Abstract
Two new diterpenoid alkaloids, heterophyllinine-A (1) and heterophyllinine-B (2), along with two known alkaloids dihydroatisine (3) and lycoctonine (4) were isolated from the roots of Aconitum heterophyllum Wall. The structure of (1) and (2), were deduced on the basis of spectral data. Compounds 1–2 inhibited acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8) enzymes in a concentration-dependent manner with percent inhibition ranging between 4.24% and 6.94 % and 79.1 % and 82.75 % for AChE and BChE, respectively indicating that compounds 1 and 2 are about thirteen times more specific to BChE than AChE.
Introduction
Genus Aconitum is a rich source of diterpenoid alkaloids, many of which exhibit a broad spectrum of biological activities. Lappaconitine hydrobromide has been used as an antiarrhythmic drug [Citation1]. Methyllycaconitine perchlorate is used in a curaremimetic preparation [Citation2]. Some aconitine and mesaconitine derivatives possess potent analgesic and anti-inflammatory activities [Citation3]. The methyllycaconitine and lycaconitine exhibited neuronal nicotinic acetylcholine receptor affinity [Citation4]. Lycaconitine, obtained from several Aconitum species, was found to be effective against multi-drug resistant cancers. Aconitum plants are widely used in Chinese and Indian traditional systems of medicine [Citation5,Citation6]. Turkish Aconitum species are used externally in the treatment of rheumatic pain and sciatica and also against body lice [Citation7]. Previously, heterophyllisine, heterophylline, heterophyllidine, heteratisine, atisine, atidine, F-dihydroatisine, hetisine, benzoylheteratisine and atisenol were reported from A. heterophyllum Citation8–11.
AChE (EC 3.1.1.7) is a key component of cholinergic brain synapses and neuromuscular junctions. The major biological role of the enzyme is the termination of impulse transmission by rapid hydrolysis of the cationic neurotransmitter acetylcholine [Citation12]. According to the cholinergic hypothesis, memory impairments in patients with the senile dementia diseases are due to a selective and irreversible deficiency in the cholinergic functions in the brain [Citation13]. The role of BChE (EC 3.1.1.8) in normal aging and brain diseases is still elusive. It has been found that BChE is present in significantly higher quantities in Alzheimer's plaques than in plaques of normal age related non-demented brains [Citation14]. The discovery of natural cholinesterase inhibitors has been a very challenging area of drug development due to the involvement of cholinesterases in Alzheimer's disease and other related dementias. We have previously reported a number of new natural inhibitors of cholinesterases (AChE and BChE) isolated from indigenous medicinal plants [Citation15,Citation16].
Herein we report the isolation and structure elucidation of two new diterpenoid alkaloids from A. heterophyllum and their cholinesterase inhibition potential.
Experimental
General experimental
Optical rotations were measured on a JASCO DIP 360 polarimeter. IR spectra were recorded on a JASCO 302-A spectrophotometer. EI-MS and HREI-MS were recorded on JMS HX 110 with data system and on JMS-DA 500 mass spectrometers. The 1H- and 13C-NMR spectra were recorded on Bruker NMR spectrometers operating at 400 MHz, (100 and 125 MHz for 13C). The chemical shifts values are reported in ppm (δ) units and the coupling constants (J) are given in Hz.
Chromatographic conditions
For TLC, precoated aluminum sheets (silica gel 60F-254, E. Merck) were used. Visualization of the TLC plates was achieved under UV at 254 and 366 nm and by spraying with Dragendorff's reagent. Solvent system; “n-hexane-acetone-diethylamine (8:2:10)”, was used to monitor the separation profile.
Plant material
The roots (5 kg, dry wt) of Aconitum heterophyllum Wall. were collected from Swat, N.W.F.P., Pakistan, at an elevation of 2000 m in August 2005 and identified by Mr. Mehboob ur Rahman, Assistant Professor, Department of Botany, Jahanzeb Post Graduate College, Saidu Sharif, Swat, NWFP, Pakistan. The voucher specimen (HA-014) is deposited in the herbarium of the botany department.
Extraction and isolation
Dried and powdered roots (5 Kg) of the plant were extracted exhaustively with n-hexane (3 × 8 L) followed by 80% EtOH (3 × 10 L) extraction at room temperature for 7 days (3-times). The filtrate was concentrated in vacuo to yield 60 g of residue. The residue was acidified to pH 1.5 with 0.5 N H2SO4 and extracted with CH2Cl2 (3 × 2 L) to afford alkaloid mixture (18 g). The acidic aqueous solution was basified (pH 8–10) by using 10% KOH (aq) and extracted with CH2Cl2 (5 × 2 L) to yield 13.8 g of alkaloid mixture. The crude basic fraction was fractionated on silica gel column (260 g) and five combined fractions were obtained. On repeated flash column chromatography using solvent system n-hexane-acetone (9:1) containing 10 drops of diethylamine per 100 ml. Heterophyllinine-A (1), Heterophyllinine-B (2), along with two known alkaloids dihydroatisine (3) and lycoctonine (4) were obtained.
Heterophyllinine-A (1)
Amorphous powder (15 mg). mp 110–112°C; [α]30D–81.1 (c 0.8, CHCl3); IR υmaxCHCl3, 3492 (OH groups), 3086, 1658, 900 (C = CH2, terminal methylene), 1376 (CCH3), 1H-NMR (400 MHz, CDCl3): see . 13C-NMR (CDCl3, 100 MHz): see , EIMS (M+ m/z): C22H33NO2, (343.518).
Table I. 1H- and 13C-NMR data of compound 1 and 2 (400 & 100 MHz, CDCl3).
Heterophyllinine-B (2)
Amorphous powder (20 mg). mp., 68–70°C; [α]30D–68.0 (c 1.0, CHCl3); IR υmaxCHCl3, 3492 (OH groups), 3086, 1658, 900 (C = CH2, terminal methylene), 1376 (CCH3), 1735 (C = O). 1H-NMR (400 MHz, CDCl3): see . 13C-NMR (CDCl3, 100 MHz): see , EIMS (M+ m/z): C24H35NO4, (401.398).
Enzyme inhibition assay
The standard operational assay protocolwas employed to determine the AChE and BChE inhibition activities of natural products, by modifying the spectrophotometric method of Ellman et al. (1961) [Citation17]. Enzymes and reagents such as electric-eel AChE (EC 3.1.1.7), horse-serum BChE (E.C 3.1.1.8), acetylthiocholine iodide, butyrylthiocholine chloride, 5, 5′-dithiobis [2-nitrobenzoic acid] (DTNB), and galanthamine were purchased from Sigma (St. Louis, MO, USA). All other chemicals were of molecular biology grade. Acetylthiocholine iodide and butyrylthiocholine chloride were used as the substrates to assay AChE and BChE activities, respectively. The reaction mixture contained 150 μL of sodium phosphate buffer (100 mM) (pH 8.0), 10 μL of DTNB, 10 μL (0.2 mM) of test-compound solution and 20 μL of AChE or BChE solution, which were mixed and incubated for 15 min. (25°C). The reaction was then initiated with the addition of 10 μL acetylthiocholine or butyrylthiocholine, respectively. The hydrolysis of acetylthiocholine and butyrylthiocholine were monitored by the formation of yellow 5-thio-2-nitrobenzoate anion resulting from the reaction of DTNB with thiocholine, catalyzed by acetylthiocholine and butyrylthiocholine, respectively at a wavelength of 412 nm (15 min.). Test-compounds and the positive control (galanthamine) were dissolved in EtOH. As the extinction coefficient of the yellow anion is known, the rate of enzymatic reaction was finally determined by applying the Ellman equation:
All the inhibition studies were conducted in 96-well micro-titer plates using Spectra Max-340 (Molecular Devices, CA, USA). Concentration of compounds that inhibited the hydrolysis of substrates (acetylthiocholine and butyrylthiocholine) by 50% (IC50) was determined by monitoring effect of various concentrations of the compound on inhibition values. The IC50 values were then calculated using the EZ-Fit Enzyme Kinetics program [Citation18].
Results and discussion
Heterophyllinine-A (1) was obtained as a white amorphous powder, and was assigned the molecular formula C22H33NO2 on the basis of EI-MS (M+ m/z = 343) and NMR spectral data. The 1H- and 13C-NMR spectra of heterophyllinine-A (1) exhibited a close resemblance to that of the known compound atidine [Citation19] except for the presence of a methane group instead of carbonyl and methylene at both C-7 and C-20. Its IR spectrum also showed characteristic signals at 3492 (OH groups), 3086, 1658, 900 (C = CH2, terminal methylene), 1376 (CCH3). In the down field region of the 1H-NMR spectrum of heterophyllinine-A (1) two broad singlets each of one proton at δ 5.03 and 4.98, were assigned to the methylene group. A broad singlet of one proton at δ 3.50 was assigned to H-15. A triplet of one proton integration at δ 2.02 (J = 11.6 Hz), was due to the C-9 methine proton. Similarly, two doublets each of one proton integration at δ 2.91 and 2.83 (J = 10.27), were assigned to the methylene protons attached at C-19. A broad singlet of one proton at δ 2.26 was assigned to the H-5, while, a multiplet of one proton at δ 2.40 was assigned to H-7. The 13C-NMR spectrum (BB, DEPT) (), showed 22 signals, including one methyl, eleven methylene, six methine, and four quaternary carbons. The 1H- 13C correlation was determined by the HMQC spectrum, while the long range 1H- 13C connectivities were obtained through HMBC technique (see HMBC of compound 1). The H-5 (δ 2.26) showed 2J and 3J correlation with C-4 (δ 39.3), C-10 (δ 50.5), C-6 (δ 28.8), and C-7 (δ 40.9), whereas H-15 (δ 3. 30), exhibited 1J and 2J correlation with C-15 (δ 77.8), C-16 (δ 156.7), and C-14 (δ 29.1), while, H-12 (δ 1.46), showed correlation with C-12 (δ 38.0), C-16 (δ 156.7), C-17 (δ 110.3), C-13 (δ 27.5), and C-11 (δ 27.5).
Thus on the basis of above spectral data, the structure of compound 1 was deduced as heterophyllinine-A.
Heterophyllinine-B (2), was obtained as a white amorphous powder, and was assigned the molecular formula C24H35NO4, on the basis of EI-MS (M+ m/z = 401), and NMR spectral data. The 1H- and 13C-NMR spectra of heterophyllinine-B (2) exhibited a close resemblance to that of the known compound isoatisine [Citation20,Citation21] except the presence of acetyl group instead of two hydroxyl groups at C-15 and at C-6. Its IR spectrum also showed characteristic signals at 3356 (OH groups), 3012, 1656, 893 (C = CH2, terminal methylene), 1385 (CCH3), 1685 (C = O). In the down field region of the 1H-NMR spectrum of heterophyllinine-B (2) two broad singlets each of one proton at δ 5.03 and 4.98, were assigned to the methylene group. A broad singlet of one proton at δ 3.49 was assigned to H-15. Similarly, a singlet of one proton integration at δ 4.01 was assigned to the methine proton attached at C-19. A broad singlet of one proton at δ 0.88 was assigned to the H-5. The 13C-NMR spectrum (BB, DEPT) (), showed 24 signals, including two methyl, eleven methylene, six methine, and five quaternary carbons. The 1H- 13C correlation was determined by the HMQC spectrum, while the long-range 1H- 13C connectivities were obtained through HMBC technique (see HMBC of compound 2). The H-5 (δ 0.88) showed 2J and 3J correlation with C-4 (δ 41.0), C-10 (δ 38.0), C-6 (δ 77.9), and C-7 (δ 41.8), whereas H-15 (δ 3.49), exhibited 1J and 2J correlation with C-15 (δ 77.9), C-16 (δ 156.8), and C-14 (δ 27.5).
Thus on the basis of above spectral data, the structure of compound 2 was deduced as heterophyllinine-B.
From the activity of both tested compounds () it can be concluded that compound 1 can be better accommodated than compound 2 for AChE and as well for BChE. Compound 1 has two hydroxyl groups at position C-15 and C-22 which facilitate penetration inside the aromatic pocket of both enzymes, while in case of compound 2 both hydroxyl groups are unavailable due to natural transformation in biological system. Therefore compound 2 is unable to penetrate into the active site of the enzymes. The size and shape of compound 1 supports its strong binding due its hydrophilic hydroxyl moieties which are readily available for hydrogen bonding with receptor, while compound 2 due to its hydrophobic nature is unable to form such strong hydrogen bonding with the target protein in both of the cases.
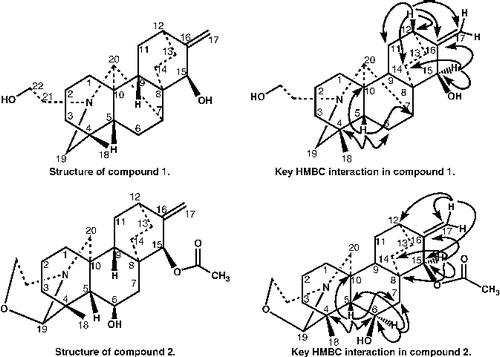
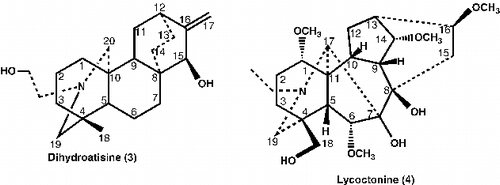
Table II. Inhibition of acetylcholinestrase (AChE) and butyrylcholinestrase (BChE) by compounds 1, 2 and galanthamine.
Acknowledgements
The research work was partially funded by the University of Peshawar, Peshawar, Pakistan and Higher Education Commission, Government of Pakistan under the promotion of research scheme. The authentication of plant material by Mr. Mehboob-ur-Rahman, Assistant Professor, Department of Botany, Jehanzeb Postgraduate College, Swat, Pakistan, is thankfully acknowledged.
References
- Atta-ur-Rahman, A Nasreen, F Akhtar, MS Shekhani, J Clardy, M Parvez, and MI Choudhary. (1997). Antifungal diterpenoid alkaloids from Delphinium denudatum. J Nat Prod 60:472–474.
- MH Benn, and JM. Jacuno In: SW Pelletier. editor. Alkaloids: Chemical and biological perspectives. Vol. 1. Chapter 4. New York: Wiley; (1984). p 153–209.
- MG Hertog, EJ Feskens, PC Hollman, MB Katan, and D Kromhout. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 342:1007–1011.
- JM Jacyno, JS Harwood, N-H Lin, JE Campbell, JP Sullivan, and MW Holladay. (1996). J Nat Prod 59:707.
- NG Bisset. (1981). Arrow poisons in China. Part II. Aconitum-botany, chemistry, and pharmacology. J Ethnopharmacol 4:247–336.
- KS Mhaskar, E Blatter, and JF Caius. (2000). Ind Med Plants 1:28.
- T Baytop. Theraphy with medicinal plants in Turkey (past and present). Istanbul University; Publication No. 3255. (1984). p 187.
- SW Pelletier, R Aneja, and KW Gopinath. (1968). 635The alkaloids of aconitum heterophyllum wall: Isolation and characterization. Phytochemsitry 7:625–629.
- SW Pelletier, MMA Abdel, FM Janet, VM Naresh, and CS Lee. (1982). Atisenol, a new entatisene diterpenoid lactone from aconitum heterophyllum. J Nat Prod 45 (6):779–781.
- WA Jacobs, and LC Craig. (1942). The aconite alkaloids. IX. The isolation of two new alkaloids from aconitum heterophyllum, heteratisine and hetisine. J Biol Chem 143:605–609.
- WA Jacobs, and LC Craig. (1943). The aconite alkaloids. xii. Benzoyl heteratisine, a new alkaloid from aconitum heterophyllum. J Biol Chem 147:571–572.
- V Tougu. (2001). Acetylcholinesterase: Mechanism of catalysis and inhibition. Curr Med Chem 1:155–170.
- EK Perry. (1986). The cholinergic hypothesis—ten years on. Br Med Bull 42:63–69.
- SQ Yu, HW Holloway, T Utsuki, A Brossi, and NH Greig. (1999). Synthesis of novel phenserine based selective inhibitors of butyrylcholinesterase for Alzheimer's disease. J Med Chem 42:1855–1861.
- Atta-ur-Rahman, Zaheer-ul-Haq, F Feroz, A Khalid, SA Nawaz, MR Khan, and MI Choudhary. (2004). Pregnane-type steroidal alkaloids of sarcococca saligna: A new class of cholinesterases inhibitors. Helv Chem Acta 87:439.
- Atta-ur-Rahman, F Feroz, SA Nawaz, MR Khan, and MI Choudhary. (2004). 735New pregnane-type steroidal alkaloids from sarcococca saligna and their cholinesterase inhibitory activity. Steroids 69:735–741. Prod 1982; 45(6): 779–781
- GL Ellman, KD Courtney, V Andres, and RM Featherstone. (1961). New and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95.
- EZ-Fit Enzyme Kinetics program. Perrella Scientific Inc., Amherst, MA, USA.
- SW Pelletier. (1965). The diterpene alkaloids. The structure of atidine. J Am Chem Soc 87:799.
- Q Jiang, and SW Pelletier. (1988). Conformation et reactivite de systemes (4.n.0) bicycliques a jonction trans—XIX: Synthese, conformation et configuration de methyl-4 et halogeno-4 bicyclo (4.2.0) octanols-3 et octanones-3 trans. Equilibres d'epimerisation des bicyclo (4.2.0) octanones α-substituees. Tetrahedron Lett 29:1865–1875.
- MG Reinecke, WH Watson, C Chen De, and WM Yan. (1987). The case of the troubling doubling. Isoatisine and 19-Epiisoatisine from the Chinese Herb Guan-Bai-Fu (Aconitum koreanum). J Org Chem 52:5051.