Abstract
The in vitro influence of potassium ion modulations, in the concentration range 2 mM–500 mM, on digoxin-induced inhibition of porcine cerebral cortex Na+/K+-ATPase activity was studied. The response of enzymatic activity in the presence of various K+ concentrations to digoxin was biphasic, thereby, indicating the existence of two Na+/K+-ATPase isoforms, differing in the affinity towards the tested drug. Both isoforms showed higher sensitivity to digoxin in the presence of K+ ions below 20 mM in the medium assay. The IC50 values for high/low isoforms 2.77 × 10− 6 M / 8.56 × 10− 5 M and 7.06 × 10− 7 M /1.87 × 10− 5 M were obtained in the presence of optimal (20 mM) and 2 mM K+, respectively. However, preincubation in the presence of elevated K+ concentration (50 – 500 mM) in the medium assay prior to Na+/K+-ATPase exposure to digoxin did not prevent the inhibition, i.e. IC50 values for both isoforms was the same as in the presence of the optimal K+ concentration. On the contrary, addition of 200 mM K+ into the medium assay after 10 minutes exposure of Na+/K+-ATPase to digoxin, showed a time-dependent recovery effect on the inhibited enzymatic activity. Kinetic analysis showed that digoxin inhibited Na+/K+-ATPase by reducing maximum enzymatic velocity (Vmax) and Km, implying an uncompetitive mode of interaction.
Introduction
Na+/K+–ATPase (EC 3.6.1.37) is a cell membrane located enzyme, which plays a key role in the active transport of monovalent cations (Na+ and K+) across the membranes [Citation1,Citation2]. The enzyme is composed of an α-subunit, which is the residence of ATP-binding, phosphorylation, Na+ and K+ binding as well as the specific inhibitor ouabain, and a β-subunit, which stabilises the K+ binding cage [Citation3]. Na+/K+-ATPase acts as a dimer (αβ–αβ). The most widely accepted view related to such a dimer acts is a “flip-flop” model, in which both α subunits show complementary conformation:
where E is the conformation of each α-subunit [Citation4]. The activity of this enzyme is very sensitive to the presence of some metal ions [Citation5,Citation6] and organic compounds of various structures, especially some drugs and pesticides Citation7-10.
The main pharmacological effect of digoxin, one of the most frequently used cardiac glycosides to improve cardiac contractility, is Na+/K+-ATPase inhibition Citation11-14. Therapeutic effect is achieved with a digoxin concentration that produces a moderate enzyme inhibition (about 30 per cent), whereas a toxic concentrations inhibit over 60 per cent of enzyme activity [Citation15].
The present study was undertaken to examine the inhibition of porcine cerebral cortex Na+/K+-ATPase activity by digoxin. Furthermore, it has been shown that hypokalaemia lowers the threshold for digoxin-induced cardiac arrhythmias i.e. it increases the likelihood of digoxin toxicity [Citation12,Citation16]. So we examined the inhibitor efficiency of digoxin in the presence of below optimal potassium concentration. Conversely, the ability of above optimal K+ concentration to prevent and recover digoxin-induced inhibition was also investigated. In addition, extensive kinetic studies were undertaken to determine the nature of enzyme inhibition by digoxin.
Material and methods
Chemicals
All chemicals were of analytical grade. Na+/K+-ATPase from porcine cortex brain with specific Na+/K+-ATPase activity 2.75 μmol Pi/h/mg protein, was purchased by Sigma Chemicals Co.
ATPase assay
The influence of digoxin, within the concentration range from 1 × 10− 10 to 1 × 10− 3 M on Na+/K+-ATPase activity was determined in a standard medium containing 50 mM Tris–HCl (pH 7.4), 1 mM EDTA, 100 mM NaCl, 20 mM KCl, 5 mM MgCl2, 2 mM ATP, 40 μg protein in a final volume of 200 μl. Incubation mixtures were preincubated for 10 min at 37°C in the presence of the desired concentration of digoxin or distilled water (control). The reaction was started by the addition of ATP, allowed to proceed for 10 min, and interrupted by the addition of ice cold HClO4 with immediate cooling on ice. Inorganic orthophosphate (Pi) liberated from the hydrolysis of ATP was measured using a modified spectrophotometric procedure [Citation5,Citation6], reading the absorbance at 690 nm.
The effect of a lower potassium concentration on the inhibitory efficiency of digoxin was assayed in the same standard medium containing 2 mM KCl.
The effect of above optimal concentration of K+ on prevention of digoxin-induced inhibition was measured under the same conditions as described above but with 50 mM, 200 mM and 500 mM KCl added to the medium before the enzyme exposure to digoxin.
The recovery of inhibited enzyme activity by K+ was examined by adding a ten-fold higher concentration of K+ to the base level already present in the experimental tube, after 10 min in cubation in the presence of digoxin at a concentration which induced complete enzyme inhibition. The degree of reactivation, dependent on the time of exposure of the sample to the higher potassium concentration, was followed within the time interval 10–60 min. For each time point there was a control sample (without digoxin) which served as a basis for calculating the per cent reactivation.
Kinetic parameters
Kinetic experiments were carried out according to the slightly modified method of Philips [Citation17]. The initial velocities were measured in the same incubation medium, as a function of rising concentrations of MgATP2 − (0.1–4.0 mM). The measurements were performed in the absence and the presence of digoxin, while maintaining the concentrations of other ions (Na+, K+, Mg2+) constant. The experimental data were fitted to the Michaelis-Menten equation by non-linear regression analysis using EZ FIT [Citation18]. Vmax and Km values with standard errors were derived from a Lineweaver-Burk plot.
Results
Influence of potassium modulations on digoxin-induced inhibition of Na+/K+-ATPase
The effect of digoxin, within the range 1 × 10− 10–1 × 10− 3 M on ATP hydrolysis catalyzed by porcine cerebral cortex Na+/K+-ATPase was investigated in the presence of optimal and a ten-fold lower concentration of K+. As shown on the response of the enzymatic activity to digoxin concentration in both cases was biphasic, and could be approximated by the sum of two overlapping sigmoid curves, separated by a plateau.
Figure 1 Inhibition of porcine cerebral cortex Na+/K+-ATPase activity by digoxin in the presence of 20mM KCl (○) and 2mM KCl (▪). The experimental curves were analysed using the PC package assuming a two site model fit [Citation9]. The values given are the mean of at least three experiments ± SEM, done in duplicate.
![Figure 1 Inhibition of porcine cerebral cortex Na+/K+-ATPase activity by digoxin in the presence of 20mM KCl (○) and 2mM KCl (▪). The experimental curves were analysed using the PC package assuming a two site model fit [Citation9]. The values given are the mean of at least three experiments ± SEM, done in duplicate.](/cms/asset/37d5c7aa-70e6-47fd-9c17-5ae0ecae7457/ienz_a_164202_f0001_b.gif)
The results indicate that the enzyme activity at each digoxin concentration, in the presence of optimal as well as a ten-fold lower concentration of K+, represent the sum of the activity of two Na+/K+-ATPase isoforms, differing in their affinity i.e. in their sensitivity towards digoxin. The first one, sensitive at a digoxin concentration below 1 × 10− 5 M, is denoted as the “high affinity” Na+/K+-ATPase isoform, while the second, sensitive at an inhibitor concentration above 1 × 10− 5 M, is denoted as the “low affinity” Na+/K+-ATPase isoform.
Experimental data presented in was analyzed by a PC software package assuming a two-site model fit [Citation9]. The activity of the “high” digoxin affinity isoenzyme was obtained by subtracting the calculated low affinity values from the experimental data. The theoretical curves for “high” and “low” digoxin affinity isoforms in the presence of optimal and a ten-fold lower concentration of K+ are presented in .
Figure 2 The theoretical curves for inhibition of “high-(1)” and “low (2) affinity” Na+/K+-ATPase isoforms induced by digoxin [Citation9]. ○ – in the presence of 20 mM KCl; ▪ in the presence of 2 mM KCl.
![Figure 2 The theoretical curves for inhibition of “high-(1)” and “low (2) affinity” Na+/K+-ATPase isoforms induced by digoxin [Citation9]. ○ – in the presence of 20 mM KCl; ▪ in the presence of 2 mM KCl.](/cms/asset/3262b430-7173-45ef-b6c1-39a27471572b/ienz_a_164202_f0002_b.gif)
Hill analysis was performed on the high and low digoxin affinity parts of the inhibition curves. The half maximum inhibition concentrations (IC50 values) as well as the values of the Hill coefficients n for the “high” and “low” inhibitor affinity isoforms respecti vely, were calculated and are summarized in .
Table I. IC50 values and Hill coefficients (n) as calculated from experimental data for “high”- and “low affinity” isoenzymes by Hill analysis in the presence of various K+ concentrations.
It can be noted that both Na+K+-ATPase isoforms are more sensitive to digoxin inhibition in the low potassium concentration sample in comparison to the sample with an optimal concentration of potassium.
On the contrary, the protective effect of above optimal concentrations of K+ is not obtained. The presence of 50 mM, 200 mM and 500 mM KCl in the reaction medium before enzyme exposure to digoxin, did not prevent the digoxin-induced inhibition, i.e. IC50 values were the same as in the presence of the optimal K+ concentration ().
Recovery of digoxin-induced inhibition of Na+K+-ATPase
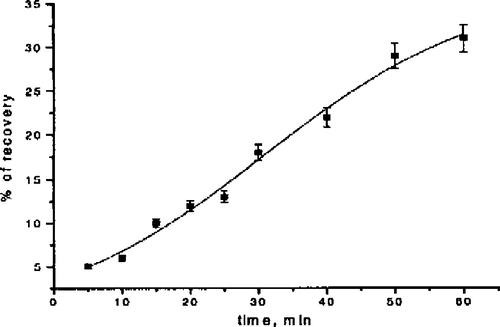
The recovery of inhibited Na+K+-ATPase by a higher potassium concentration was examined by adding a ten-fold larger potassium concentration (200 mM KCl) following the incubation period with digoxin at a concentration (1 mM) which induced complete enzyme inhibition. It was shown that exposure of inhibited enzyme to a ten-fold larger potassium concentration induced the recovery of enzymatic activity within the time interval of 10–60 minutes (). The degree of reactivation vs. incubation time was linearly proportional to the time of exposure. However, complete recovery was not achieved even after 60 minutes.
Kinetic analysis
In order to evaluate the nature of the Na+/K+-ATPase inhibition induced by digoxin, kinetic parameters Km and Vmax were determined by varying the concentration of MgATP2 − . The kinetic properties of the enzyme were determined in the presence of 2 × 10− 6 M of digoxin, a concentration chosen from the inhibition curves as the concentration that inhibits the enzyme activity in the high affinity concentration range. The dependence of the initial reaction rate vs. substrate concentration in the presence and the absence of inhibitor exhibited typical Michaelis–Menten kinetics which is presented in . Kinetic constants were calculated from the experimental data by a Lineweaver-Burk transformation (, ) and are summarized in . It is clearly apparent that digoxin decreased the Km and Vmax values of the enzyme to the same extent.
Figure 4 Porcine cerebral cortex Na+/K+-ATPase activity dependence on (MgATP2 − ) in the absence (▪) and presence of 2 × 10− 6 M digoxin (○). The values given are the mean of at least three experiments ± SEM, done in duplicate. The Lineweaver-Burk transformation of the data is shown in the inset. *Taken from ref. [Citation9].
![Figure 4 Porcine cerebral cortex Na+/K+-ATPase activity dependence on (MgATP2 − ) in the absence (▪) and presence of 2 × 10− 6 M digoxin (○). The values given are the mean of at least three experiments ± SEM, done in duplicate. The Lineweaver-Burk transformation of the data is shown in the inset. *Taken from ref. [Citation9].](/cms/asset/c1ea9f2a-48dc-4480-bc9b-461f9ea0f6ac/ienz_a_164202_f0004_b.gif)
Table II. Kinetic analysis of porcine cerebral cortex Na+/K+-ATPase activity in the absence and presence of inhibitor (2 × 10−6 M).
Discussion
The present study showed that digoxin, as previously reported for rat brain [Citation19], inhibits porcine cerebral cortex Na+/K+–ATPase in a dose – dependent manner. Additionally, biphasic inhibitory curves were obtained which indicated the interference of two distinct inhibitor binding sites. The heterogeneity of digoxin binding sites has been reported in rat brain and beef heart Na+/K+–ATPase and was related to two distinct isoforms of the α – subunit Citation19-21. The high affinity to digoxin can be attributed to the α3—isoform, which is known to be the most sensitive to cardiac glycosides [Citation9,Citation22,Citation23]. Moreover, it is well known that the α3—isoform is especially abundant in brain [Citation24,Citation25] as well as some other vertebrate tissues [Citation15]. The low affinity to digoxin can be attributed to the α1—isoform which shows the lowest affinity toward cardiac glycosides and is present in almost every animal cell [Citation26].
Our study clearly showed that the inhibitory effect of digoxin also depends on the concentration of potassium in the reaction mixture. Two differential effects in the presence of various K+ concentrations were achieved: 1) the decreasing of the IC50 value of digoxin-induced inhibition (K+ concentration below 20 mM) and 2) a time-dependent recovery effect of enzymatic activity (concentration above 20 mM). What is more, both isoforms showed higher sensitivity towards digoxin in the presence of a ten-fold lower potassium concentration. It is interesting to point out that Hill values were elevated for both isoforms in below optimal K+ concentrations in comparison to optimal conditions, which is an indication of a positive cooperative effect for digoxin binding. As suggested earlier, there are two possible mechanisms which could explain the increased sensitivity of Na+/K+–ATPase in the below optimal potassium conditions. On the one hand, it has been reported Citation27-29 that below optimal potassium concentrations reduce the rate of Na+/K+–ATPase turnover number and subsequently exacerbate the sodium pump inhibition caused by cardiac glycosides. On the other hand, certain authors [Citation30] suggest that digoxin binds to the potassium binding sites, i.e. that digoxin competes with potassium for the same binding site. Therefore, digoxin binding and its physiological effects would be augmented in the presence of low potassium concentrations.
However, since the results of our study show that elevated potassium concentrations did not display protective effect in vitro, decreases the likelihood of digoxin and potassium competing for a common binding site. In fact, it is more probable that low potassium concentrations in the presence of optimal Na+, Mg2+ and ATP favour the E2 conformation of the catalytic α-subunit, which has a higher affinity towards digoxin [Citation7]. Furthermore, this hypothesis is concordant with the results of kinetic analysis which confirmed that digoxin is an uncompetitive inhibitor in comparison to ATP as a substrate. In other words, digoxin binds to the α-subunit of Na+/K+–ATPase posterior to Na+ and ATP binding, resulting in the formation of a stable phosphoenzyme/digoxin complex [E2 –P digoxin] and, ultimately, prevents its conversion to the E1 conformation and consequently, according to a “flip-flop” model [Citation4], conversion of the other catalytic subunit to the E2 conformation.
Moreover, it has been recognized that digoxin-induced inhibition is not only reversible, but also that the enzyme could be reactivated by means of an appropriate specific antidigoxin antibody [Citation19]. Our study has shown that elevated potassium concentrations are also able to reactivate the inhibited enzyme to a certain extent, and that the reactivation is proportional to the time of exposure to increased potassium concentration. Furthermore, it can be assumed that higher potassium concentrations (in contrast to low potassium concentration) move the chemical balance between E1 and E2 Na+/K+-ATPase conformations in favour of E1 and, consequently, enable the continuation of the digoxin-impeded enzymatic cycle.
To summarize, it is quite plausible that factors such as Mg2+and Na+ concentrations as well as ATP availability also affect digoxin-induced inhibition of Na+/K+-ATPase, which will be the subject of future studies.
Acknowledgements
The Ministry of Science, Technologies and Development of the Republic of Serbia, Proj. No 1991, provided the financial support for this study.
References
- Vasilets LA, Schwarz W. Structure–function relationships of cation binding in Na/K-ATPase. Biochim Biophys Acta 1993; 1154: 201–222
- Rodrigez G de Lores Aranaiz, Pena C. Characterization of synaptosomal membrane Na,K-ATPase inhibitors. Neurochem Int 1995; 27: 319–327
- Schuurmans Stekhoven FMAH. E31-K352, the minimal cation binding moiety of Na,K-ATPase. Biochem Biophys Res Commun 1998; 245: 366–369
- Repke KR, Schoen R. Flip-flop model of (NaK)-ATPase function. Acta Biol Med Ger 1973; 31: K19–K30
- Vujisić Lj, Krstić D, Vučetić J. Chemical aspect of the influence of cobalt ions on ATPase activty. J Serb Chem Soc 2000; 65(7)507–515
- Vasić V, Jovanović D, Krstić D, Nikezić G, Horvat A, Vujisić Lj, Nedeljković N. Prevention and recovery of CuSO4-induced inhibition of Na,K-ATPase and Mg-ATPase in rat brain synaptosomes by EDTA. Toxicol Lett 1999; 110: 95–104
- Scheiner-Bobis G. The sodium pump; its molecular properties and mechanics of ion transport. Eur. J. Biochem. 2002; 269: 2424–2433
- Scheiner-Bobis G. Sanguinarine induces K+ outflow from yeast cells expressing mammalian sodium pumps. Naunyn-Schmiedeberg's Arch Pharmacol 2000; 363: 203–208
- Krstić D, Krinulović K, Joksić G, Spasojević-Tišma V, Momić T, Vasić V. Effects of digoxin and gitoxin on the enzymatic activity and kinetic parameters of Na+/K+-ATPase. J Enz Inhib Med Chem 2004; 19(5)409–415
- Jovanović D, Vasić V, Nikolić V, Ćetković, Nikezić G. Effect of the organophosphate chlorpyrifos on the SPMs Na,K-ATPase and Mg-ATPase. Arch Toxicol Kinet Xenobiot Metab 2000; 8(3)152–154
- Thomas R, Gray P, Andrews J. Digitalis: Its mode of action, receptor, and structure–activity relationships. Adv Drug Res 1990; 19: 312–562
- Kelly R. Cardiac glycosides and congestive heart failure. Am J Cardiol 1990; 65: 33
- Clausen T. Clinical and therapeutic significance of the Na+/K+ pump. Clin Sci 1998; 1: 3–17
- Piasick M. Therapy of heart failure Clin Pharm. 824. Available: http://www.emedicine.com/EMERG/topic 108.
- Melero CP, Maderade M, Feliciano AS. A short review on cardiac steroids and their aminoguanidine analogues. Molecules 2000; 5: 51–81
- Li-Shaw-He. Digoxin revisited. Q J Med 1998; 91: 259–264
- Philips TD, Hayes AW, Ho IK, Desiah D. Effects of rubrotoxin B on the kinetics of cationic and substrate activation of Na, K-ATPase and p-nitrophenylphosphatase. J Biol Chem 1978; 253: 3487–3493
- Perrella FW. EZ-FIT A practical curve fitting microcomputer program for the analysis of enzyme kinetic data on IBM-PC. Anal Biochem 1988; 174: 437–447
- Cano NJ, Sabouraud AE, Debray M, Scherrmann JMG. Dose-dependent reversal of digoxin-inhibited activity of an in vitro Na,K-ATPase model by digoxin-specific antibody. Tox Lett 1996; 85: 107–111
- Erdmann E, Werden K, Brown L. Multiplicity of cardiac glucoside receptors in the heart. Trends Biochem Sci 1985; 293–295
- O'Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium–potassium adenosine triphosphate. Arch Biochem Biophys 1994; 310: 32–39
- Quadri L, Ferrandi M. Involvement of the Na+,K+-ATPase and its inhibitors in cardiovascular diseases. Exp Opin Ther Pat 1998; 8(1)39–52
- Hoffman JF, Wickrema A, Potapova O, Milanick M, Yingst DR. Na pump isoforms in human erythroid progenitor cells and mature erythrocytes. Proc Natl Acad Sci USA 2002; 99(22)14572–14577
- McGrail KM, Phillips JM. Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: Both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. 1991; 11: 381–391
- Hieber V, Siegel GJ, Fink DJ, Beaty MW, Mata M. Differential distribution of (Na,K)-ATPase alpha isoforms in the central nervous system. Cell Mol Neurobiol 1991; 11: 253–262
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am J Physiol 1998; 275(Renal. Physiol)F633–F650
- Kenneth TK. Toxicity, digitalis, http/www.emedicine.com/ped/topic590.html
- Gennari J. Hypokalaemia. N Eng J Med 1998; 339(7)451–458
- Weiner D, Wingo S. Hypokalaemia—consequences, causes and correction. J Am Coc Nephr 1997; 8(7)1179–1188
- Mohamad NKM. Digoxin poisoning, http/www.prn2.usm.my/mainsite/bulletin/2000/prn30.html