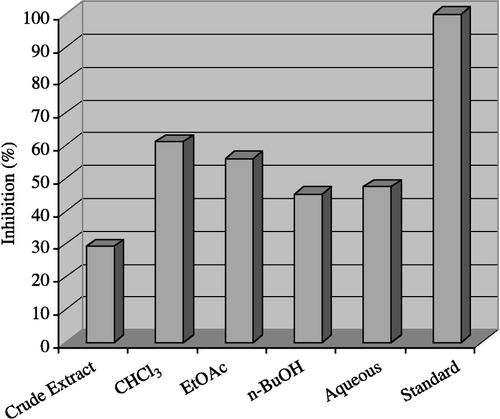
The results obtained with the crude extract of colchicum luteum and its subsequent fractions for inhibitory activitys against acetylcholinesterase are shown in . The crude methanolic extract exhibited low (29.30%) inhibition, while moderate activity was displayed by the n-butanol fraction (45.10%) and aqueous fraction (47.30%), but good inhibitory activity (61.20%) and (56.10%) were found with the chloroform and ethyl acetate fractions respectively.
Figure 1 Inhibitory activity of Crude Extract and Fractions of Colchicum luteum Baker on acetylcholinesterase at 40 μg/200 μL.
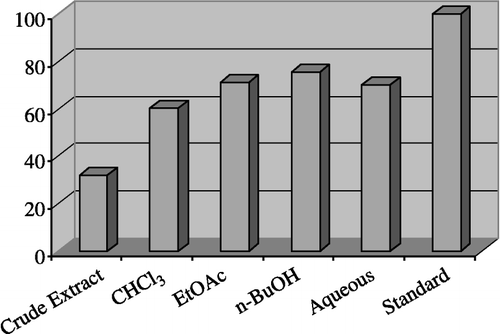
The inhibitory activity against Butyrylcholinesterase are shown in . Excellent result (75.50%) were shown by the n-butanol fraction, ethyl acetate fraction (71.10%) and aqueous fraction (70%), while (60.30%) and (32%) inhibition was noted for the chloroform and crude extract respectively.
Figure 2 Inhibitory activity of Crude Extract and Fractions of Colchicum luteum Baker on butyrylcholinesterase at 40 μg/200 μL.
showed the enzyme inhibition investigation found in the current study against lipoxygenase. Overall, outstanding inhibition was displayed by the crude extract and subsequent fractions of colchicum luteum against lipoxygenase (); crude extract (80.10% inhibition), chloroform fraction (87.10%), ethyl acetate fraction (89.40%), n-butanol fraction (84.25%) and aqueous fraction (82.50%). However, no inhibitory activity was found against urease at a concentration of 1 g/200 μL.
Figure 3 Inhibitory activity of Crude Extract and Fractions of Colchicum luteum Baker on lipoxygenase at 240 μg/200 μL.
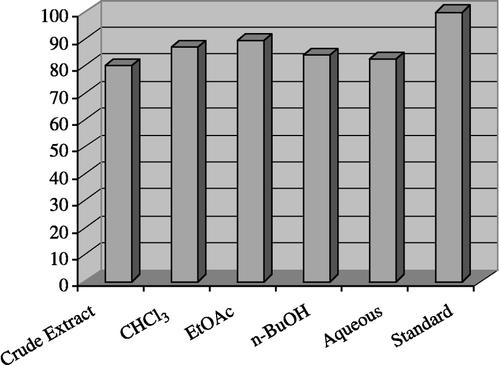
From the results shown in , it can be seen that the crude extract and subsequent fractions of colchicum luteum Baker have a strong potential to inhibit lipoxygenase and the plant is a potential target for activity-guided isolation of its active constituents for the possible treatment of a variety of disorders including bronchial asthma, inflammation, cancer and autoimmune disease.
References
- Bertram G Katzung. Basic and clinical pharmacology9th edn. 2004; 102
- Robbins, Kummar. Basic pathology4th edn. 1987; 747
- Taylor P. Development of acetylcholinesterase inhibitors in the therapy of alzheimer's disease. Neurology 1998; 51: 30–35
- Ballard CG. Eur Neurol 2002; 47: 64–70
- J Ruof J, Mittendorf T Pirk, von der Schulenburg JM. Health Policy (New York) 2002; 60: 59–66
- Millard CB, Broomfield CA. J Neurochem 1995; 64: 1909–1918
- Yu QS, Holloway HW, Utsuki T, Brossi A, Greig NH. Synthesis of novel phenserinebased selective inhibitors of butyrylcholinesterase for alzheimer's disease. J Med Chem 1999; 42: 1855–1861
- Lepley RA, Fitzpatrick FA. J Biol Chem 1994; 269: 24163–24168
- Steinhilber D. 5-Lipoxygenase, a target for anti inflammation drugs Revisited Curr Med. Chem 1999; 6: 71–85
- Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 2002; vol. 5: 799–807
- Mobley HLT, Hausinger RP. Microbial urease: Significance, regulation and molecular characterization. Micro. Rev 1989; 53: 85–100
- Mobley HLT, Island MD, Hausinger RP. Molecular biology of microbial urease. Micro Rev 1995; 59: 451–480
- Bremen JM. Recent research on the problems in the use of urea as a nitrogen fertilizer. Fert Res 1995; 42: 321–329
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants (Including the Supplement). Council of Scientific and Industrial Research, New Delhi. 1986.
- Shinwari ZK, Khan AA, Nakaike T. Medicinal and other useful plants of the District Swat Pakistan, Al Aziz Communications, Peshawar, Pakistan 2003. p 55.
- Yusupov MK, Sadykov AS, Nauchin Tashkentsk Tt. Gos. Univ. im. v.I. Lenin 1962, Estestv. Nauki, No. 203, 3
- Ellman GL, Courtney V K D, Jr., Andres RM. Featherstone, A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; vol. 7(7)88–95
- Tappel AL. Meth Enzymol 1962; Vol 5: 539–542
- Weatherburn MW. Phenol-hypochlorite reaction for the determination of the ammonia. Anal Chem 1967; Vol.39: 971–974
- Bannerman RH. Traditional medicine in modern health care. World Health Forum 1982; Vol. 3(1)8–13
- Kattak SG, Gilani SN, Ikram M. J Ethnopharmacol 1985; 14: 45–51