Abstract
Novel series of bis- and tris-pyrrolo[1,2-a]quinoxaline derivatives 1 were synthesized and tested for in vitro activity upon the intraerythrocytic stage of W2 and 3D7 Plasmodium falciparum strains. Biological results showed good antimalarial activity with IC50 in the μM range. In attempting to investigate the large broad-spectrum antiprotozoal activities of these new derivatives, their properties toward Leishmania donovani were also investigated and revealed their selective antiplasmodial profile. In parallel, the in vitro cytotoxicity of these molecules was assessed on the human HepG2 cell line. Structure–activity relationships of these new synthetic compounds are discussed here. The bis-pyrrolo[1,2-a]quinoxalines 1n and 1p were identified as the most potent antimalarial candidates with selectivity index (SI) of 40.6 on W2 strain, and 39.25 on 3D7 strain, respectively. As the telomeres of the parasite could constitute an attractive target, we investigated the possibility of targeting Plasmodium telomeres by stabilizing the Plasmodium telomeric G-quadruplexes through a FRET melting assay by our new compounds.
Introduction
Malaria remains as one of the most devastating infectious diseases of the developing world. Malaria remains a major cause of public health problem in about 95 countries mainly located in the tropical zone of the globe (notably Africa, South-East Asia and also Eastern Mediterranean region)Citation1; while approximately 3.2 billion people are at risk of being infected with malaria and developing disease. The latest figures on the incidence and mortality of malaria show that, despite progress in the implementation of preventive measures such as insecticide-treated mosquito nets and intermittent preventive treatments, this parasitic disease is still estimated to affect over 214 million people and to account for 438,000 deaths in 2015, of which approximately 80% are concentrated in just fifteen countries, mainly in Africa. The death toll is particularly high in children under five and pregnant women of the World Health Organization (WHO) African regionCitation1. Five species of protozoan parasites belonging to the Plasmodium genus, namely, falciparum, malariae, vivax, ovale and knowlesi cause malaria in human beings; from which, P. falciparum is the most dangerous of these speciesCitation2–4.
The increasing prevalence of multiple drug resistant strains of P. falciparum in most malaria endemic areas has significantly reduced the efficacy of the current antimalarial drugs. Nowadays, the only fully effective antimalarial drugs utilize artemisinin and its derivatives, notably parenteral artesunate now recommended as the first line of treatment of severe malaria for at least 24 h and until oral medication could be toleratedCitation5. Artemisinin and its derivatives are also used in combination with several different partner drugs (including lumefantrine, mefloquine, amodiaquine, sulfadoxine/pyrimethamine and piperaquine) in artemisinin-based combination therapies (ACTs), now recommended as the first line of treatment of uncomplicated Plasmodium falciparum malaria and as the second part of treatment for 3 days of severe malaria in endemic areasCitation5. However, over the last decade evidence has grown that artemisinin resistance has emerged and spread within Southeast Asia, first on the Cambodia-Thailand border in 2009Citation6,Citation7, but now across a widening area of the Greater Mekong Subregion. Rapid scientific advances in understanding of this problem have taken place within the last five yearsCitation8–10 and defined mutations in “K13” gene of P. falciparum associated to reduced ring-stage susceptibility to artemisininsCitation11. Therefore, new antimalarial agents with new mechanisms of action are required to overcome the emergence of resistance and to control an ever-increasing number of epidemics caused by the malaria parasiteCitation12. Although current efforts in antimalarial drug discovery are focused on identification of new biological targets, continued research on new 4-aminoquinoline derivatives is still warranted. This is because the hemoglobin degradation pathway in P. falciparum has a proven history as an excellent therapeutic target to which the parasite has difficulty in evolving resistance. In spite of resistance to chloroquine, previous work has shown that modification of the lateral side chain of chloroquine results in aminoquinoline derivatives that avoid the chloroquine resistance mechanismCitation13,Citation14.
A possibility to overcome this multidrug-resistant mechanism is to design new quinoline-based drugs which will not be recognized by the protein system involved in drug efflux. In this regard, two series of compounds that show promise in this regard are the bisquinoline and bisacridine antimalarial drugs A and B ()Citation15–18. These drugs show much lower resistance indices than chloroquine, indicating that the bisquinoline or bisacridine structures are less efficiently excluded by drug-resistant parasites.
Figure 1. Structure of chloroquine, bisquinolines A, bisacridines B, bispyrrolo[1,2-a]quinoxalines C, and new synthesized substituted bis- and trispyrrolo[1,2-a]quinoxaline derivatives 1a–t.
![Figure 1. Structure of chloroquine, bisquinolines A, bisacridines B, bispyrrolo[1,2-a]quinoxalines C, and new synthesized substituted bis- and trispyrrolo[1,2-a]quinoxaline derivatives 1a–t.](/cms/asset/e3d9ef42-4bd5-44d2-adb5-f28af31f1425/ienz_a_1268608_f0001_b.jpg)
The pyrrolo[1,2-a]quinoxaline heterocyclic framework constitutes the basis of an important class of compounds possessing interesting biological activities. These compounds have been reported as key intermediates for the assembly of several heterocycles including antipsychotic agents, anti-HIV agents, adenosine A3 receptor modulatorsCitation19, antiparasitic agentsCitation20–25 and antitumor agentsCitation26–30. In the course of our work devoted to discover new compounds employed in the antiparasitic chemotherapy, we previously identified some series of substituted pyrrolo[1,2-a]quinoxaline derivatives designed as interesting bioactive isosteres of quinoline derivativesCitation20–24.Thus, taking into account our experience in the field of the synthesis of new antimalarial heterocyclic compounds based on our pyrrolo[1,2-a]quinoxaline heterocyclic coreCitation20–24,Citation27–30, we decided to incorporate a benzyl group in position 4 of the heterocyclic skeletonof our previously described bispyrrolo[1,2-a]quinoxalines CCitation20 to broaden the structural diversity of these derivatives, and mainly to increase the aromatic surface of these designed compounds ().
Hence, we described here the synthesis of new bis- or trispyrrolo[1,2-a]quinoxaline derivatives 1a–t () and reported on their in vitro antiplasmodial activity against the chloroquine-sensitive (3D7) and the chloroquine-resistant (W2) strains of the malaria parasite Plasmodium falciparum. As these new compounds were designed as quinoline-like bio-isosteres, and as the quinoline skeleton is the fundamental unit of many antiprotozoan drugs, these pyrrolo[1,2-a]quinoxaline derivatives were also investigated on another medically important protozoan, Leishmania donovani, in order to evaluate the specificity of their antiparasitic activity. Leishmaniasis is an infectious disease caused by protozoan parasites belonging to the genus Leishmania. Leishmania parasites exist in two major morphological stages: extracellular flagellated promastigotes in the digestive tract of their sandfly vector, which is the infective stage and immobile intracellular amastigotes in the cells of their host’ mononuclear phagocytic system. Leishmaniasis presents various clinical aspects including cutaneous leishmaniasis, the most common form, muco-cutaneous leishmaniasis and visceral leishmaniasis, the most severe form, lethal in untreated patients. Leishmania donovani is one of the major causative agents of human visceral leishmaniasis which represents a public health problem: 0.2 to 0.4 million visceral leishmaniasis cases occur each year and more than 90% of global visceral leishmaniasis cases occur in just five countries: India, Bangladesh, Sudan, Brazil and EthiopaCitation31. The current treatment of the disease is based on a limited number of chemotherapeutic agents (meglumine antimoniate, sodium stibogluconate, pentamidine, amphotericin B and miltefosine) which present many limits, notably characterized by a high toxicity and costCitation32,Citation33. In addition, the in vitro cytotoxicity of these new molecules was assessed on the human HepG2 cell line, in order to determine their selectivity index. Moreover, as the telomeres of the parasite could constitute an attractive target, we also investigated the possibility of targeting Plasmodium telomeres by stabilizing the Plasmodium telomeric G-quadruplexes through a FRET melting assay by our new synthesized compounds. Indeed, telomerase activity has been identified in gametocytes and during the transition to erythrocytic stage of P. falciparumCitation34. The telomeric 3' G-overhang region of P. falciparum is comprised of repeated degenerate unit 5′GGGTTYA3′ (where Y may be T or C)Citation35 which can fold into intramolecular G-quadruplexCitation36.
Materials and methods
Chemistry
Commercial reagents were used as received without additional purification. Melting points were determined with an SM-LUX-POL Leitz hot-stage microscope (Leitz GMBH, Midland, ON) and are uncorrected. IR spectra were recorded on a NICOLET 380FT-IR spectrophotometer (Thermo Electron Scientific Instruments LLC, Madison, WI). NMR spectra were recorded with tetramethylsilane as an internal standard using a BRUKER AVANCE 300 spectrometer (Bruker BioSpin, Wissembourg, France). Splitting patterns have been designated as follows: s = singlet; bs = broad singlet; d = doublet; t = triplet; q = quartet; qt = quintet, dd = double doublet; ddd = double double doublet; dt = double triplet; m = multiplet. Analytical TLC were carried out on 0.25 precoated silica gel plates (POLYGRAM SIL G/UV254) and visualization of compounds after UV light irradiation. Silica gel 60 (70–230 mesh) was used for column chromatography. Microwave experiments were carried out using a focused microwave reactor (CEM Discover, Saclay, France). High resolution mass spectra (electrospray in positive mode, ESI+) were recorded on a Waters Q-TOF Ultima apparatus. Mass spectra were recorded on an Ultraflex III TOF/TOF system (Bruker Daltonics, Bremen, Germany), equipped with 200 Hz smartbeam laser (355 nm) and operating in reflectron positive ion mode. Mass spectra were acquired over the m/z range 300–5000 by accumulating data from 1000 laser shots for each spectrum. The instrumental conditions employed to analyze molecular species were the following: ion source 1: 25.08 kV; ion source 2: 21.98 kV, lens: 11.03 kV, pulsed ion extraction: 30 ns, reflector: 26.39 kV, reflector 2: 13.79 kV. Matrix suppression was activated by deflection mode: suppression up to 450 Da. Mass calibration was performed for each sample with a peptide calibration mixture (8206195, Peptide Calibration Standard, Bruker Daltonics). The instrument was controlled using Bruker's flexControl 3.4 software and mass spectra were analyzed in Bruker's FlexAnalysis 3.4 software (Bruker Daltonics, Billerica, MA).
General procedure for the synthesis of 4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehydes and 3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehydes8
To suspension of 4-chloropyrrolo[1,2-a]quinoxaline 7 (3.3 mmol), and Pd(PPh3)4 (0.164 mmol) in a mixture of toluene/EtOH (50/3 mL) under nitrogen were added K2CO3 (3.6 mmol) and phenylboronic acid (3.6 mmol). The reaction mixture was refluxed for 24 h, and the cooled suspension was extracted with CH2Cl2 (3 × 70 mL). The organic layer was washed with a saturated solution of NaCl (90 mL), and the combined organic extracts were dried over sodium sulfate, filtered, and evaporated under reduced pressure. The crude residue was triturated in ethanol. The resulting precipitate was filtered, washed with ethanol, and purified by column chromatography on silica gel using dichloromethane as eluent gave the pure product 8.
4–(9-Methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde (8d)
Yellow crystals, Yield: 85%, mp =162–164 °C; IR νmax (KBr)/cm−1 1705 (CHO); 1H NMR δ (300 MHz, CDCl3) 10.15 (s, 1H, CHO), 8.90 (dd, 1H, J = 2.70 and 1.20 Hz, H-1), 8.17 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 8.08 (d, 2H, J = 8.40 Hz, H-3′ and H-5′), 7.70 (dd, 1H, J = 8.10 and 1.20 Hz, H-6), 7.42 (t, 1H, J= 8.10 Hz, H-7), 7.13 (dd, 1H, J = 8.10 and 1.20 Hz, H-8), 7.00 (dd, 1H, J = 3.90 and 1.20 Hz, H-3), 6.90 (dd, 1H, J = 3.90 and 2.70 Hz, H-2), 4.13 (s, 3H, OCH3). MALDI-TOF MS m/z [M + H]+ Calcd for C19H15N2O2: 303.113, Found: 303.127.
4–(3-Ethoxypyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde (8e)
Yellow crystals, Yield: 68%, mp =170–172 °C; IR νmax (KBr)/cm−1 1700 (CHO); 1H NMR δ (300 MHz, CDCl3) 10.14 (s, 1H, CHO), 8.00–7.98 (m, 4H, H-2′, H-3′, H-5′ and H-6′), 7.94 (dd, 1H, J = 8.10 and 1.40 Hz, H-6), 7.84 (d, 1H, J = 3.15 Hz, H-1), 7.81 (dd, 1H, J= 8.10 and 1.40 Hz, H-9), 7.50 (ddd, 1H, J = 8.10, 8.10 and 1.40 Hz, H-7), 7.43 (ddd, 1H, J = 8.10, 8.10 and 1.40 Hz, H-8), 6.54(d, 1H, J = 3.15 Hz, H-2), 3.97(q, 2H, J = 6.90 Hz, OCH2), 1.17 (t, 3H, J = 6.90 Hz, CH3). MALDI-TOF MS m/z [M + H]+ Calcd for C20H17N2O2: 317.129, Found: 317.135.
3-(Pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde (8 g)
Yellow crystals, Yield: 68%, mp =147–150 °C; IR νmax (KBr)/cm−1 1700 (CHO); 1H NMR δ (300 MHz, CDCl3) 10.18 (s, 1H, CHO), 8.56 (s, 1H, H-2′), 8.32 (d, 1H, J = 7.80 Hz, H-6), 8.09 (dd, 1H, J = 2.85 and 1.30 Hz, H-1), 8.08–8.05 (m, 2H, H-4′ and H-6′), 7.93 (d, 1H, J = 7.80 Hz, H-9), 7.75 (t, 1H, J = 7.65 Hz, H-5′), 7.56 (t, 1H, J = 7.80 Hz, H-7), 7.53 (t, 1H, J = 7.80 Hz, H-8), 7.02 (dd, 1H, J = 3.90 and 1.30 Hz, H-3), 6.96 (dd, 1H, J = 3.90 and 2.85 Hz, H-2). MALDI-TOF MS m/z [M + H]+ Calcd for C18H13N2O: 273.103, Found: 273.127.
3–(8-Methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde (8 h)
Beige crystals, Yield: 87%, mp =135–138 °C; IR νmax (KBr)/cm−1 1705 (CHO); 1H NMR δ (300 MHz, CDCl3) 10.16 (s, 1H, CHO), 8.54 (dd, 1H, J = 1.50 and 1.50 Hz,H-2′), 8.30 (ddd, 1H, J = 7.65, 1.50 and 1.50 Hz, H-4′), 8.05 (ddd, 1H, J = 7.65, 1.50 and 1.50 Hz, H-6′), 8.02 (d, 1H, J = 9.15 Hz, H-6), 7.95 (dd, 1H, J = 2.70 and 1.50 Hz, H-1), 7.73 (t, 1H, J = 7.65 Hz, H-5′),7.32 (d, 1H, J = 2.70 Hz, H-9), 7.10(dd, 1H, J = 9.15 and 2.70 Hz, H-7), 6.99 (dd, 1H, J = 3.90 and 1.50 Hz, H-3), 6.95 (dd, 1H, J = 3.90 and 2.70 Hz, H-2), 4.01 (s, 3H, CH3O). MALDI-TOF MS m/z [M + H]+ Calcd for C19H15N2O2: 303.113, Found: 303.135.
General procedure for bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine and bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine 9a-p, bis{N-[4-(indolo[1,2-a]quinoxalin-6-yl)benzylidene]-3-aminopropyl}methylamine 9q and bis{N-[(4-phenylpyrrolo[1,2-a]quinoxalin-2-yl)methylidene]-3-aminopropyl}piperazine 9r
To a solution of diamine (2.7 mmol) in ethanol (15 mL) was added (pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde 8a–h or (indolo[1,2-a]quinoxalin-6-yl)benzaldehyde 8i or 4-phenylpyrrolo[1,2-a]quinoxaline-2-carboxaldehyde 8j (5.4 mmol). The reaction mixture was then heated under reflux for 5 h and then evaporated to dryness under reduced pressure. After cooling, the residue was extracted with dichloromethane (40 mL). The organic layer was dried over sodium sulfate and evaporated to dryness. The products were then used without further purification.
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}amine (9a)
Yellow crystals, Yield: 93%, mp =107–109 °C; 1H NMR δ (300 MHz, CDCl3) 8.31 (s, 2H, 2 HC = N), 8.03–7.80 (m, 8H, 2 H-2′, 2 H-6′, 2 H-6 and 2 H-1), 7.63 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.54–7.34 (m, 4H, 2 H-7 and 2 H-8), 6.96 (dd, 2H, J = 4.05 and 1.30 Hz, 2 H-3), 6.86 (dd, 2H, J = 4.05 and 2.70 Hz, 2 H-2), 4.09 (s, 1H, NH), 3.73–3.20 (m, 4H, 2 CH2), 2.88–2.31 (m, 4H, 2 CH2), 2.11–1.85 (m, 4H, 2 CH2).
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9b)
Yellow oil, Yield: 89%; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.05 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 8.02 (dd, 2H, J = 8.25 and 1.40 Hz, 2 H-6), 7.97 (dd, 2H, J = 2.70 and 1.30 Hz, 2 H-1), 7.90 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.85 (dd, 2H, J = 8.25 and 1.40 Hz, 2 H-9), 7.52 (ddd, 2H, J = 8.25, 8.25 and 1.40 Hz, 2 H-7), 7.44 (ddd, 2H, J = 8.25, 8.25 and 1.40 Hz, 2 H-8),6.96 (dd, 2H, J = 4.05 and 1.30 Hz, 2 H-3), 6.87 (dd, 2H, J = 4.05 and 2.70 Hz, 2 H-2), 3.74 (t, 4H,J = 7.10 Hz, 2 CH2), 2.53 (t, 4H, J = 7.10 Hz, 2 CH2), 2.32 (s, 3H, CH3), 1.96 (qt, 4H, J = 7.10 Hz, 2 CH2).
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9c)
Pale-yellow crystals, Yield: 78%, mp =177–180 °C; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.07 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 8.04 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-6), 7.97 (dd, 2H, J = 2.70 and 1.25 Hz, 2 H-1), 7.91 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.90 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-9), 7.55 (ddd, 2H, J = 8.10, 8.10 and 1.20 Hz, 2 H-7), 7.49 (ddd, 2H, J = 8.10, 8.10 and 1.20 Hz, 2 H-8), 7.01 (dd, 2H, J = 3.90 and 1.25 Hz, 2 H-3), 6.93 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.73 (t, 4H, J = 6.90 Hz, 2 CH2), 2.78–2.50 (m, 8H, 4 CH2 pip.), 2.54 (t, 4H, J = 6.90 Hz, 2 CH2), 1.99 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9d)
Yellow crystals, Yield: 96%, mp =67–69 °C; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.03 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.91 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.89 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.78(d, 2H, J = 9.00 Hz, 2 H-9), 7.49 (d, 2H, J = 2.90 Hz, 2 H-6), 7.14 (dd, 2H, J = 9.00 and 2.90 Hz, 2 H-8), 6.94 (dd, 2H, J = 4.20 and 1.20 Hz, 2 H-3), 6.85 (dd, 2H, J = 4.20 and 2.70 Hz, 2 H-2), 3.93 (s, 6H, 2 CH3O), 3.74 (t, 4H, J = 6.90 Hz, 2 CH2), 2.52 (t, 4H, J = 6.90 Hz, 2 CH2), 2.31 (s, 3H, CH3), 1.96 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9e)
Yellow crystals, Yield: 96%, mp =78–80 °C; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.07 (d, 4H, J = 8.20 Hz, 2 H-2′ and 2 H-6′), 7.97 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.91 (d, 4H, J = 8.20 Hz, 2 H-3′ and 2 H-5′), 7.83 (d, 2H, J = 9.00 Hz, 2 H-9), 7.53 (d, 2H, J = 3.00 Hz, 2 H-6), 7.17 (dd, 2H, J = 9.00 and 3.00 Hz, 2 H-8), 6.99 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.90 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.95 (s, 6H, 2 CH3O), 3.73 (t, 4H, J = 6.90 Hz, 2 CH2), 2.71–2.45 (m, 8H, 4 CH2 pip.), 2.50 (t, 4H, J = 6.90 Hz, 2 CH2), 1.97 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9f)
Yellow crystals, Yield: 96%, mp =61–63 °C; 1H NMR δ (300 MHz, CDCl3) 8.40 (s, 2H, 2 HC = N), 8.02 (d, 4H, J = 8.20 Hz, 2 H-2′ and 2 H-6′), 7.94 (d, 2H, J = 9.00 Hz, 2 H-6), 7.88(d, 4H, J = 8.20 Hz, 2 H-3′ and 2 H-5′), 7.87 (dd, 2H, J = 2.75 and 1.20 Hz, 2 H-1), 7.26 (d, 2H, J = 2.70 Hz, 2 H-9), 7.05 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.95 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.86 (dd, 2H, J = 3.90 and 2.75 Hz, 2 H-2), 3.99 (s, 6H, 2 CH3O), 3.73 (t, 4H, J = 6.90 Hz, 2 CH2), 2.51 (t, 4H, J = 6.90 Hz, 2 CH2), 2.31 (s, 3H, CH3), 1.96 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9 g)
Pale-yellow crystals, Yield: 97%, mp =218–221 °C; 1H NMR δ (300 MHz, CDCl3) 8.40 (s, 2H, 2 HC = N), 8.06 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.99 (d, 2H, J = 9.00 Hz, 2 H-6), 7.93 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.90 (d, 4H, J= 8.40 Hz, 2 H-3′ and 2 H-5′), 7.31 (d, 2H, J = 2.70 Hz, 2 H-9), 7.09 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.98 (dd, 2H, J = 4.05 and 1.20 Hz, 2 H-3), 6.93 (dd, 2H, J = 4.05 and 2.70 Hz, 2 H-2), 4.00 (s, 6H, 2 CH3O), 3.73 (t, 4H, J = 6.90 Hz, 2 CH2), 2.72–2.43 (m, 8H, 4 CH2 pip.), 2.53 (t, 4H, J = 6.90 Hz, 2 CH2), 1.98 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(9-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9 h)
Yellow crystals, Yield: 96%, mp =62–64 °C; 1H NMR δ (300 MHz, CDCl3) 8.82 (dd, 2H, J = 2.75 and 1.45 Hz, 2 H-1), 8.41 (s, 2H, 2 HC = N), 8.03 (d, 4H, J = 8.25 Hz, 2 H-2′ and 2 H-6′), 7.90 (d, 4H, J = 8.25 Hz, 2 H-3′ and 2 H-5′), 7.66 (dd, 2H, J = 8.15 and 1.20 Hz, 2 H-8), 7.36 (t, 2H, J = 8.15 Hz, 2 H-7), 7.05 (dd, 2H, J = 8.15 and 1.20 Hz, 2 H-6), 6.98 (dd, 2H, J = 4.10 and 1.45 Hz, 2 H-3), 6.83 (dd, 2H, J = 4.10 and 2.75 Hz, 2 H-2), 4.09 (s, 6H, 2 CH3O), 3.73 (t, 4H, J = 6.90 Hz, 2 CH2), 2.51 (t, 4H, J = 6.90 Hz, 2 CH2), 2.31 (s, 3H, CH3), 1.96 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(9-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9i)
Pale-yellow crystals, Yield: 94%, mp =79–82 °C; 1H NMR δ (300 MHz, CDCl3) 8.87 (dd, 2H, J = 2.70 and 1.35 Hz, 2 H-1), 8.40 (s, 2H, 2 HC = N), 8.05 (d, 4H, J = 8.25 Hz, 2 H-2′ and 2 H-6′), 7.90 (d, 4H, J = 8.25 Hz, 2 H-3′ and 2 H-5′), 7.69 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-8), 7.40 (t, 2H, J = 8.10 Hz, 2 H-7), 7.09 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-6), 7.00 (dd, 2H, J = 4.20 and 1.35 Hz, 2 H-3), 6.87 (dd, 2H, J = 4.20 and 2.70 Hz, 2 H-2), 4.12 (s, 6H, 2 CH3O), 3.72 (t, 4H, J = 6.90 Hz, 2 CH2), 2.68–2.44 (m, 8H, 4 CH2 pip.), 2.52 (t, 4H, J = 6.90 Hz, 2 CH2), 1.98 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4–(3-ethoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9j)
Yellow crystals, Yield: 97%, mp =37–39 °C; 1H NMR δ (300 MHz, CDCl3) 8.39 (s, 2H, 2 HC = N), 7.91 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 7.86 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.82(d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.76 (d, 2H, J = 3.00 Hz, 2 H-1), 7.73 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-9), 7.43 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-7), 7.35 (ddd, 2H, J = 7.80, 7.80and 1.50 Hz, 2 H-8), 6.48 (d, 2H, J = 3.00 Hz, 2 H-2), 3.92 (q, 4H, J = 6.90 Hz, 2 OCH2), 3.72 (t, 4H, J = 6.90 Hz, 2 CH2), 2.50 (t, 4H, J = 6.90 Hz, 2 CH2), 2.29 (s, 3H, CH3), 1.95 (qt, 4H, J = 6.90 Hz, 2 CH2), 1.17 (t, 6H, J = 6.90 Hz, 2 CH3).
Bis{N-[4–(3-ethoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9k)
Yellow oil, Yield: 97%; 1H NMR δ (300 MHz, CDCl3) 8.38 (s, 2H, 2 HC = N), 7.93 (dd, 2H, J= 7.80 and 1.50 Hz, 2 H-6), 7.88 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.83 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.80 (d, 2H, J = 3.00 Hz, 2 H-1), 7.78 (dd, 2H, J= 7.80 and 1.50 Hz, 2 H-9), 7.47 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-7), 7.41 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-8), 6.53 (d, 2H, J = 3.00 Hz, 2 H-2), 3.96 (q, 4H, J = 6.90 Hz, 2 OCH2), 3.71 (t, 4H, J = 7.05 Hz, 2 CH2), 2.68–2.37 (m, 8H, 4 CH2 pip.), 2.48 (t, 4H, J = 7.05 Hz, 2 CH2), 1.96 (qt, 4H, J = 7.05 Hz, 2 CH2), 1.19 (t, 6H, J = 6.90 Hz, 2 CH3).
Bis{N-[4–(2-phenylpyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9 l)
Orange crystals, Yield: 95%, mp =116–119 °C; 1H NMR δ (300 MHz, CDCl3) 8.43 (s, 2H, 2 HC = N), 8.29 (d, 2H, J = 1.50 Hz, 2 H-1), 8.11 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 8.07 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 7.94 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-9),7.92 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.74–7.70 (m, 4H, 2 H-2” and 2 H-6”), 7.58 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-7), 7.51 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-8), 7.43 (t, 4H, J = 7.20 Hz, 2 H-3” and 2 H-5”), 7.36–7.30 (m, 2H, 2 H-4”), 7.25 (d, 2H, J = 1.50 Hz, 2 H-3), 3.74(t, 4H, J = 6.90 Hz, 2 CH2), 2.67–2.40 (m, 8H, 4 CH2 pip.), 2.52 (t, 4H, J = 6.90 Hz, 2 CH2), 1.99 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9m)
Yellow oil, Yield: 97%; 1H NMR δ (300 MHz, CDCl3) 8.43 (s, 2H, 2 HC = N), 8.30 (t, 2H, J = 1.50 Hz, 2 H-2′), 8.09–8.03 (m, 4H, 2 H-6 and 2 H-4′), 8.02 (dd, 2H, J = 2.70 and 1.35 Hz, 2 H-1), 7.93–7.86 (m, 4H, 2 H-6′ and 2 H-9), 7.63–7.44 (m, 4H, 2 H-5′, 2 H-7 and 2 H-8),7.03 (dd, 2H, J = 3.90 and 1.35 Hz, 2 H-3), 6.90 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.71 (t, 4H, J = 6.90 Hz, 2 CH2), 2.47 (t, 4H, J = 6.90 Hz, 2 CH2), 2.29 (s, 3H, CH3), 1.93 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9n)
Yellow oil, Yield: 98%; 1H NMR δ (300 MHz, CDCl3) 8.42 (s, 2H, 2 HC = N), 8.32 (s, 2H, 2 H-2′), 8.09–8.03 (m, 6H, 2 H-6, 2 H-4′ and 2 H-1), 7.98–7.90 (m, 4H, 2 H-6′ and 2 H-9), 7.63–7.46 (m, 4H, 2 H-5′, 2 H-7 and 2 H-8), 7.03–7.01 (m, 2H, 2 H-3), 6.93–6.91 (m, 2H, 2 H-2), 3.71 (t, 4H, J = 6.90 Hz, 2 CH2), 2.65–2.42 (m, 8H, 4 CH2 pip.), 2.48 (t, 4H, J = 6.90 Hz, 2 CH2), 1.95 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}methylamine (9o)
Yellow oil, Yield: 98%; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.29 (dd, 2H, J = 1.50 and 1.50 Hz, 2 H-2′), 8.03 (ddd, 2H, J = 7.35, 1.50 and 1.50 Hz, 2 H-4′), 7.97 (d, 2H, J = 9.00 Hz, 2 H-6), 7.93 (ddd, 2H, J = 7.35, 1.50 and 1.50 Hz, 2 H-6′), 7.89 (dd, 2H, J = 2.85 and 1.35 Hz, 2 H-1), 7.56 (t, 2H, J = 7.65 Hz, 2 H-5′), 7.30 (d, 2H, J = 2.70 Hz, 2 H-9), 7.07 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.97 (dd, 2H, J = 3.90 and 1.35 Hz, 2 H-3), 6.89 (dd, 2H, J = 3.90 and 2.85 Hz, 2 H-2), 3.99 (s, 6H, 2 CH3O),3.71 (t, 4H, J = 7.05 Hz, 2 NCH2), 2.50 (t, 4H, J = 7.05 Hz, 2 NCH2), 2.30 (s, 3H, NCH3), 1.93 (qt, 4H, J = 7.05 Hz, 2 CH2).
Bis{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}piperazine (9p)
Orange oil, Yield: 55%; 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 2H, 2 HC = N), 8.30 (dd, 2H, J = 1.50 and 1.50 Hz, 2 H-2′), 8.05 (ddd, 2H, J = 7.40, 1.50 and 1.50 Hz, 2 H-4′), 8.01 (d, 2H, J = 9.00 Hz, 2 H-6), 7.94 (ddd, 2H, J = 7.40, 1.50 and 1.50 Hz, 2 H-6′), 7.91 (dd, 2H, J = 2.70 and 1.35 Hz, 2 H-1), 7.59 (t, 2H, J = 7.40 Hz, 2 H-5′), 7.29 (d, 2H, J = 2.70 Hz, 2 H-9), 7.08 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.98 (dd, 2H, J = 3.90 and 1.35 Hz, 2 H-3), 6.92 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 4.00 (s, 6H, 2 CH3O),3.70 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.62–2.42 (m, 8H, 4 NCH2 pip.), 2.48 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.95 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[4-(indolo[1,2-a]quinoxalin-6-yl)benzylidene]-3-aminopropyl}methylamine (9q)
Yellow crystals, Yield: 84%, mp =226–229 °C; 1H NMR δ (300 MHz, CDCl3) 8.57 (d, 2H, J = 8.00 Hz, 2 H-11), 8.53 (d, 2H, J = 8.10 Hz, 2 H-1), 8.44 (s, 2H, 2 HC = N), 8.15–8.08 (m, 6H, 2 H-2′, 2 H-6′ and 2 H-4), 7.97–7.92 (m, 6H, 2 H-3′, 2 H-5′ and 2 H-8), 7.70–7.57 (m, 4H, 2 H-2 and 2 H-3), 7.51–7.45 (m, 4H, 2 H-9 and 2 H-10), 7.26 (s, 2H, 2 H-7), 3.76 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.87–2.55 (m, 12H, 4 NCH2 pip. and 2 NCH2), 2.09 (qt, 4H, J = 6.90 Hz, 2 CH2).
Bis{N-[(4-phenylpyrrolo[1,2-a]quinoxalin-2-yl)methylidene]-3-aminopropyl}piperazine (9r)
Beige crystals, Yield: 60%, mp >250 °C; 1H NMR δ (300 MHz, CDCl3) 8.44 (s, 2H, 2 HC = N), 8.32 (d, 2H, J = 1.10 Hz, 2 H-1), 8.06 (dd, 2H, J =7.80 and 1.60 Hz, 2 H-6), 8.02–7.99 (m, 4H, 2 H-2′ and 2 H-6′), 7.90 (dd, 2H, J = 7.80 and 1.60 Hz, 2 H-9), 7.59–7.53 (m, 8H, 2 H-3′, 2 H-4′, 2 H-5′ and 2 H-7), 7.50 (ddd, 2H, J = 7.80, 7.80, 1.60 Hz, 2 H-8), 7.29 (d, 2H, J = 1.10 Hz, 2 H-3), 3.67 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.69–2.43 (m, 8H, 4 NCH2 pip.), 2.47 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.94 (qt, 4H, J = 6.90 Hz, 2 CH2).
General procedure for tris{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}amines 9s-t
To a solution of triamine (1.8 mmol) in ethanol (15 mL) was added (pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde 8i–j (5.4 mmol). The reaction mixture was then heated under reflux for 5 h and then evaporated to dryness under reduced pressure. After cooling, the residue was extracted with dichloromethane (40 mL). The organic layer was dried over sodium sulfate and evaporated to dryness. Products were then used without further purification.
Tris{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}amine (9s)
Orange oil, Yield: 82%; 1H NMR δ (300 MHz, CDCl3) 8.42 (s, 2H, 3 HC = N), 7.99 (d, 6H, J = 8.40 Hz, 3 H-2′ and 3 H-6′), 7.88 (d, 6H, J = 8.40 Hz, 3 H-3′ and 3 H-5′), 7.86 (dd, 3H, J = 2.70 and 1.20 Hz, 3 H-1), 7.73 (d, 3H, J = 9.00 Hz, 3 H-9), 7.45 (d, 3H, J = 2.80 Hz, 3 H-6), 7.11 (dd, 3H, J = 9.00 and 2.80 Hz, 3 H-8), 6.90 (dd, 3H, J = 4.05 and 1.20 Hz, 3 H-3), 6.80 (dd, 3H, J = 4.05 and 2.70 Hz, 3 H-2), 3.91 (s, 9H, 3 CH3O), 3.76 (t, 6H, J = 6.90 Hz, 3 CH2), 2.64 (t, 6H, J = 6.90 Hz, 3NCH2), 1.96 (qt, 6H, J = 6.90 Hz, 3 CH2).
Tris{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylidene]-3-aminopropyl}amine (9t)
Orange oil, Yield: 71%; 1H NMR δ (300 MHz, CDCl3) 8.40 (s, 3H, 3 HC = N), 8.27 (dd, 3H, J = 1.50 and 1.50 Hz, 3 H-2′), 7.99 (ddd, 3H, J = 7.80, 1.50 and 1.50 Hz, 3 H-4′), 7.98(d, 3H, J = 9.00 Hz, 3H-6), 7.92 (ddd, 3H, J = 7.80, 1.50 and 1.50 Hz, 3H-6′), 7.86 (dd, 3H, J = 2.85 and 1.35 Hz, 3 H-1), 7.52 (t, 3H, J = 7.80 Hz, 3 H-5′), 7.25 (d, 3H, J = 2.70 Hz, 3 H-9), 7.04 (dd, 3H, J = 9.00 and 2.70 Hz, 3 H-7), 6.94 (dd, 3H, J = 4.05 and 1.35 Hz, 3 H-3), 6.87 (dd, 3H, J = 4.05 and 2.85 Hz, 3 H-2), 3.98 (s, 9H, 3 CH3O),3.71 (t, 6H, J = 6.90 Hz, 3 NCH2), 2.61 (t, 6H, J = 6.90 Hz, 3 NCH2), 1.92 (qt, 6H, J = 6.90 Hz, 3 CH2).
N, N′- [oxybis(2, 1-ethanediyloxy-2, 1-ethanediyl)] bis-3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylimine 9u
To a solution of 1,11-diamino-3,6,9-trioxaundecane (0.56 mmol) in ethanol (12 mL) was added 3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzaldehyde 8j (1.12 mmol). The reaction mixture was then heated under reflux for 5 h and then evaporated to dryness under reduced pressure. After cooling, the residue was extracted with dichloromethane (35 mL). The organic layer was dried over sodium sulfate and evaporated to dryness. Product 9u was then used without further purification. Yellow oil, Yield: 97%; 1H NMR δ (300 MHz, CDCl3) 8.39 (s, 2H, 2 HC = N), 8.29 (dd, 2H, J = 1.50 and 1.50 Hz, 2 H-2′), 8.05–7.87 (m, H, 2 H-4′, 2 H-6, 2 H-6′ and 2 H-1), 7.59–7.55 (m, 2H, 2 H-5′),7.27 (d, 2H, J = 2.70 Hz, 2 H-9), 7.06 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.96 (m, 2H, 2 H-3), 6.88 (m, 2H, 2 H-2), 3.95 (s, 6H, 2 CH3O), 3.81–3.79 (m, 8H, 4OCH2), 3.64–3.62 (m, 8H, 4 OCH2).
General procedure for bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine and bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine 1a-p, bis{N-[4-(indolo[1,2-a]quinoxalin-6-yl)benzyl]-3-aminopropyl} piperazine 1q and bis{N-[(4-phenylpyrrolo[1,2-a]quinoxalin-2-yl)methyl]-3-aminopropyl}piperazine 1r and tris{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine 1s-t and N,N′-[oxybis(2,1-ethanediyloxy-2,1-ethanediyl)]bis-3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylamine 1u
To a solution of compound 9 (1.26 mmol) in methanol (40 mL) was added portion-wise at 0 °C sodium borohydride (10.1 mmol; 8 equiv.). The reaction mixture was then heated under reflux for 4 h and then evaporated to dryness under reduced pressure. After cooling, the residue was triturated in water and extracted with dichloromethane (85 mL). The organic layer was separated, dried over sodium sulfate and evaporated to dryness. The residue were then purified by column chromatography on silica gel using dichloromethane/methanol (90/10, v/v) as eluent to give the pure product 1.
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine (1a)
Yellow oil, Yield: 91%; 1H NMR δ (300 MHz, CDCl3) 8.02 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 7.96 (d, 4H, J = 8.20 Hz, 2 H-2′ and 2 H-6′), 7.93 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.82 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-9), 7.48 (d, 4H, J = 8.20 Hz, 2 H-3′ and 2 H-5′), 7.47–7.39 (m, 4H, 2 H-7 and 2 H-8), 6.98 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.86 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.89 (s, 4H, 2 NCH2), 3.73 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.71(t, 4H, J = 6.90 Hz, 2 NCH2), 1.84 (bs, 3H, 3 NH), 1.77 (qt, 4H, J = 6.90 Hz, 2 CH2) 13C NMR δ (100 MHz, CDCl3) 155.3 (C-4), 141.8 (C-3a), 139.0 (C-5a), 138.7 (C-4′), 137.6 (C-1′), 131.5 (C-8), 130.3 (C-2′ and C-6′), 130.1 (C-3′ and C-5′), 128.8 (C-7), 128.5 (C-9a), 126.6 (C-6), 116.0 (C-1), 115.4 (C-2), 115.0 (C-9), 110.0 (C-3), 54.5 (NCH2), 50.0 (NCH2), 48.9 (NCH2), 27.7 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C42H42N7: 644.350, Found: 644.342.
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1b)
Orange oil, Yield: 94%; 1H NMR δ (300 MHz, CDCl3) 8.00 (dd, 2H, J = 8.00 and 1.35 Hz, 2 H-6), 7.96 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.95 (dd, 2H, J = 2.70 and 1.30 Hz, 2 H-1), 7.83 (dd, 2H, J = 8.00 and 1.35 Hz, 2 H-9), 7.49 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.47–7.39 (m, 4H, 2 H-7 and 2 H-8), 6.97 (dd, 2H, J = 3.90 and 1.30 Hz, 2 H-3), 6.86 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.88 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 7.10 Hz, 2 NCH2), 2.43 (t, 4H, J = 7.10 Hz, 2 NCH2), 2.24 (s, 3H, NCH3), 1.73 (qt, 4H, J = 7.10 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.2 (C-4), 140.2 (C-3a), 139.3 (C-5a), 139.2 (C-4′), 137.5 (C-1′), 131.5 (C-8), 130.4 (C-2′ and C-6′), 130.3 (C-3′ and C-5′), 128.8 (C-7), 128.4 (C-9a), 126.6 (C-6), 116.0 (C-1), 115.4 (C-2), 115.0 (C-9), 110.0 (C-3), 57.3 (NCH2), 54.1 (NCH2), 48.6 (NCH2), 43.4 (NCH3), 27.0 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C43H44N7: 658.366, Found: 658.362.
Bis{N-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1c)
Colorless oil, Yield: 61%; 1H NMR δ (300 MHz, CDCl3) 8.04 (dd, 2H, J = 8.25 and 1.35 Hz, 2 H-6), 8.00 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.98 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.89 (dd, 2H, J = 8.25 and 1.35 Hz, 2 H-9), 7.53 (ddd, 2H, J = 8.25, 8.25 and 1.35 Hz, 2 H-7), 7.50 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.45 (ddd, 2H, J = 8.25, 8.25 and 1.35 Hz, 2 H-8),7.01 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.91 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.91 (s, 4H, 2 NCH2), 2.74 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.58–2.39 (m, 8H, 4 NCH2 pip.), 2.44 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.76 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.2 (C-4), 140.8 (C-3a), 139.3 (C-5a), 139.2 (C-4′), 137.6 (C-1′), 131.6 (C-8), 130.3 (C-2′ and C-6′), 130.2 (C-3′ and C-5′), 128.9 (C-7), 128.5 (C-9a), 126.7 (C-6), 116.1 (C-1), 115.4 (C-2), 115.0 (C-9), 110.0 (C-3), 58.5 (NCH2), 54.4 (NCH2), 54.2 (NCH2), 49.5 (NCH2), 26.5 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C46H49N8: 713.408, Found: 713.412.
Bis{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1d)
Pale-yellow crystals, Yield: 88%, mp =65–67 °C; 1H NMR δ (300 MHz, CDCl3) 7.95 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.90 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.76 (d, 2H, J = 9.00 Hz, 2 H-9), 7.49 (d, 2H, J = 2.70 Hz, 2 H-6), 7.48 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.11 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-8), 6.96 (dd, 2H, J = 4.05 and 1.20 Hz, 2 H-3), 6.84 (dd, 2H, J = 4.05 and 2.70 Hz, 2 H-2), 3.91 (s, 6H, 2 CH3O), 3.89 (s, 4H, 2 NCH2), 2.72 (t, 4H, J = 7.05 Hz, 2 NCH2), 2.43 (t, 4H, J = 7.05 Hz, 2 NCH2), 1.73 (qt, 4H, J = 7.05 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 158.5 (C-7), 155.9 (C-4), 143.7 (C-5a), 138.7 (C-3a), 138.5 (C-4′), 130.1 (C-2′ and C-6′), 129.6 (C-3′ and C-5′), 126.4 (C-1′), 122.8 (C-9a), 117.9 (C-9), 115.9 (C-8), 115.6 (C-1), 115.0 (C-2), 112.6 (C-3), 109.7 (C-6), 57.6 (NCH2), 57.1 (OCH3), 55.2 (NCH2), 49.5 (NCH2), 43.7 (NCH3), 29.0 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C45H48N7O2: 718.387, Found: 718.399.
Bis{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1e)
Pale-yellow crystals, Yield: 93%, mp =51–53 °C; 1H NMR δ (300 MHz, CDCl3) 7.97 (d, 4H, J = 8.20 Hz, 2 H-2′ and 2 H-6′), 7.93 (dd, 2H, J= 2.75 and 1.25 Hz, 2 H-1), 7.79 (d, 2H, J = 9.00 Hz, 2 H-9), 7.52 (d, 2H, J = 2.70 Hz, 2 H-6), 7.49 (d, 4H, J = 8.20 Hz, 2 H-3′ and 2 H-5′), 7.13 (dd, 2H, J= 9.00 and 2.70 Hz, 2 H-8), 6.98 (dd, 2H, J = 3.90 and 1.25 Hz, 2 H-3), 6.87 (dd, 2H, J = 3.90 and 2.75 Hz, 2 H-2), 3.93 (s, 6H, 2 CH3O), 3.89 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.60–2.35 (m, 8H, 4 NCH2 pip.), 2.44 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.76 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 158.5 (C-7), 155.8 (C-4), 142.8 (C-5a), 138.8 (C-3a), 138.7 (C-4′), 130.1 (C-2′ and C-6′), 129.8 (C-3′ and C-5′), 126.4 (C-1′), 122.8 (C-9a), 118.0 (C-9), 116.0 (C-8), 115.7 (C-1), 115.1 (C-2), 112.7 (C-3), 109.7 (C-6), 58.5 (NCH2), 57.1 (OCH3), 54.8 (NCH2), 54.6 (NCH2), 49.5 (NCH2), 27.7 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C48H53N8O2: 773.429, Found: 773.428.
Bis{N-[4–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1f)
Yellow crystals, Yield: 71%, mp =71–73 °C; 1H NMR δ (300 MHz, CDCl3) 7.95 (d, 2H, J = 9.00 Hz, 2 H-6), 7.93 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.86 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.47 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.26 (d, 2H, J = 2.70 Hz, 2 H-9), 7.04 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.95 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.87 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.96 (s, 6H, 2 CH3O), 3.88 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.43 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.25 (s, 3H, NCH3), 1.73 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 160.4 (C-8), 153.0 (C-4), 143.3 (C-3a), 138.6 (C-5a), 132.7 (C-6), 132.0 (C-4′), 130.0 (C-2′ and C-6′), 129.6 (C-3′ and C-5′), 126.2 (C-1′), 126.6 (C-9a), 115.4 (C-7), 115.3 (C-1), 114.1 (C-2), 109.5 (C-3), 98.8 (C-9), 57.6 (NCH2), 57.1 (OCH3), 55.2 (NCH2), 49.3 (NCH2), 43.7 (NCH3), 29.0 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C45H48N7O2: 718.387, Found: 718.399.
Bis{N-[4–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1 g)
Yellow oil, Yield: 97%; 1H NMR δ (300 MHz, CDCl3) 7.96 (d, 2H, J = 9.00 Hz, 2 H-6), 7.94 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.89 (dd, 2H, J = 2.75 and 1.20 Hz, 2 H-1), 7.47 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.29 (d, 2H, J = 2.70 Hz, 2 H-9), 7.06 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.97 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.89 (dd, 2H, J = 3.90 and 2.75 Hz, 2 H-2), 3.97 (s, 6H, 2 CH3O), 3.88 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.57–2.34 (m, 8H, 4 NCH2 pip.), 2.43 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.74 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 160.4 (C-8), 153.0 (C-4), 143.3 (C-3a), 138.6 (C-5a), 132.7 (C-6), 132.0 (C-4′), 130.0 (C-2′ and C-6′), 129.6 (C-3′ and C-5′), 126.2 (C-1′), 126.7 (C-9a), 115.5 (C-7), 115.4 (C-1), 114.2 (C-2), 109.5 (C-3), 98.9 (C-9), 58.4 (NCH2), 57.2 (OCH3), 55.1 (NCH2), 54.7 (NCH2), 49.4 (NCH2), 28.3 (CH2). MALDI-TOF MS m/z [M + H]+Calcd for C48H53N8O2: 773.429, Found: 773.446.
Bis{N-[4–(9-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1 h)
Orange-pale oil, Yield: 92%; 1H NMR δ (300 MHz, CDCl3) 8.83 (dd, 2H, J = 2.70 and 1.35 Hz, 2 H-1), 7.95 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.66 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-8), 7.48 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.37 (t, 2H, J = 8.10 Hz, 2 H-7), 7.04 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-6), 7.00 (dd, 2H, J = 4.10 and 1.35 Hz, 2 H-3), 6.82 (dd, 2H, J = 4.10 and 2.70 Hz, 2 H-2), 4.08 (s, 6H, 2 CH3O), 3.89 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.43 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.25 (s, 3H, NCH3), 1.73 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.9 (C-9), 151.1 (C-4), 143.5 (C-3a), 139.8 (C-5a), 138.5 (C-4′), 130.1 (C-2′ and C-6′), 129.6 (C-3′ and C-5′), 127.3 (C-9a), 125.7 (C-7), 123.9 (C-6), 123.7 (C-1), 119.8 (C-1′), 114.3 (C-2), 110.2 (C-8), 109.4 (C-3), 57.6 (OCH3), 57.5 (NCH2), 55.2 (NCH2), 49.3 (NCH2), 43.7 (NCH3), 28.9 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C45H48N7O2: 718.387, Found: 718.416.
Bis{N-[4–(9-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1i)
Pale-yellow crystals, Yield: 94%, mp =66–68 °C; 1H NMR δ (300 MHz, CDCl3) 8.84 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.96 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.67 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-8), 7.48 (d, 4H, J = 8.10 Hz, 2 H-3′ and 2 H-5′), 7.37 (t, 2H, J = 8.10 Hz, 2 H-7), 7.05 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-6), 7.00 (dd, 2H, J = 4.00 and 1.20 Hz, 2 H-3), 6.85 (dd, 2H, J = 4.00 and 2.70 Hz, 2 H-2), 4.09 (s, 6H, 2 CH3O), 3.89 (s, 4H, 2 NCH2), 2.71 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.57–2.31 (m, 8H, 4 NCH2 pip.), 2.43 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.74 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.9 (C-9), 151.1 (C-4), 143.5 (C-3a), 139.8 (C-5a), 138.5 (C-4′), 130.1 (C-2′ and C-6′), 129.6 (C-3′ and C-5′), 127.3 (C-9a), 125.7 (C-7), 123.7 (C-6), 123.5 (C-1), 119.8 (C-1′), 114.3 (C-2), 110.2 (C-8), 109.4 (C-3), 58.4 (NCH2), 57.6 (OCH3), 55.1 (NCH2), 54.7 (NCH2), 49.4 (NCH2), 28.3 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C48H53N8O2: 773.429, Found: 773.446.
Bis{N-[4–(3-ethoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1j)
Yellow oil, Yield: 85%; 1H NMR δ (300 MHz, CDCl3) 7.91 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 7.79–7.74 (m, 8H, 2 H-2′, 2 H-6′, 2 H-1 and 2 H-9), 7.43–7.34 (m, 4H, 2 H-3′, 2 H-5′, 2 H-7 and 2 H-8), 6.48 (d, 2H, J = 3.00 Hz, 2 H-2), 3.92 (q, 4H, J = 6.90 Hz, 2 OCH2), 3.89 (s, 4H, 2 NCH2), 2.68 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.43 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.24 (s, 3H, NCH3), 1.72 (qt, 4H, J = 6.90 Hz, 2 CH2), 1.16 (t, 6H, J = 6.90 Hz, 2 CH3). 13C NMR δ (100 MHz, CDCl3) 156.2(C-4), 145.7 (C-3a), 143.6 (C-5a), 138.2 (C-4′), 137.5 (C-1′), 131.1 (C-6), 130.9 (C-2′ and C-6′), 128.5 (C-3′ and C-5′), 128.3 (C-8), 128.0 (C-9a), 126.4 (C-7), 114.0 (C-1), 113.4 (C-3),113.1 (C-9), 102.2 (C-2), 68.7 (OCH2), 57.6 (NCH2), 55.2 (NCH2), 49.0 (NCH2), 43.6 (NCH3), 28.8 (CH2), 16.1 (CH3). MALDI-TOF MS m/z [M + H]+ Calcd for C47H52N7O2: 746.418, Found: 746.472.
Bis{N-[4–(3-ethoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1k)
Yellow oil, Yield: 76%; 1H NMR δ (300 MHz, CDCl3) 7.93 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 7.80–7.75 (m, 8H, 2 H-2′, 2 H-6′, 2 H-1 and 2 H-9), 7.47 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-7), 7.42 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.36 (ddd, 2H, J = 7.80, 7.80 and 1.50 Hz, 2 H-8), 6.51 (d, 2H, J = 3.00 Hz, 2 H-2), 3.94 (q, 4H, J = 6.90 Hz, 2 OCH2), 3.88 (s, 4H, 2 NCH2), 2.68 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.56–2.31 (m, 8H, 4 NCH2 pip.), 2.42 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.73 (qt, 4H, J = 6.90 Hz, 2 CH2), 1.17 (t, 6H, J = 6.90 Hz, 2 CH3). 13C NMR δ (100 MHz, CDCl3) 156.2 (C-4), 145.7 (C-3a), 142.6 (C-5a), 138.2 (C-4′), 137.5 (C-1′), 131.2 (C-6), 130.9 (C-2′ and C-6′), 128.5 (C-3′ and C-5′), 128.4 (C-8), 128.0 (C-9a), 126.4 (C-7), 114.0 (C-1), 113.5 (C-3), 113.2 (C-9), 102.3 (C-2), 68.8 (OCH2), 58.4 (NCH2), 55.1 (NCH2), 54.7 (NCH2), 49.1 (NCH2), 28.3 (CH2), 16.1 (CH3). MALDI-TOF MS m/z [M + H]+ Calcd for C50H57N8O2: 801.460, Found: 801.505.
Bis{N-[4–(2-phenylpyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1 l)
Pale-yellow crystals, Yield: 79%, mp =81–83 °C; 1H NMR δ (300 MHz, CDCl3) 8.27 (d, 2H, J = 1.20 Hz, 2 H-1), 8.07 (dd, 2H, J = 7.80 and 1.30 Hz, 2 H-6), 8.03 (d, 4H, J = 8.10 Hz, 2 H-2′ and 2 H-6′), 7.94 (dd, 2H, J = 7.80 and 1.30 Hz, 2 H-9),7.71 (d, 4H, J = 7.50 Hz, 2 H-2” and 2 H-6”), 7.58–7.41 (m, 8H, 2 H-3′, 2 H-5′, 2 H-7 and 2 H-8), 7.45 (t, 4H, J = 7.80 Hz, 2 H-3” and 2 H-5”),7.35–7.30 (m, 2H, 2 H-4”), 7.26 (d, 2H, J = 1.20 Hz, 2 H-3), 2.73(t, 4H, J = 6.90 Hz, 2 NCH2), 2.65–2.38 (m, 8H, 4 NCH2 pip.), 2.45 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.76 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 154.6 (C-4), 146.6 (C-3a), 140.0 (C-5a), 137.6 (C-4′), 135.4 (C-1”), 131.7 (C-8), 131.2 (C-1′), 130.6 (C-3” and C-5”), 130.4 (C-2′ and C-6′), 129.7 (C-7), 129.2 (C-4”), 128.6 (C-3” and C-5”), 128.1 (C-9a), 127.6 (C-3′ and C-5′), 126.9 (C-6), 118.7 (C-2), 115.0 (C-9), 112.8 (C-1), 107.2 (C-3), 58.5 (NCH2), 54.0 (NCH2), 53.2 (NCH2), 49.5 (NCH3), 31.1 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C58H57N8: 865.470, Found: 865.454.
Bis{N-[3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1m)
Pale-yellow oil, Yield: 83%; 1H NMR δ (300 MHz, CDCl3) 8.04 (dd, J = 7.80 and 1.80 Hz, 2 H-6), 7.99 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.94 (t, 2H, J = 1.50 Hz, 2 H-2′), 7.89–7.85 (m, 4H, 2 H-6′ and 2 H-9), 7.55–7.42 (m, 8H, 2 H-4′, 2 H-5′, 2 H-7 and 2 H-8), 6.99 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.88 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.88 (s, 4H, 2 NCH2), 2.70 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.40 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.22 (s, 3H, NCH3), 1.72 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.8 (C-4), 142.2 (C-3a), 139.8 (C-5a), 137.6 (C-3′), 131.5 (C-8), 131.0 (C-7), 130.0 (C-2′), 129.7 (C-5′), 128.8 (C-4′),128.6 (C-6′), 128.5 (C-1′), 126.7 (C-9a), 126.6 (C-6), 116.0 (C-1), 115.4 (C-2), 115.0 (C-9), 110.1 (C-3), 57.4 (NCH2), 55.3 (NCH2), 49.4 (NCH2), 43.6 (NCH3), 28.8 (CH2).MALDI-TOF MS m/z [M + H]+ Calcd for C43H44N7: 658.366, Found: 658.395.
Bis{N-[3-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1n)
Pale-yellow oil, Yield: 96%; 1H NMR δ (300 MHz, CDCl3) 8.05 (dd, J = 7.80 and 1.80 Hz, 2 H-6), 8.01 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.96 (t, 2H, J = 1.50 Hz, 2 H-2′), 7.92–7.86 (m, 4H, 2 H-6′ and 2 H-9), 7.56–7.43 (m, 8H, 2 H-4′, 2 H-5′, 2 H-7 and 2 H-8), 7.00 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.90 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.91 (s, 4H, 2 NCH2), 2.69 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.58–2.34 (m, 8H, 4 NCH2 pip.), 2.34 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.95 (bs, 2H, 2 NH), 1.68 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.8 (C-4), 142.2 (C-3a), 139.9 (C-5a), 137.6 (C-3′), 131.5 (C-8), 131.0 (C-7), 130.0 (C-2′), 129.7 (C-5′), 128.9 (C-4′), 128.6 (C-6′), 128.5 (C-1′), 126.8 (C-9a), 126.7 (C-6), 116.0 (C-1), 115.4 (C-2), 115.0 (C-9), 110.1 (C-3), 58.4 (NCH2), 55.2 (NCH2), 54.6 (NCH2), 49.5 (NCH2), 28.2 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C46H49N8: 713.408, Found: 713.447.
Bis{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}methylamine (1o)
Yellow oil, Yield: 97%; 1H NMR δ (300 MHz, CDCl3) 7.95 (d, 2H, J = 8.70 Hz, 2 H-6), 7.90 (dd, 2H, J = 1.50 and 1.50 Hz, 2 H-2′), 7.87–7.83 (m, 2H, 2 H-4′), 7.85 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1), 7.47–7.43 (m, 4H, 2 H-5′ and 2 H-5′), 7.25 (d, 2H, J = 2.70 Hz, 2 H-9), 7.03 (dd, 2H, J = 8.85 and 2.70 Hz, 2 H-7), 6.94 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.86 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.94 (s, 6H, 2 CH3O),3.86 (s, 4H, 2 NCH2), 2.68 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.39 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.20 (s, 3H, NCH3), 1.69 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 160.4 (C-8), 153.1 (C-4), 141.8 (C-3a), 140.0 (C-5a), 132.7 (C-6), 131.9 (C-3′), 130.8 (C-2′), 129.9 (C-5′), 129.7 (C-4′), 129.2 (C-1′), 128.6 (C-6′), 126.6 (C-9a), 115.5 (C-7), 115.4 (C-1), 114.2 (C-2), 109.5 (C-3), 98.8 (C-9), 57.5 (NCH2), 57.1 (OCH3), 55.2 (NCH2), 49.3 (NCH2), 43.6 (NCH3), 28.6 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C45H48N7O2: 718.387, Found: 718.433.
Bis{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazine (1p)
Yellow oil, Yield: 75%; 1H NMR δ (300 MHz, CDCl3) 7.97 (d, 2H, J = 9.00 Hz, 2 H-6), 7.93 (dd, 2H, J = 1.50 and 1.50 Hz, 2 H-2′), 7.88 (dd, 2H, J = 2.70 and 1.20 Hz, 2 H-1),7.88–7.84 (m, 2H, 2 H-4′), 7.50–7.46 (m, 4H, 2 H-5′ and 2 H-5′), 7.28 (d, 2H, J = 2.70 Hz, 2 H-9), 7.06 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.96 (dd, 2H, J = 4.00 and 1.20 Hz, 2 H-3), 6.88 (dd, 2H, J = 4.00 and 2.70 Hz, 2 H-2), 3.96 (s, 6H, 2 CH3O),3.90 (s, 4H, 2 NCH2), 2.69 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.53–2.34 (m, 8H, 4 NCH2 pip.), 2.33 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.20 (bs, 2H, 2 NH), 1.68 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 160.5 (C-8), 153.2 (C-4), 142.0 (C-3a), 140.0 (C-5a), 132.7 (C-6), 132.0 (C-3′), 130.7 (C-2′), 130.0 (C-5′), 129.6 (C-4′), 129.2 (C-1′), 128.6 (C-6′), 126.7 (C-9a), 115.5 (C-7), 115.4 (C-1), 114.2 (C-2), 109.5 (C-3), 98.9 (C-9), 58.4 (NCH2), 57.2 (OCH3), 55.2 (NCH2), 54.6 (NCH2), 49.5 (NCH2), 28.1 (CH2). MALDI-TOF MS m/z [M + H]+Calcd for C48H53N8O2: 773.429, Found: 773.464.
Bis{N-[4-(indolo[1,2-a]quinoxalin-6-yl)benzyl]-3-aminopropyl}piperazine (1q)
Yellow oil, Yield: 86%; 1H NMR δ (300 MHz, CDCl3) 8.55 (dd, 2H, J = 8.10 and 1.20 Hz, 2 H-11), 8.51 (dd, 2H, J = 8.70 and 0.90 Hz, 2 H-1), 8.09 (dd, 2H, J = 8.00 and 1.65 Hz, 2 H-4), 8.00 (d, 4H, J = 8.40 Hz, 2 H-2′ and 2 H-6′), 7.95 (d, 2H, J = 7.80 Hz, 2 H-8), 7.67–7.60 (m, 4H,2 H-2 and 2 H-3), 7.53 (d, 4H, J = 8.40 Hz, 2 H-3′ and 2 H-5′), 7.48–7.43 (m, 4H, 2 H-9 and 2 H-10), 7.27 (s, 2H, 2 H-7), 3.92 (s, 4H, 2 NCH2), 2.75 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.63–2.41 (m, 8H, 4 NCH2 pip.), 2.46 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.96 (bs, 2H, 2 NH), 1.77 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 157.3 (C-6), 142.8 (C-6a), 138.6 (C-11a), 137.7 (C-4a), 134.4 (C-4′), 131.9 (C-3), 131.6 (C-1′), 130.5 (C-8a), 130.2 (C-2′ and C-6′), 129.9 (C-3′ and C-5′), 129.7 (C-2), 125.8 (C-4), 125.6 (C-1), 124.1 (C-9), 124.0 (C-10), 116.0 (C-8 and C-11), 103.8 (C-7), 58.5 (NCH2), 54.6 (NCH2), 49.5 (NCH2), 27.6 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C54H53N8: 813.439, Found: 813.432.
Bis{N-[(4-phenylpyrrolo[1,2-a]quinoxalin-2-yl)methyl]-3-aminopropyl}piperazine (1r)
Pale-yellow crystals, Yield: 70%, mp =62–64 °C; 1H NMR δ (300 MHz, CDCl3) 8.04 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-6), 8.03–7.97 (m, 4H, 2 2 H-2′ and 2 H-6′), 7.98 (d, 2H, J = 1.10 Hz, 2 H-1), 7.85 (dd, 2H, J = 7.80 and 1.50 Hz, 2 H-9), 7.57–7.53 (m, 8H, 2 H-3′, 2 H-4′, 2 H-5′ and 2 H-7), 7.47 (ddd, 2H, J = 7.80, 7.80, 1.50 Hz, 2 H-8), 6.94 (d, 2H, J = 1.10 Hz, 2 H-3), 3.95 (s, 4H, 2 NCH2), 2.75 (t, 4H, J = 6.90 Hz, 2 NCH2), 2.61–2.35 (m, 8H, 4 NCH2 pip.), 2.40 (t, 4H, J = 6.90 Hz, 2 NCH2), 1.93 (bs, 2H, 2 NH), 1.72 (qt, 4H, J = 6.90 Hz, 2 CH2). 13C NMR δ (100 MHz, CDCl3) 155.2 (C-4), 139.5 (C-3a), 137.5 (C-5a), 131.6 (C-7), 131.4 (C-8), 130.1 (C-3′ and C-5′), 130.0 (C-2′ and C-6′), 129.2 (C-6), 128.0 (C-1′), 127.0 (C-9a), 126.7 (C-4′), 115.3 (C-9), 114.2 (C-2), 109.9 (C-1), 109.8 (C-3), 58.2 (NCH2), 54.3 (NCH2), 54.1 (NCH2), 49.3 (NCH2), 46.4 (NCH2), 24.8 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C46H49N8: 713.408, Found: 713.412.
Tris{N-[4–(7-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine (1s)
Yellow oil, Yield: 94%; 1H NMR δ (300 MHz, CDCl3) 7.94 (d, 6H, J = 8.40 Hz, 3 H-2′ and 3 H-6′), 7.87 (dd, 3H, J = 2.70 and 1.35 Hz, 3 H-1), 7.74 (d, 3H, J = 9.00 Hz, 3 H-9), 7.48 (d, 6H, J = 8.40 Hz, 3 H-3′ and 3 H-5′), 7.47 (d, 3H, J = 2.70 Hz, 3 H-6), 7.09 (dd, 3H, J = 9.00 and 2.70 Hz, 3 H-8), 6.93 (dd, 3H, J = 4.05 and 1.35 Hz, 3 H-3), 6.81 (dd, 3H, J= 4.05 and 2.70 Hz, 3 H-2), 3.90 (s, 9H, 3 CH3O), 3.88 (s, 6H, 3 NCH2), 2.70 (t, 6H, J = 6.90 Hz, 3 CH2), 2.52 (t, 6H, J = 6.90 Hz, 3 CH2), 1.71 (qt, 6H, J = 6.90 Hz, 6 CH2). 13C NMR δ (100 MHz, CDCl3) 158.5 (C-7), 155.8 (C-4), 143.1 (C-5a), 138.76 (C-3a and C-4′), 130.1 (C-2′ and C-6′), 129.8 (C-3′ and C-5′), 126.4 (C-1′), 122.8 (C-9a), 117.9 (C-9), 115.9 (C-8), 115.6 (C-1), 115.0 (C-2), 112.6 (C-3), 109.7 (C-6), 57.1 (OCH3), 55.1 (NCH2), 53.7 (NCH2), 49.2 (NCH2), 28.4 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C66H67N10O3: 1047.540, Found: 1047.613.
Tris{N-[3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine (1t)
Orange oil, Yield: 79%; 1H NMR δ (300 MHz, CDCl3) 7.94 (d, 3H, J = 9.00 Hz, 3 H-6), 7.89 (dd, 3H, J = 1.50 and 1.50 Hz, 3 H-2′), 7.86–7.80 (m, 6H, 3 H-4′and 3 H-1), 7.45–7.42 (m, 6H, 3 H-6′ and 3 H-5′), 7.23 (d, 3H, J = 2.70 Hz, 3 H-9), 7.03 (dd, 3H, J = 9.00 and 2.70 Hz, 3 H-7), 6.93 (dd, 3H, J = 3.90 and 1.20 Hz, 3 H-3), 6.85 (dd, 3H, J = 3.90 and 2.70 Hz, 3 H-2), 3.96 (s, 9H, 3 CH3O), 3.83 (s, 6H, 3NCH2), 2.65 (t, 6H, J = 6.90 Hz, 3 NCH2), 2.47 (t, 6H, J = 6.90 Hz, 3 NCH2), 1.63 (qt, 6H, J = 6.90 Hz, 3 CH2). 13C NMR δ (100 MHz, CDCl3) 160.4 (C-8), 153.1 (C-4), 141.9 (C-3a), 140.0 (C-5a), 132.7 (C-6), 132.0 (C-3′), 130.8 (C-2′), 129.9 (C-5′), 129.7 (C-4′), 129.2 (C-1′), 128.6 (C-6′), 126.6 (C-9a), 115.4 (C-7), 115.3 (C-1), 114.1 (C-2), 109.5 (C-3), 98.7 (C-9), 57.1 (OCH3), 55.3 (NCH2), 53.7 (NCH2), 49.3 (NCH2), 28.4 (CH2). MALDI-TOF MS m/z [M + H]+ Calcd for C66H67N10O3: 1047.540, Found: 1047.598.
N,N′-[oxybis(2,1-ethanediyloxy-2,1-ethanediyl)]bis-3–(8-methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzylamine (1u)
Yellow oil, Yield: 92%; 1H NMR δ (300 MHz, CDCl3) 7.95 (d, 3H, J = 9.00 Hz, 2 H-6), 7.93 (dd, 3H, J = 1.50 and 1.50 Hz, 2 H-2′), 7.88–7.83 (m, 4H, 2 H-4′and 2 H-1), 7.50–7.45 (m, 4H, 2 H-6′ and 2 H-5′), 7.26 (d, 2H, J = 2.70 Hz, 2 H-9), 7.05 (dd, 2H, J = 9.00 and 2.70 Hz, 2 H-7), 6.95 (dd, 2H, J = 3.90 and 1.20 Hz, 2 H-3), 6.87 (dd, 2H, J = 3.90 and 2.70 Hz, 2 H-2), 3.95 (s, 6H, 2 CH3O), 3.91 (s, 4H, 2 NCH2), 3.64–3.58 (m, 12H, 6 OCH2), 2.83 (t, 4H, J = 5.40 Hz, 2 NCH2), 2.18 (bs, 2H, 2 NH). 13C NMR δ (100 MHz, CDCl3) 160.5 (C-8), 153.1 (C-4), 141.3 (C-3a), 140.0 (C-5a), 132.7 (C-6), 132.0 (C-3′), 131.0 (C-2′), 129.9 (C-5′ and C-4′), 129.2 (C-1′), 128.7 (C-6′), 126.7 (C-9a), 115.4 (C-7), 115.3 (C-1), 114.2 (C-2), 109.6 (C-3), 98.9 (C-9), 71.8 (OCH2), 71.6 (OCH2), 71.5 (OCH2), 56.7 (OCH3), 54.8 (NCH2), 49.8 (NCH2). MALDI-TOF MS m/z [M + H]+ Calcd for C46H49N6O5: 765.376, Found: 765.457.
In vitro antiplasmodial activity
The in vitro antiplasmodial activities were tested over concentrations ranging from 39 nM to 40 μM against culture-adapted Plasmodium falciparum reference strains 3D7 and W2. The former strain is susceptible to chloroquine (CQ) but displays a decreased susceptibility to mefloquine (MQ) while the latter is considered as resistant to chloroquine. The parasites were cultivated in RPMI medium (Sigma-Aldrich, Lyon, France) supplemented with 0.5% Albumax I (Life Technologies corporation, Paisley, United Kingdom), hypoxanthine (Sigma-Aldrich), gentamicin (Sigma-Aldrich), and human erythrocytes and were incubated at 37 °C in a candle jar, as described previouslyCitation37. The P. falciparum drug susceptibility test was carried out in 96-well flat bottom sterile plates under a final volume of 250 μL. After a 48 h incubation with the drugs, quantities of DNA in treated and control cultures of parasites in human erythrocytes were compared according to the SYBR Green I (Sigma-Aldrich) fluorescence-based methodCitation38,Citation39. Briefly, after incubation, plates were frozen at -20 °C until use. They were then left to thaw for 2 h at room temperature and 100 μL of the homogenized culture were transferred to 96-well flat bottom sterile black plates (Nunc Inc) already containing 100 μL of the SYBR Green I lysis buffer (2xSYBR Green, 20 mM Tris base pH 7.5, 5 mM EDTA, 0.008% w/v saponin, 0.08% w/v Triton X-100). A negative control, controls treated by solvents (DMSO and H2O, typically) and positive controls (chloroquine and mefloquine) were added to each set of experiments. Plates were incubated for 1 h at room temperature and then read on a fluorescence plate reader (Tecan, Austria) using excitation and emission wavelengths of 485 and 535 nm, respectively. Concentrations inhibiting 50% of the parasite′s growth (half maximal inhibitory concentration or IC50 values) were then calculated from the obtained experimental results using a regression program available on lineCitation40.
In vitro antileishmanial activity
L. donovani (MHOM/IN/00/DEVI) used in this study was provided by the CNR Leishmania (Montpellier, France). The effects of the tested compounds on the growth of L. donovani (MHOM/IN/00/DEVI)promastigotes were assessed by MTT assayCitation41 .Briefly, promastigotes in log-phase in Schneider′s medium supplemented with 20% fetal calf serum (FCS), 2 mM L-glutamine and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), were incubated at an average density of 106 parasites/mL in sterile 96-well plates with various concentrations of compounds dissolved in DMSO (final concentration less than 0.5% v/v), in duplicate. Appropriate controls treated by DMSO and pentamidine or amphotericin B (reference drugs purchased from Sigma-Aldrich) were added to each set of experiments. After a 72 h incubation period at 27 °C, parasite metabolic activity was determined. Each plate-well was then microscope-examined for detecting possible precipitate formation. 20 μL of MTT 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (5 mg/mL) were added to each well followed by incubation for another 4 h. The enzyme reaction was then stopped by addition of 100 μL of 50% isopropanol – 10% sodium dodecyl sulfateCitation42 .Plates were shaken vigorously (300 rpm) for 10 minutes and the absorbance measured in a plate reader at 570 nm in a BIO-TEK ELx808 Absorbance Microplate Reader. Inhibitory concentration 50% (IC50) was defined as the concentration of drug required to inhibit by 50% the metabolic activity of L. donovani promastigotes compared to the control. IC50 were calculated by non-linear regression analysis processed on doseresponse curves, using TableCurve 2D V5.0 software. IC50 values represent the mean value calculated from three independent experiments.
Cytotoxicity evaluation
A cytotoxicity evaluation was realized according to the method of MosmannCitation41 with slight modificationsto determine the cytotoxic concentrations 50% (CC50) and using doxorubicin as a cytotoxic reference-compound. These assays were performed toward the human HepG2 cell line (HepG2 CC50). HepG2 (hepatocarcinoma cell line purchased from ATCC, ref HB-8065) is a commonly used human-derived hepatocarcinoma cell line that has shown characteristics similar to those of primary hepatocytes. These cells express many of the hepatocyte-specific metabolic enzymes, thus enabling the cytotoxicity of tested product metabolites to be evaluated. Briefly, cells in 100 μL of complete RPMI medium, [RPMI supplemented with 10% FCS, 1% l-glutamine (200 mM) and penicillin (100 U/mL)/streptomycin (100 μg/mL)] were inoculated into each well of 96-well plates and incubated at 37 °C in a humidified 6% CO2. After 24 h incubation, 100 μL of medium with various product concentrations dissolved in DMSO (final concentration less than 0.5% v/v) were added and the plates were incubated for 72 h at 37 °C. Duplicate assays were performed for each sample. Each plate-well was then microscope-examined for detecting possible precipitate formation before the medium was aspirated from the wells. 100 μL of MTT solution (0.5 mg/mL in medium without FCS) were then added to each well. Cells were incubated for 2 h at 37 °C. After this time, the MTT solution was removed and DMSO (100 μL) was added to dissolve the resulting blue formazan crystals. Plates were shaken vigorously (300 rpm) for 5 minutes. The absorbance was measured at 570 nm with 630 nm as reference wavelength spectrophotometer using a BIO-TEK ELx808 Absorbance Microplate Reader. DMSO was used as blank and doxorubicin (Sigma Aldrich) as positive control. Cell viability was calculated as percentage of control (cells incubated without compound). The 50% cytotoxic concentration was determined from the dose-response curve by using the TableCurve 2D V5.0 software (Systat Software, San Jose, CA).
FRET melting experiments
FRET melting experiments were performed with dual-labeled oligonucleotides mimicking the Plasmodium telomeric sequences FPf1T [FAM-5′(GGGTTTA)3-GGG3′-TAMRA] and FPf8T [FAM-5′(GGGTTCA)3GGG3′-TAMRA] and the human telomeric sequence F21T [FAM-(GGGTTA)3-GGG3′- TAMRA]Citation36,Citation43. The oligonucleotides were prefolded in 10 mM lithium cacodylate buffer (pH 7.2), with 10 mM KCl and 90 mM LiCl (K+ condition). The FAM emissions were recorded at 516 nm using a 492-nm excitation wavelength in the absence and presence of a single compound as a function of temperature (25 to 95 °C) in 96-well microplates by using a Stratagene MX3000P real-time PCR device at a rate of 1 °C.min−1. The data obtained were normalized between 0 and 1, and the temperature required for half-denaturationof oligonucleotides corresponded to the emission value of 0.5 was calculated as ΔTm. Each experiment was performed in duplicate with 200 nM of labeled oligonucleotide and 0, 1, 2, or 5 μM of compound under K+ condition. For each compound, two independent experiments were carried out. The data were plotted using OriginPro 9.1 software (OriginLab Corporation, Northampton, MA).
Results and discussion
Chemistry
The reported bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine or piperazine derivatives 1a–p were synthesized in seven steps from 2-nitroaniline (Scheme 1). Preparation of 1–(2-nitrophenyl)pyrroles 3a–d was performed according to the Clauson-Kaas reaction run under micro-wave irradiation starting from 2-nitroaniline and 2,5-dimethoxytetrahedrofuran in acetic acid. This pathway partially involved synthetic methodologies already described by our groupCitation20–23,Citation27. The resulting 1–(2-nitrophenyl)pyrrole intermediates 3a–d was subsequently reduced into the attempted 1–(2-aminophenyl)pyrroles 4a–d using a sodium borohydride-copper (II) sulfate in ethanol at room temperature. This NaBH4-CuSO4 system was found to be quite powerful in reducing our aromatic nitro group with excellent yield (73–85%). The reaction of 4a–d with triphosgene in toluene gave the lactams 5a–dCitation22. Reduction of the nitro moiety of 6a–b with iron in hot glacial acetic acid produced the spontaneous ring closure onto the ester to afford the desired the lactams 5e–f through a one-pot reduction-cyclization stepCitation27,Citation25.
The lactams 5a–f were subsequently chlorodehydroxylated with phosphorous oxychloride, leading to the 4-chloropyrrolo[1,2-a]quinoxalines 7a–f.
Coupling chloro derivatives 7a–f with 3- or 4-formylphenylboronic acid in the presence of Pd(PPh3)4 as a catalyst under Suzuki-Miyaura cross-coupling conditions proceeded to afford the substituted benzaldehydes 8a–hCitation22,Citation27,Citation28,Citation30. Reaction of primary amines, such as 3,3′-diamino-N-methyldipropylamine or N-(3-aminopropyl)-1,3-propanediamine or 1,4-bis(3-aminopropyl)piperazine), with the latter 7a–f gave the di-imines 9a–p, reduced into the bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amines 1a–p using sodium borohydride in methanol. The same pathway was used for the synthesis of bisindoloquinoxaline 1q and bis(4-phenyl)pyrroloquinoxaline 1r from aldehydes 8i and 8j, respectively (Schemes 2 and 3).
This strategy was also used to prepare the tris{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amines 1s–t (Scheme 4) and N,N′-[oxybis(2,1-ethanediyloxy-2,1-ethanediyl)]bis-(pyrrolo[1,2-a]quinoxalin-4-yl)benzylamine 1u (Scheme 5).
All these quinoxaline compounds 1a–u were then converted into their ammonium oxalate salts by treatment with oxalic acid in refluxing isopropanol ().
Table 1. Physical properties of the final amines 1a–t.
Biological activity
In vitro antimalarial activity
All new substituted bis- and trispyrrolo[1,2-a]quinoxaline derivatives 1a–u were evaluated for their antimalarial activity in vitro on P. falciparum CQ-resistant strain W2 (IC50 CQ =0.20 μM) and the CQ-sensitive and MQ decrease susceptibility strain 3D7 (IC50 CQ =0.08 μM). As shown in , they were found to have IC50 between 0.05–4.20 μM on W2, and 0.04–8.96 μM on 3D7 P. falciparum strains, respectively.
Table 2. In vitro sensitivity of compounds 1a-u on P. falciparum and L. donovani strains, and cytotoxicity on the HepG2 cell line.
For the pyrroloquinoxalines unsubstituted on their heterocyclic moiety (compounds 1a–c), compound 1a joined by a bis-(3-aminopropyl)amine linker was found more active (up to 4 to 5 times) on the W2 strain than its counterparts with bis-(3-aminopropyl)methylamine (compounds 1b) or bis-(3-aminopropyl)piperazine linkages (compounds 1c): i.e. IC50 = 0.85 μM for 1a versus 4.20 and 3.40 μM for 1b and 1c, respectively.As a general rule against the W2 strain, the introduction of a methoxy substituent in position 7, 8 or 9 of the bis-pyrrolo[1,2-a]quinoxaline skeleton linked in position 4 of the benzyl ring (compounds 1d–i) strongly increased the antiparasitic activity in comparison with their respective unsubstituted bispyrrolo[1,2-a]quinoxaline homologs 1b–c: i.e. IC50 = 0.13–0.44 μM for 1d–i and 3.40–4.20 μM for 1b–c. Moreover, introduction of an ethoxy substituent in position C-3 of the pyrrolo[1,2-a]quinoxaline moieties joined by a bis-(3-aminopropyl) piperazine linker (compound 1k) was found more active (up to 38 times) than its non-substituted homolog 1c (IC50 = 0.09 μM for 1k in comparison of 3.40 μM for 1c). In addition, 1k was found to be 2.2 times more active than the reference drug CQ (IC50 = 0.20 μM). In contrast, the addition of a phenyl in position 2 of the pyrrolo[1,2-a]quinoxaline moiety (compound 1 l) reduced considerably the antimalarial activity on the W2 strain (IC50 = 1.39 μM).
The bis-pyrrolo[1,2-a]quinoxalines 1m–n, which are linked with polyaminoalkyl chains on position 3 of the benzyl moieties, increased the antimalarial activity up to 35 and 68 times when compared to their non-substituted counterparts linked in position 4 (compounds 1b–c). Thus, compound 1n bearing a diaminopropylpiperazine linker exhibited the most potent antiplasmodial activity (IC50 = 0.05 μM) against the CQ-resistant strain (W2). Nevertheless, in these subseries in which the polyaminoalkyl side chain was anchored in position 3 of the benzyl ring, the introduction of a methoxy substituent on the pyrrolo[1,2-a]quinoxaline heterocycles (compounds 1o–p) seemed to be less detrimental against drug-resistant strain W2 in comparison with their analogs 1d–i. The bis-pyrrolo[1,2-a]quinoxaline 1r, structural isomer of derivative 1c, was also found to have a significant activity against the W2 strain (IC50 = 0.75 μM). In addition, the tris{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amines 1s–t showed similar antimalarial activities when compared with their bis-homologs 1d–i (IC50 = 0.19–0.29 μM for 1s–t versus 0.13–0.44 μM for 1d–i). Moreover, the replacement of the polyaminoalkyl linker by a 1,11-diamino-3,6,9-trioxaundecane chain (compound 1u) showed the same level of antimalarial activity against the W2 strain with IC50 = 0.25 μM.
Against the CQ-sensitive strain (3D7)and in the subseries of the pyrrolo[1,2-a]quinoxalines unsubstituted on their heterocyclic moieties (compounds 1a–c), compound 1c joined by a bis-(3-aminopropyl)piperazine linker was found more active than its structural analogs with bis-(3-aminopropyl)amine (compound 1a) or bis-(3-aminopropyl)methylamine linkages (compound 1b): i.e. IC50 = 0.80 μM for 1c versus 3.42 and 1.20 μM for 1a and 1b, respectively. Surprisingly, most of the pyrrolo[1,2-a]quinoxalines 1d–g and 1i bearing a methoxy substituent on the heterocyclic skeleton exhibited moderate antimalarial activity against 3D7 strain with IC50 ranging from 1.28 to 5.85 μM, in the exception of compound 1 h which showed an interesting IC50 of 0.25 μM against this Plasmodium strain. As against the W2 strain, replacement of the methoxy function by an ethoxy one in position 3 of the pyrrolo[1,2-a]quinoxaline moieties (compounds 1j–k) increased the anti P. falciparum activity against the 3D7 strain (IC50 = 0.26 and 0.19 μM for 1j and 1k, respectively). The introduction of a phenyl ring in position 2 of the pyrrolo[1,2-a]quinoxaline moiety (compound 1 l) reduced considerably the antimalarial activity on the 3D7 strain (IC50 = 8.96 μM). Similar observations could be made with the structural analogs 1q–r which showed moderate activity against 3D7 strain (IC50 = 1.00–7.22 μM). In the subseries in which the polyaminoalkyl side chain was anchored in position 3 of the benzyl ring, the piperazine substituted bis-pyrrolo[1,2-a]quinoxaline 1p was found to be two times more active than the reference drugs chloroquine and mefloquine (IC50 = 0.040 μM for 1p versus IC50 = 0.080 μM for the references). The tris-pyrrolo[1,2-a]quinoxalines 1s–t showed similar antimalarial activities when compared with their methoxy substituted bis-homologs 1o–p (IC50 = 0.20–0.24 μM for 1s–t versus 0.04–0.18 μM for 1o–p). Compound 1u bearing diaminotrioxaundecane linkage exhibited similar activity against the 3D7 strain than those observed for chloroquine and mefloquine (IC50–0.08 μM).
In vitro antileishmanial activity against promastigote forms
In order to increase the biological profile of molecules 1a–u, complementary analyses were performed. Notably, P. falciparum belonging to coccidian protozoan parasites, their in vitro biological activity on flagellate protozoan parasites like Leishmania donovani was evaluated (). In comparison with amphotericin B and pentamidine used as reference drugs (IC50 = 0.1 μM and 5.5 μM, respectively), most of the tested compounds 1 were found active against the promastigote forms of L. donovani. It must be noticed that a majority of these bis-pyrrolo[1,2-a]quinoxaline derivatives 1 (compounds 1a, 1c, 1d–j and 1m–q)with IC50 ranging from 1.0 to 4.26 μM were found slightly more potent than pentamidine, one of the reference compounds (IC50 = 5.5 μM). In particular, bis{N-[4-(indolo[1,2-a]quinoxalin-6-yl)benzyl]-3-aminopropyl}piperazine 1q was found to be 9.65 times more active than pentamidine (IC50 = 0.57 μM versus IC50 = 5.5 μM). In our previously described antileishmanial seriesCitation21,Citation22, we have noticed that introduction of a methoxy group in positions 7 and 8 of the pyrrolo[1,2-a]quinoxaline moiety generally increased the antileishmanial activity. In this new series, we also decided to introduce a methoxy or an ethoxy to evaluate the influence of such groups for their in vitro antileishmanial activity upon the L. donovani strain. Meanwhile, the presence of these alkoxy groups in the 7, 8, 9 or 3 position seems not to be detrimental for the activity against L. donovani as illustrated by results obtained for compounds 1d–k and 1o–p (IC50 = 1.07–4.26 μM) in comparison with unsubstituted compounds 1a, 1c and 1m–n (IC50 = 1.0–3.71 μM). The same observation could be done for the nature of the polyalkylamine linker between the two benzylpyrrolo[1,2-a]quinoxaline moieties that seemed also not to be crucial, with the exception of piperazine substituted 1 g, which showed a better antileishmanial activity in vitro compared to its structural methylamine analog 1f (IC50 = 4.25 μM versus 1.07 μM for 1 g) upon the L. donovani strain.
Moreover, in the subseries in which the polyaminoalkyl side chain was anchored in position 3 of the benzyl ring, the various bis-pyrrolo[1,2-a]quinoxalines 1m–p were found less beneficial for the in vitro antiparasitic activity with an IC50 of 2.80–3.71 μM in comparison with their analogs 1a–c and 1f–g. In addition, the tri-substitution of the tris(3-aminopropyl)amine linker by the pyrrolo[1,2-a]quinoxaline core (compounds 1s–t) led to a decrease in the antileishmanial activity (IC50 > 10 μM) in comparison with their bis-pyrrolo[1,2-a]quinoxaline analogs 1d–e and 1o–p.
Cytotoxicity and selectivity index
In order to assess their selectivity of action, the cytotoxicity of the antimalarial compounds 1a-u was also evaluated in vitro upon one human cell line. The cytotoxic concentrations 50% (CC50) on the metabolizing human hepatocyte HepG2 cell line allowed access to the corresponding selectivity indexes (SI) defined as ratio of cytotoxic and antimalarial activities (SI = CC50/W2 or 3D7 IC50). The SI could validate their real potential as selective antiparasitic agents. The results concerning the cytotoxicity and SI data are presented in . As expected, most of the active bis- and trispyrrolo[1,2-a]quinoxalines 1 showed significant level of cytotoxicity against the HepG2 cell line (IC50 = 1.25–3.38 μM). Considering the W2 P. falciparum strain, the selectivity indexes varied between 0.37 and 40.6, and SI ranged from 0.53 to 39.25 by taking into account the 3D7 strain. This SI led to the identification of compound 1n with SI of 40.6 on W2 strain, and SI of 39.25 for derivative 1p on 3D7 strain. We could notice that these last bioactive compounds 1n and 1p were bis-pyrrolo[1,2-a]quinoxalines linked by a 1,4-bis(3-aminopropyl)piperazine moiety in position 3 of their benzyl ring. On the other hand, the bis-pyrrolo[1,2-a]quinoxaline 1u is also interesting with SI =20.26, on the 3D7 malaria strain. However, according to the SI values, these compounds appeared to be toxic; thus, new modulations by replacing the benzyl substituted heterocyclic moiety to synthesize new candidates could be developed for forthcoming pharmacological investigations.
FRET-melting experiments
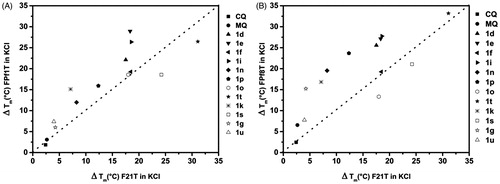
As these new compounds presented the same requirements as those required for G-4 stabilizing ligands, we have also investigated their ability of targeting P. falciparum telomeres as a potential strategy to interfere with human protozoan parasite infections. In fact, the telomeres of the parasite could constitute an attractive target. They are composed of repetitions of a degenerate motif (5′GGGTTYA3′, where Y could be T or C), which is different from the human one (5′GGGTTA3′). This antimalarial approach based on targeting P. falciparum telomeres has been previously described by our teamCitation36. Thus, in this report, we investigated the stabilization of Plasmodium telomeric G-quadruplexes by these new compounds through a FRET melting assay. For this evaluation, the two Plasmodium telomeric sequences (FPf1T and FPf8T) and the human one (F21T), in potassium, were used (two different Plasmodium telomeric repeats were used to fit the degenerate consensus). Thus, we used this FRET melting assay to study the interactions of some of our most bioactive compounds 1 and also the reference drugs (chloroquine – CQ and mefloquine – MQ) with these three telomeric sequences. For better visualization, a plot of ΔTm values induced on FPf1T or FPf8T versus the ΔTm induced on F21T is presented in , allowing us to classify the more active compounds 1 in various subsets. Almost all the tested compounds displayed roughly the same stabilization profiles for the two Plasmodium telomeric sequences FPf1T and FPf8T and slightly better than that of the human one F21T, except 1s and 1t for FPf1T and 1o and 1s for FPf8T.The reference compounds CQ and MQ, never evaluated on these parasitic sequences before our work, showed very slight stabilization on these three telomeric sequences. These preliminary results mean that most of the tested bis-pyrrolo[1,2-a]quinoxalines 1 have a moderate preference for Plasmodium over human telomeric quadruplexes, excepted bispyrroloquinoxaline 1o. Surprisingly, the tris-pyrrolo[1,2-a]quinoxalines 1s–t were found more active on the human telomeric sequence F21T in comparison with their bis-analogues1d–e and 1o–p. In contrast, the bis{N-[4-(methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazines 1e and 1i were found to be more active on the Plasmodium sequences with the higher ΔTm. These last data could benow considered as a promising starting point for the further development and optimization of new and potent antimalarial compounds as well as promising stabilizers for the telomeric Plasmodium DNA sequences. As very little is known about telomere regulation in P. falciparum, further studies should be now investigated both on parasitic and human sequences which presented different response or time-response to telomere perturbation.
Conclusion
In the present report, we have described the synthesis and the antimalarial activity of new bis- and trispyrrolo[1,2-a]quinoxaline derivatives 1 in which aromatic nuclei are joined by various aliphatic polyamines linker. These new compounds were then tested for their in vitro antiparasitic activity on (i) two P. falciparum strains (the CQ-resistant W2 and CQ-sensitive 3D7); and (ii) the promastigote forms of L. donovani strain. Among these new synthesized molecules, few of them were identified as potential in vitro antiplasmodial hits, displaying IC50 ranging from 0.04 to 0.09 μM on the W2 and 3D7 strains of P. falciparum. Thus, the most promising antimalarial results were obtained for the two bis-pyrrolo[1,2-a]quinoxalines 1n and 1p linked by a 3-(aminopropyl)piperazine chain in position 3 of their benzyl moieties. These compounds 1n and 1p were identified as the most potent antimalarial candidates with selectivity index (SI) of 40.6 on W2 strain, and 39.25 on 3D7 strain, respectively. In addition, biological results showed activity against the promastigote forms of L. donovani with IC50 ranging from 0.57 to 4.26 μM. In parallel, the in vitro cytotoxicity of these new molecules was assessed on the human HepG2 cell line. Structure-activity relationships of these new synthetic compounds are here also discussed, as well as their relative ability of targeting P. falciparum telomeres as potential mechanism of action. Thus, as the telomeres of the parasite could constitute an interesting target, we have also established the possibility of targeting Plasmodium telomeres by stabilizing the Plasmodium telomeric G-quadruplexes through a FRET melting assay. These results led us to conclude that the two bis{N-[4-(methoxypyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}piperazines 1e and 1i seemed to be able to discriminate between Plasmodium and human telomeric quadruplexes. By taking into account the biological and biophysical results and also the structure–activity relationships of these new series and those previously described, our overall results could be now considered as a promising starting point for the further development and optimization of new and potent antiprotozoan compounds.
Acknowledgements
We thank the DGA and ANR (project ANR-12-ASTR-003) for the financial support of this study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aponte J, Aregawi M, Cibulskis R, et al. World malaria report. World Health Organization. Geneva: WHO Press; 2015.
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg 2001;64:1–11.
- Klan SM, Waters AP. Malaria parasite transmission stages: an update. Trends Parasitol 2004;20:575–80.
- World Health Organization. 2010. Available from: http://www.who.int/drugresistance/en/ [last accessed 10 Jun 2016].
- World Health Organization. Guidelines for the treatment of malaria. 3rd ed. Geneva: WHO Press; 2015.
- Yeung S, Socheat D, Moorthy VS, Mills AJ. Artemisinin resistance on the Thai-Cambodian border. Lancet 2009;374:1418–19.
- Müller O, Sié A, Meissner P, et al. Artemisinin resistance on the Thai-Cambodian border. Lancet 2009;374:1419.
- Winzeler EA, Manary MJ. Drug resistance genomics of the antimalarial drug artemisinin. Genome Biol 2014;15:544.
- Fairhurst RM. Understanding artemisinin-resistant malaria: what a difference a year makes. Curr Opin Infect Dis 2015;28:417–25.
- Tilley L, Straimer J, Gnadig NF, et al. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol 2016;32:682–96.
- Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014;505:50–5.
- Biot C, Chibale K. Novel approaches to antimalarial drug discovery. Infect Disord Drug Targets 2006;6:173–204.
- De D, Krogstad FM, Cogswell FB, Krogstad DJ. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am J Trop Med Hyg 1996;55:579–83.
- Ridley RG, Hfheinz W, Matile H, et al. 4-Aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother 1996;40:1846–54.
- Deshpande S, Kuppast B. 4-Aminoquinolines: an overview of antimalarial chemotherapy. Med Chem 2016;6:1–11.
- Kumar S, Singh RK, Patial B, et al. Recent advances in novel heterocyclic scaffolds for the treatment of drug-resistant malaria. J Enzyme Inhib Med Chem 2016;31:173–86.
- Manohar S, Tripathi M, Rawat DS. 4-aminoquinoline based molecular hybrids as antimalarials: an overview. Curr Top Med Chem 2014;14:1706–33.
- O'Neill PM, Ward SA, Berry NG, et al. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr Top Med Chem 2006;6:479–507.
- Huang A, Ma C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini Rev Med Chem 2013;13:607–16.
- Guillon J, Grellier P, Labaied M, et al. Synthesis, antimalarial activity, and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines, and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J Med Chem 2004;47:1997–2009.
- Guillon J, Forfar I, Mamani-Matsuda M, et al. Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo[1,2-a]quinoxalines as antileishmanial agents. Bioorg Med Chem 2007;15:194–210.
- Guillon J, Forfar I, Desplat V, et al. Synthesis of new 4-(E)-alkenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents by Suzuki-Miyaura cross-coupling reactions. J Enzyme Inhib Med Chem 2007;22:541–9.
- Guillon J, Moreau S, Mouray E, et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity. Bioorg Med Chem 2008;16:9133–44.
- Guillon J, Mouray E, Moreau S, et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity-Part II. Eur J Med Chem 2011;46:2310–26.
- Ronga L, Del Favero M, Cohen A, et al. Design, synthesis and biological evaluation of novel 4-alkapolyenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents-part III. Eur J Med Chem 2014;81:378–93.
- Desplat V, Geneste A, Begorre M-A, et al. Synthesis of new pyrrolo[1,2-a]quinoxaline derivatives as potential inhibitors of Akt kinase. J Enzyme Inhib Med Chem 2008;23:648–58.
- Desplat V, Moreau S, Gay A, et al. Synthesis and evaluation of the antiproliferative activity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt kinase. Part II. J Enzyme Inhib Med Chem 2010;25:204–15.
- Desplat V, Moreau S, Belisle-Fabre S, et al. Synthesis and evaluation of the antiproliferative activity of novel isoindolo[2,1-a]quinoxaline and indolo[1,2-a]quinoxaline derivatives. J Enzyme Inhib Med Chem 2011;26:657–67.
- Guillon J, Le Borgne M, Rimbault C, et al. Synthesis and biological evaluation of novel substituted pyrrolo[1,2-a]quinoxaline derivatives as inhibitors of the human protein kinase CK2. Eur J Med Chem 2013;65:205–22.
- Desplat V, Vincenzi M, Lucas R, et al. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur J Med Chem 2016;113:214–27.
- Alvar J, Vélez ID, Bern C, the WHO Leishmaniasis Control Team, et al. Leishmaniasis worldwide and global estimates of its incidence. PLos One 2012;7:e35671.
- WHO Technical report series n°975, Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis, 2012:116.
- Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. Visceral Leishmaniasis Treatment: what do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist 2012;2:11–19.
- Bottius E, Bakhsis N, Scherf A. Plasmodium falciparum telomerase: de novo telomere addition to telomeric and nontelomeric sequences and role in chromosome healing. Mol Cell Biol 1998;18:919–25.
- Raj DK, Das BR, Dash AP, Supakar PC. Identification of telomerase activity in gametocytes of Plasmodium falciparum. Biochem Biophys Res Commun 2003;309:685–8.
- De Cian A, Grellier P, Mouray E, et al. Plasmodium telomeric seqsuences: structure, stability and quadruplex targeting by small compounds. ChemBioChem 2008;9:2730–9.
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semi-automated microdilution technique. Antimicrob Agents Chemother 1979;16:710–18.
- Bennett TN, Paguio M, Gligorijevic B, et al. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 2004;48:1807–10.
- Bacon DJ, Latour C, Lucas C, et al. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob Agents Chemother 2007;51:1172–8.
- Kaddouri H, Nakache S, Houzé S, et al. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother 2006;50:3343–9.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Emami SA, Zamanai Taghizadeh Rabe S, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran J Basic Med Sci 2012;15:807–11.
- De Cian A, Guittat L, Kaiser M, et al. Fluorescence-based melting assays for studying quadruplex ligands. Methods 2007;42:183–95.

![Scheme 1. Synthesis of bispyrrolo[1,2-a]quinoxalines 1a–p.](/cms/asset/af3b31e8-5492-4632-ad5d-52beea90c870/ienz_a_1268608_sch0001.gif)
![Scheme 2. Synthesis of bisindolo[1,2-a]quinoxaline 1q.](/cms/asset/b029329e-43e3-4cc2-8d92-cdbf229810ca/ienz_a_1268608_sch0002.gif)
![Scheme 3. Synthesis of bispyrrolo[1,2-a]quinoxaline 1r.](/cms/asset/53a123e7-203e-451d-b0c7-e83695761ac8/ienz_a_1268608_sch0003.gif)
![Scheme 4. Synthesis of trispyrrolo[1,2-a]quinoxalines 1s–t.](/cms/asset/89bbb435-b73e-4fb6-b814-0ac6d89c1b69/ienz_a_1268608_sch0004.gif)
![Scheme 5. Synthesis of bispyrrolo[1,2-a]quinoxaline 1u.](/cms/asset/ffd3cd08-5ee4-43fa-b30a-dfa164e42376/ienz_a_1268608_sch0005.gif)