Abstract
Urease from the seeds of pigeonpea was competitively inhibited by boric acid, butylboronic acid, phenylboronic acid, and 4-bromophenylboronic acid; 4-bromophenylboronic acid being the strongest inhibitor, followed by boric acid>butylboronic acid>phenylboronic acid, respectively. Urease inhibition by boric acid is maximal at acidic pH (5.0) and minimal at alkaline pH (10.0), i.e., the trigonal planar B(OH)3 form is a more effective inhibitor than the tetrahedral anionic form. Similarly, the anionic form of phenylboronic acid was least inhibiting in nature.
Introduction
Urease is found in a wide range of organisms and has been isolated from various bacteria, higher plants, fungi and some invertebrates [Citation1]. Plant urease sps(urea amidohydrolase, EC 3.5.1.5) is a hexameric protein (540 kDa) consisting of six identical subunits Citation2-4 and a nickel-dependent metalloenzyme, which catalyzes the hydrolysis of urea to ammonia and carbamate. The carbamate then spontaneously hydrolyzes to form carbonic acid and a second molecule of ammonia. At physiological pH, the carbonic acid proton dissociates and the ammonia molecules equilibrate with water becoming protonated, resulting in a net increase in pH.
The rate of the catalyzed hydrolysis is 1014-fold the rate of an uncatalyzed reaction. The urease from pigeonpea has been recently purified in our laboratory [Citation4] and the comparison of the N-terminal amino acid sequence shows a high similarity with plant ureases. This urease has been used successfully in many analytical applications Citation5-12.
Worldwide consumption of urea is over 85 million tonnes (US$14 billion) and is growing at a rate of almost 3% a year. China and India alone account for 56% of total consumption (according to a report by mindbranch.com). Continued growth is expected owing to urea's high-analysis safety and its ability to be applied as a dry or urea-containing solution. Urea breakdown begins as soon as it is applied to the soil. If the soil is totally dry, no reaction occurs. But with the enzyme urease, plus any small amount of soil moisture, urea normally hydrolyzes and converts to ammonium and carbon dioxide. This can occur in 2–4 days and occurs quicker on high pH soils.
To make efficient use of urea and ammonium fertilizers, reduce nitrate runoff, leaching, and the emission of ammonia and greenhouse gases [Citation13], the incorporation of urease inhibitors and nitrification inhibitors into urea- and ammonium-containing fertilizers should be recommended as a best management practice. There are also reports that use of urease inhibitors can lead to potential phytotoxicity [Citation14] but this should not preclude their use to eliminate the adverse effects of urea fertilizers on seed germination and seedling growth in soil because the NH3 produced through hydrolysis is much more detrimental to plant growth than is the urea accumulation induced by urease inhibitors. The ability to curb urea volatilization losses and ammonium forms of nitrogen could be the next frontier in achieving nitrogen efficiency.
Apart from agricultural importance, studies on urease inhibition remain an important area of medicinal research since these studies could lead to the discovery of drugs useful in a variety of physiological conditions. Urease inhibitors have recently attracted much attention as potential new anti-ulcer drugs. Ureolytic microbial infections of the urinary tract at elevated pH lead to deposition of urinary salts known as stones [Citation15]. It has been estimated that 20–40% of urinary stones arise as a result of infection by ureolytic microorganisms [Citation16]. Urease inhibitors have been proposed as a potentially effective method to combat urease-induced stone formation [Citation16]. Boric acid has been used as a urine preservative for biochemical analysis and as a microbiological for over 20 years and concentrations of up to 20 g/L have been recommended for this purpose [Citation17]. Mazurkiewicz et al. [Citation18], have recommended that boric acid should not be added to urine at concentrations above 2 g/L for the analysis of urea by methods employing urease. It should be avoided as a urinary preservative when urea is to be measured by urease based-analyzers [Citation19].
Finally, studies on urease inhibitors can provide insight into the detailed mechanism of catalysis. Ironically, urease was the first enzyme crystallized but its mechanism of action is still largely not understood. Recently, Benini et al. [Citation20] have thrown some light on the catalytic mechanism of urease inhibition by boric acid, where the boric acid replaces the labile water molecules in the active site and can be considered a substrate analogue. In the past decade, there has been a huge increase of interest in boronic acid compounds. Such an interest stems from the tremendous importance of boronic acids in synthetic organic chemistry and the use of boronic acid themselves as biological agents and which may serve as leads for the development of therapeutic agents for the treatment of beta-lactam-resistant infections [Citation21]. Inhibition by boronic acids has been studied with some bacterial ureases [Citation22], however, little work has been carried out on plant ureases and to our knowledge the present study on the inhibition of pigeonpea urease by boric acid and boronic acids forms the first report.
Materials and methods
Urease (urea amidohydrolase, EC 3.5.1.5) was isolated from dehusked pigeonpea (Cajanus cajan) seeds procured from the local market. Boric acid (BA), urea (enzyme grade) and Tris were purchased from Sisco Research Laboratories, Mumbai, India, butylboronic acid (BBA) from Sigma Chemical Co., St. Louis, MO, USA, 4-bromophenylboronic acid (4-BPBA) was from Aldrich and phenylboronic acid (PBA) was from John Baker Inc. USA; Nessler's reagent from HiMedia Laboratories, Mumbai, India. All other chemicals used were of analytical grade. All solutions were prepared in Milli Q (Millipore, USA) water.
Urease purification and activity measurements
Urease was purified according to Das et al. [Citation4]. The enzyme used in the present study has a specific activity of 1250–1750 Units mg− 1 protein (varied from batch to batch). One unit of urease activity liberates 1 μmol of NH3 from urea per min at pH 7.3 and 27°C. All activity measurements were done in triplicate. The amount of NH3 liberated on incubating the free and immobilized enzyme with 0.2 M urea for a fixed time period at an enzyme-saturating concentration of urea was determined using Nessler's reagent; the yellow–orange colour produced was measured spectrophotometrically at 405 nm (ATI-UNICAM UV–Vis spectrophotometer, UK). The amount of NH3 liberated in the test solution was calculated by calibrating the reagent with standard NH4Cl solution.
Protein estimation
Protein was estimated by the method of Lowry et al. [Citation23].
Inhibition studies
Stock solutions of inhibitors except for 4-BPBA were prepared in 50 mM Tris-acetate buffer, pH 7.3 and were suitably diluted for the experiments, whereas a stock solution of 4-BPBA was prepared in absolute ethanol. The activity of urease was determined in the presence of varying concentrations of these inhibitors. Inhibition constants (Ki) were calculated by the method of Dixon [Citation24].
Inhibition studies at different pH
Enzyme activity was determined in three different buffers, pH 5.0 (0.05 M MES), pH 7.0 (0.05 M Tris–acetate) and pH 10.0 (0.05 M Carbonate). The stock solutions of substrate (urea) and inhibitors (boric acid and phenylboronic acid) were also prepared in the respective pH buffers. In order to determine which form (acidic, neutral and alkaline) of the inhibitor is more effective, enzyme activity with and without inhibitor was carried out at the respective pH and the percent (%) net effect of inhibitor was equal to % residual activity without inhibitor minus % percent residual activity with inhibitor.
Results and discussion
Boric acid inhibition
As shown in , boric acid is a strong competitive inhibitor of pigeonpea urease and exhibits a Ki of 0.35 ± 0.15 mM at pH 7.3. It has also been reported to be a strong competitive inhibitor in the case of jackbean [Citation25,Citation26], P. mirabilis [Citation22] and K. aerogenes urease [Citation27]. The comparison of Ki values has been presented in . Boric acid acts as a competitive inhibitor for many enzymes like prostate specific antigen [Citation28], Streptomyces griseus proteinase [Citation29] and boric acid also reversibly inhibits the second step of pre-mRNA splicing [Citation30].
Figure 1 Dixon plots for the boric and phenylboronic acids. The competitive inhibition constant (Ki) was determined for each inhibitor based on the urease activity at urea concentrations of 0.1, 0.2 M and an enzyme concentration of 2.07 μg/mL by using standard assay conditions as described in the Materials and Methods. (a) Boric acid (b) Butylboronic acid (c) Phenylboronic acid and (d) 4-Bromophenylboronic acid.
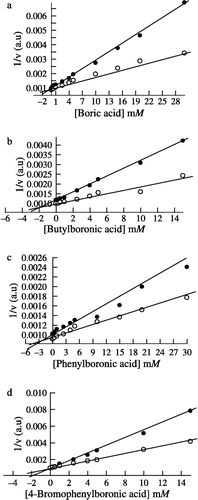
Table I. Competitive inhibition constant (Ki) values for boric and boronic acids as urease inhibitors.
Boronic acids inhibition
The three boronic acids (BBA, PBA and 4-BPBA) were examined for inhibitory action on pigeonpea urease and all were found to inhibit competitively. The Ki value at pH 7.3 for BBA is 1.8 ± 0.2 mM (), PBA is 2.5 ± 0.4 mM () and 4-BPBA is 0.3 ± 0.1 mM (). Among the above tested boronic acids, 4-BPBA was found to be the most potent competitive inhibitor, a similar trend having been also observed for P. mirabilis [Citation22] and K. aerogenes urease [Citation26]. However, PBA was found to be a weak inhibitor, similar to the case of K. aerogenes where the Ki is 10 mM [Citation26] which was several-fold greater than pigeonpea and P. mirabilis urease. The Ki values of these inhibitors for other ureases were compared with pigeonpea urease (). In the case of several proteases the boronic acids are thought to inhibit by reacting with an active site serine group [Citation31].
Inhibition at different pH
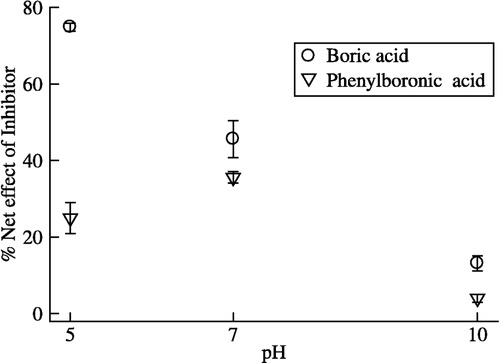
In the present experiment the net inhibitory effect of boric acid, which existed in two forms (boric acid and borate) in relative proportions under different pH conditions was studied. As the pH rises, boric acid is converted to borate and as the pH drops, borate is converted back to boric acid. Therefore, at acidic (5.0) and neutral pH (7.0), the boric acid would be in the greater proportion and little to negligible amounts of borate form would exist; in an alkaline pH (10.0), the situation would be vice versa. The percentage net effect of boric acid on urease activity at pH 5.0 and 7.0 was 74 and 42%, respectively, and at pH 10, the net effect was only 13%. Similarly, with PBA, the net effect was 21, 35 and 4%, respectively. These results () clearly showed that for boric acid the trigonal planar B(OH)3 form is a more effective inhibitor than the tetrahedral form. This pH-dependence of inhibition was also observed in the case of P. mirabilis [Citation22], but it was vice versa in the case of manganese-dependent human liver arginase where borate behaved as an S-hyperbolic I-hyperbolic non-competitive inhibitor [Citation32].
Figure 2 pH-dependence of boric acid and phenylboronic acid inhibition of pigeonpea urease. The percent (%) net effect of inhibitor was determined by the formula mentioned in the text. The enzyme activity was carried out in three different buffers, pH 5.0 (0.05 M MES), pH 7.0 (0.05 M Tris–acetate) and pH 10.0 (0.05 M Carbonate).
Conclusion
Boric acid, BBA, PBA and 4-BPBA are competitive inhibitors of pigeonpea urease. The trigonal planar B(OH)3 form is a more effective inhibitor than the tetrahedral form. The competitive nature of inhibition along with low-toxicity and non-volatility of these compounds could make them attractive options as urease inhibitors.
Acknowledgements
Reddy K R C is thankful to the Indian Council of Medical Research, New Delhi for financial assistance in the form of Junior Research Fellowship.
References
- Mobley HLT, Hausinger P. Microbiol. Rev. 1989; 53: 85
- Hirai M, Kawai-Hirai R, Hirai T, Ueki T. Eur. J. Biochem. 1993; 215: 55
- Polacco JC, Havir EA. J. Biol. Chem. 1979; 254: 1707
- Das N, Kayastha AM, Srivastava PK. Phytochem. 2002; 61: 513
- Das N, Prabhakar P, Kayastha AM, Srivastava RC. Biotechnol. Bioeng. 1997; 54: 619
- Das N, Kayastha AM, Malhotra P. Biotechnol. Appl. Biochem. 1998; 1: 25
- Das N, Kayastha AM. World. J. Microbiol. Biotechnol. 1998; 14: 927
- Kayastha AM, Srivastava PK. Appl. Biochem. Biotechnol. 2001; 96: 41
- Kayastha AM, Srivastava PK, Miksa B, Slomkowski S. J. Bioact. Compat. Polym. 2003; 18: 113
- Reddy KRC, Srivastava PK, Dey PM, Kayastha AM. Biotechnol. Appl. Biochem. 2004; 39: 323
- Srivastava PK, Kayastha AM, Srinivasan. Biotechnol. Appl. Biochem. 2001; 34: 55
- Srivastava PK, Kayastha AM, Jagannadham MV. J. Biochem. Mol. Biol. Biophys. 2002; 6: 1
- Zhengping W, van Cleemput O, Liantie L, Baert L. Biol. Fertil. Soils 1991; 11: 101
- Krogmeier MJ, McCarty GW, Bremner JM. Proc. Natl. Acad. Sci. USA. 1989; 86: 1110
- Griffith DP, Musher DM, Itin C. Investig. Urol 1976; 13: 346
- Rosenstein IJ, Hamilton-Miller JM. Crit. Rev. Microbiol. 1984; 11: 1
- Porter IA, Brodie J. Br. Med. J 1969; 2: 353
- Mazurkiewicz JC, Bingham SA, Runswick S, Ang BCN. Ann. Clin. Biochem. 1993; 30: 215
- Jones GRD. Ann. Clin. Biochem 1997; 34: 430
- Benini S, Rypniewski WR, Wilson KS, Mangani S, Ciurli S. J. Am. Chem. Soc. 2004; 126: 3714
- Weston GS, Blazquez J, Baquero F, Shoichet BK. J. Med. Chem. 1998; 41: 4577
- Breitenbach JM, Hausinger RP. Biochem. J 1988; 250: 917
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J. Biol. Chem. 1951; 193: 265
- Dixon M. Biochem. J 1953; 55: 170
- Krajewska B, Zaborska W, Leszo M. J. Mol. Catal-B 1997; 3: 231
- Krajewska B, Zaborska W, Leszko M, Brzózka Z. Polish J. Chem. 1999; 73: 359
- Todd MJ, Hausinger RP. J. Biol. Chem 1991; 266: 10260
- Gallardo-Williams MT, Maronpot RR, Wine RN, Brunssen SH, Chapin RE. Prostate 2003; 54: 44
- Bauer C-A, Petterson G. Eur. J. Biochem 1974; 45: 473
- Shomron N, Ast G. FEBS Lett 2003; 552: 219
- Farr-Jones S, Smith SO, Kettner CA, Griffin RG, Bachovchin W. Proc. Natl. Acad. Sci. USA 1989; 86: 6922
- Carvajal N, Salas M, Lopez V, Uribe E, Herrera P, Cerpa J, Fuentes M. J. Inorg. Biochem 1999; 77: 163