Abstract
Inhibition of NF-κB signalling has been demonstrated as a therapeutic option in treating inflammatory diseases and cancers. Herein, we synthesized novel dissymmetric 3,5-bis(arylidene)-4-piperidones (BAPs, 83–102) and characterized fully. MTT and ELISA assay were performed to screen the anti-hepatoma and anti-inflammation properties. 96 showed the most potential bioactivity. 96 could promote HepG2 apoptosis through up-regulating the expression of C-Caspase-3 and Bax, down-regulating the expression of Bcl-2, while markedly inhibit LPS or TNF-α-induced activation of NF-κB through both inhibiting the phosphorylation of IκBα and p65, and preventing the p65 nuclear translocation to exhibit both anti-hepatoma and anti-inflammatory activities. Molecular docking verified that simulated 96 can effectively bond to the active site of Bcl-2 and NF-κB/p65 proteins. 96 inhibited xenografts growth by reducing the expression of TNF-α and Bcl-2 in the tumour tissue. This study suggested that 96 could be developed as a potential multifunctional agent for treatment of inflammatory diseases and cancers.
Introduction
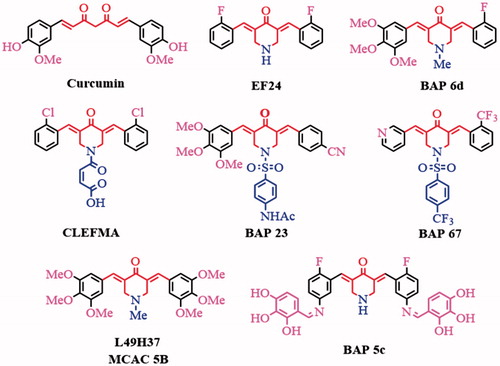
Curcumin (), a major active ingredient of turmeric, has been demonstrated showing anti-inflammatory and anti-cancer activitiesCitation1. However, due to its poor solubility, unstability and low bioavailability, its clinical utility is limitedCitation2,Citation3. Therefore, a lot of its analogues were developed. 3,5-bis(arylidene)-4-piperidone derivatives (BAPs) possess the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore leads a priorchemosensitivity to cancer cells rather than normal cellsCitation4,Citation5. 3,5-Bis(2-flurobenzylidene)piperidin-4-one (EF24, ) inhibits cancer growth and metastasis through inhibiting NF-κB pathwaysCitation6,Citation7. CLEFMA induces cell apoptosis of lung cancer xenografts by inhibiting Bcl-2 expression and up-regulating Bax expressionCitation8. L49H37 (or MCAC 5B, ) exhibits better inhibitory effects on cell cycle of pancreatic stellate cells (PSCs), as well as anti-lung cancer activity, and chemosensitization based on ROS-mediated JNK pathway activation and NF-кB pathway inhibitionCitation9–Citation11.
In recent years, we have focused on improving modifying the structure of BAPs to optimize the anti-inflammation and anti-hepatoma capability by inhibiting the NF-κB signalling. We have reported some symmetric or dissymmetric N-substituted-BAPsCitation12–Citation17and Schiff-base substituted BAPsCitation18–21 with improved anti-hepatoma and anti-inflammation activities. For example, the dissymmetric BAP (3E,5E)-3-(2-fluorobenzylidene)-5-(3,4,5-trimethoxybenzylidene)-1-methylpiperidin-4-one(BAP 6d, ) with different substituent groups on both sides can potentially inhibit HepG2 and THP-1 growth, which were proved by inducing cell apoptosis using flow cytometry and suppressing the growth of HepG2 xenograftsCitation12. The further optimized and synthesized dissymmetric N-phenylsulphonyl-BAPs 23 and 76 can prevent the nuclear translocation of NF-κB induced by inflammatory cytokines (lipopolysaccharide (LPS), TNF-α) and exhibit both anti-inflammation and anti-cancer capability in hepatic carcinoma cell linesCitation13,Citation14. However, the bioactivity is still not satisfied and the toxicity to the normal cell may be optimized.
Schiff-base compounds with active –C=N groups exhibit a range of biological activities, including antibacterial, antiviral, anti-cancer and anti-inflammatory propertiesCitation22,Citation23. We tried to incorporate of amino-substituted BAPs and aromatic aldehydes substituted by -X or -OH to construct novel symmetricCitation18 (such as BAP 5c) or dissymmetric Schiff-base substituted BAPs with desired anti-cancer and anti-inflammation activity. In this study, a series of novel dissymmetric BAPs 83–102 were synthesized, characterized and evaluated as potential anti-hepatoma and anti-inflammatory agents.
Experimental
Materials and methods
N-Methyl-4-piperidinone, stannous chloride and allaromatic aldehydes were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) and used as obtained without further purification. Compounds (3E,5E)-3–(2-fluorobenzylidene)-1-methyl-5–(3-nitrobenzylidene)piperidin-4-one (83), (3E,5E)-3-(3-aminobenzylidene)-5-(2-fluorobenzylidene)-1-methylpiperidin-4-one (92) were prepared based on a literatureCitation19. NMR data were collected using a Bruker Avance 400 MHz for 1H NMR with chemical shifts δ relative to TMS, while Citation13C NMR data were collected at 100 MHz on a Bruker Avance 400 MHz spectrometer or 150 MHz on a Bruker Avance 600 MHz spectrometer (Bruker Scientific, Billerica, MA). The HREIMS data were obtained on a Finnigan-MAT-95 mass spectrometer (Bruker Scientific, Billerica, MA).
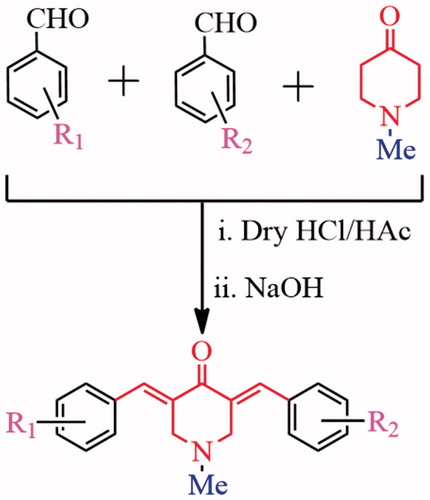
Preparation of 84-91
N-Methyl-4-piperidinone (1.13 g, 10.0 mmol) and two kinds of aromatic aldehydes (10.0 mmol, respectively) mixed with 20 ml glacial acetic acid. Dry hydrogen chloride gas was bubbled into this mixture for about 45 min (min), and the mixture was continuously stirred at room temperature (RT) for 24 h (monitored by TLC). The precipitate was collected, and then dissolved in distilled water (50 ml). Aqueous NaOH solution was added until the pH of the system was adjusted to 7. The system was filtered and subsequently washed by distilled water to provide a yellow precipitate. The precipitate was purified on silica gel by column using methanol/petroleum ether/EtOAc (10:10:1, v/v/v) as the eluent to afford yellow powders 84–91.
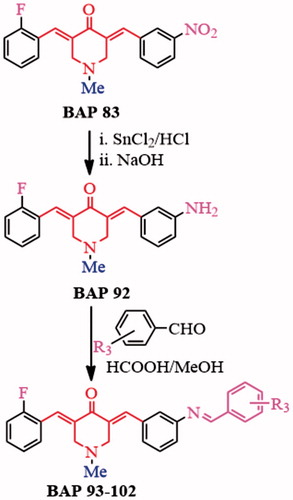
Preparation of 93-102
BAP 92 (0.32 g, 1.0 mmol) and aromatic aldehydes (1.0 mmol) were dissolved in methanol (5 ml). A drop of formic acid was added into the mixture. The mixture was stirred for 3 h at ambient temperature (monitored by TLC). The precipitate was collected and recrystallized from methanol to afford yellow crystals of 93–102.
Anticancer testing with MTT method
Compounds 83–102 were screened against human neoplastic cell lines, such as human breast cancer cells (MCF-7), human colon cancer cells (HCT116), human lung carcinoma cells (A549), human gastric adenocarcinoma cells (SGC7901), human liver hepatocellular carcinoma cell line (HepG2), human cervical carcinoma cells (HeLa), human acute mononuclear granulocyte leukaemia (THP-1), and human normal hepatic cell line (LO2) using modified MTT assayCitation18. All the cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in DMEM or 1640. Positive controls (Doxorubicin (DOX) and Curcumin) and BAPs were initially dissolved in dimethyl sulphoxide (DMSO). Experimental concentration of DMSO was always 0.1% (v/v). The concentrations of BAPs and positive controls were 50, 20, 10, 5, 2.5, 1, 0.5, 0.1, 0.05, and 0.01 μg/mL.
The cells (1 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h. Cells treated with serial concentrations of drugs were incubated for another 24 h. After the media removed, 20 μL of MTT (5 mg/mL) solution was added and incubated for 4 h at 37 °C. The MTT solution was removed and then 150 μL of DMSO was added. The optical density was measured with a multi-well plate reader (TECAN, Mӓnnedorf, Switzerland) at 570 nm. The results are expressed as a decrease in the cell viability (%) in comparison to untreated controls. The concentration of each compound was examined in triplicate, and the IC50 values are expressed in .
Table 1. Cytotoxicity of BAPs, Curcumin, and DOX.
Anti-inflammation testing of BAPs
The anti-inflammatory activities of BAPs were evaluated by ELISA. After pretreated with 30 μM of positive drug pyrrolidinedithiocarbamate (PDTC) or 6 μM of BAPs for 2 h, cells were treated with LPS (1.0 μg/mL) for 22 h. The culture media were collected and centrifuged for detecting the expression levels of TNF-α by an ELISA kit (eBioScience, San Diego, CA).
Western blot assay
The HepG2 cells were treated with BAP 96 at different concentrations (1.0, 2.0, and 4.0 µM) for 3 h, and washed twice by PBS. The cells were lysed and the extracted proteins of cell lysates were separated by 10% SDS-PAGE gel electrophoresis, then transferred onto polyvinylidene fluorides membranes. The membranes were probed with antibodies and visualized using an enhanced chemiluminescence (ECL) detection kit.
Molecular docking study
The study was performed under CDOCKER and LibDock protocol of Discovery Studio 2017 R2. The 3D structure of all BAPs was generated and minimized. The crystal structures of Bcl-2 (PDB1YSW) and NF-κB/p65 (PDB 1MY5) were obtained from Protein Data Bank. They were optimized by hydrogenation, dehydration and CHARMm force field. The ligand of the Bcl-2 protein was removed, then the BAPs were place into the site sphere. Similarly, NF-κB/p65 protein was defined as receptor, and the site sphere was selected on the p65 NLS polypeptide, and then BAP 96 was placed. Molecular docking scoring is obtained by CDOCKER and LibDock program, respectively. The types of interactions between proteins and BAPs were analyzed at the end of the molecular docking.
Apoptosis assay using annexin V-FITC staining
After treated with BAP 96 (1.0, 2.0, and 4.0 μM) and DMSO for 24 h. HepG2 cells were washed and added with 500 μL of Binding Buffer, 5 μL of Annexin V-EGFP and 5 μL of Propidiumlodide and mix (BD Biosciences, San Jose, CA) according to the manufacturer’s instruction. The mixed solution was gently vortexed and incubated in the dark at RT for 15 min. The results were detected by flow cytometry (BD FACS Calibur, San Jose, CA) within 1 h.
Immunofluorescence staining
RAW264.7 cells were pretreated in 96-well plates with LPS (1 μg/mL) for 15 min and then treated with 96 for 2 h. Similarly, HepG2 cells were pretreated with TNF-α (10 ng/mL) for 15 min and then treated with 96 for 4 h. The cells were fixed with 2% paraformaldehyde for 15 min at 37 °C and permeabilized using 0.1% Triton X-100 for 10 min at RT. After blockage with 3% BSA for 30 min, the cells were incubated with anti-NF-κB p65 antibody (NOVUS BIOLOGICALS, Colorado, USA) at 4 °C overnight. After washing, the anti-mouse Alexa488-conjugated secondary antibody (Jackson Immuno Research, West Grove, PA) was incubated for 30 min at RT. After washed with PBS, the cells were incubated in 4-6-diamidino-2-phenyindole (DAPI; Sigma, St. Louis, MO) for 15 min in the dark at RT. The cell images were simultaneously viewed using a microscopy system in High Throughput Screen (Operetta, PerkinElmer, Waltham, MA).
In vivo anti-hepatoma efficacy
The HepG2 cells xenografts were established using the Balb/c nude mice (female, 7-week-old, 17–19 g, n = 30) (Shanghai Laboratory Animal Center, Shanghai Shi, China) were used to investigate the anti-hepatoma effect of 96. Animal experiments were reviewed and approved by the Binzhou Medical University Experimental Animal Committee. Briefly, HepG2 cells (1 × 107/0.2 ml PBS/mouse) were inoculated subcutaneously. Three days after the inoculation, the mice were randomly divided into five groups: the saline treated group (Control), 96 (0.2 mg/kg) group, 96 (1.0 mg/kg) treated group, 96 (5.0 mg/kg) treated group, and DOX (1.0 mg/kg) treated group. 96 were dissolved in saline containing 1% of DMSO and administered intraperitoneally every 2 d for 20 days. DOX was also administered intraperitoneally every 2 d for 20 d. The body weight and HepG2 xenografts volumes were recorded from the day of treatment, and xenografts volumes were calculated using the equation: tumour volume = length×(width)Citation2×π/6. At the end of the experiment, mice were sacrificed and the xenografts were obtained for western blotting, HE staining and immunohistochemistry staining.
Western blotting was performed to detect the expression of Bcl-2, Bax and C-caspase-3 in HepG2 xenografts. Fresh HepG2 xenografts tissues were lysed and the obtained proteins were processed as the protein obtained from the HepG2 cells.
The HepG2 xenografts tissues were embedded using the paraffin, and sectioned for HE staining and immunohistochemistry staining. HE staining was performed routinely. The immunohistochemistry staining carried out as following. Briefly, the processed sections were incubated with the primary antibodies: Bcl-2 and TNF-α (NOVUS BIOLOGICALS) at 4 °C overnight. After washing, the anti-rabbit peroxidase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO) was incubated for 1 h at RT. After washing with PBS, the cells were visualized using the 3,3'-diaminobenzidine (DAB) solution under the light microscope. The cell images were taken using a light microscopy (Olympus, Tokyo, Japan).
Results and discussion
Synthesis and structural characterization
As shown in Scheme 1, BAPs 83–91 were synthesized by a step synthesis protocol of Claisen–Schmidt condensationCitation12. N-Methyl-4-piperidinone and two aromatic aldehydes in 1:1:1 ratio interacted under the catalysis of dry HCl gas to generate three components, including two symmetric BAPs and a dissymmetric BAP. The dissymmetric BAP can be purified on silica gel by column. The yields of 83–91 can reach ca. 40%∼47%. Their structures were confirmed by 1H NMR, 13C NMR, IR and HRMS. BAPs 83–91 provide the different electronic environment in two sides of BAPs because there are dissymmetric structure characteristics in BAPs, which will cause significant differences in the NMR signals. For example, 1H NMR signals of –CH=C–C=O in two sides of BAPs split into two groups of unimodal signals, and chemical shifts are in the range of 8.24–7.69 ppm. Similarly, 1H NMR signals of –CH2 groups in central piperidone ring are also divided into two groups, which are in the range of 3.86–3.57 ppm. In 13C NMR spectra, the carbon atoms of –CH2 groups likewise appear in different chemical shifts (56.90–56.12 ppm). According to FTIR spectra, the characteristic bands of –C=O group are shown in around 1682–1663 cm−1. The strong bands of –C=C group in α,β-unsaturated ketone and aromatic ring are shown in around 1626–1577 cm−1.
BAP 92 was obtained through nitro-reduction of 83 using stannous chloride according to a reported methodCitation19.The other aromatic aldehydes were following introduced into –NH2 fragment of 92 through Schiff-base condensation to generate BAPs 93–102 (Scheme 2), whose yields can reach ca. 71–88%. The structures, dissymmetric structure characteristics as well as different signals of BAPs 93–102 were confirmed by 1H NMR, 13C NMR, IR and HRMS. For example, the chemical shift of about 7.67–7.89 ppm should correspond to two groups of proton signals of –CH = C–C=O in two sides of BAPs. In addition, the single band in about 8.69–8.96 ppm appeared in the 1H NMR spectra, which proved the form of –CH=N bond. For 93, 96, 98–102, ortho-hydroxyl-substituted aromatic aldehydes were grafted to Schiff-base BAPs, and the chemical shift of 13.49–12.08 ppm should correspond to ortho-hydroxyl proton from target BAPs. In BAPs 98–100, other proton signals of hydroxyl also can be found in 9.78–8.53 ppm. Additionally, HRMS results displayed that the molecular weight are basically consistent with the calculated results, which further confirmed the correctness of their structures.
Cytotoxicity and anti-inflammatory activity analysis of BAPs
The cytotoxicity activities against human carcinoma cell lines MCF-7, HCT116, A549, SGC7901, HepG2, HeLa, THP-1 and toxicity toward LO2 cell lines for BAPs were evaluated by MTT method. DOX and Curcumin were used as positive controls. The selectivity index (SI) is the quotient of the IC50 values towards LO2 cells. The results are shown in .
For dissymmetric 83–92, IC50 values for about 26% of target BAPs against human carcinoma cell lines were lower than 5.0 μM, while especially for 2-F-substituted 83 and 92, this ratio can reach about 86%. Structurally, 83 and 92 contain the electron-withdrawing 3-NO2group and electron-donating –NH2 substitutes, respectively. This is basically consistent with previous studies, which showed that dissymmetric BAPs containing electron-donating and electron-withdrawing groups demonstrated more potent anti-cancer activitiesCitation12. More significantly, SI of 83 and 92 were greater than 10 for both HepG2 and THP-1. Especially for 92, the IC50 values against HepG2 and THP-1 are only 1.1 μM and 1.2 μM, respectively, while SI can reach 17.2 and 15.8.
In order to improve the anti-cancer activities and reduce the toxicity, a series of Schiff-base substituted BAPs 93–102 were synthesized based on Schiff-base condensation reaction between 92 and aromatic aldehydes. The IC50 values of about 47% of target BAPs whose are lower than 5.0 μM. For HCT116 cell, IC50 values of 2-OH-4-F-substituted 93 and 2-OH-4-Br-substituted 96 are slightly lower than 92. For A549 cell, IC50 values of 96 and 2,3,4-OH-substituted 100 are slightly lower than 92. For MCF-7, SGC7901, HepG2, HeLa and THP-1, BAP 96 displayed distinctly lower IC50 value than that of 92 and the other Schiff-base substituted BAPs. In terms of SI of BAPs, SI for 96 against A549, HepG2 and THP-1 towards LO2 cells can reach 12.4, 19.9 and 18.1, which were slightly higher compared to 92. Structural analysis displayed that R3 substituents, such as 2-OH-5-Br (96), 2-OH-5-F (93), and 2,3,4-OH (100), contributed to the improvement of anti-hepatoma activity. However, the other groups have little effect on the improvement of anti-hepatoma activity.
TNF-α is a common pro-inflammatory factors that can be produced by LPS-stimulated RAW264.7 macrophageCitation14,Citation24. In order to evaluate the anti-inflammatory activity of BAPs, the effect of BAPs on TNF-α production in LPS-stimulated RAW264.7 cells was detected by ELISA. As shown in , the cell toxicity tested by MTT indicated that all BAPs were not significantly toxic to RAW264.7 cells at 6.0 μM. As shown in , the anti-inflammatory results showed that some of BAPs had better anti-inflammatory activity than PDTC. In the presence of BAPs, the secretion of TNF-α was significantly decreased about 58% from LPS-stimulated RAW264.7 cells. For dissymmetric 83–92, nine BAPs did not decrease the secretion of inflammatory cytokines compared with that of PDTC at concentration of 6.0 μM. Only 92 presented slightly lower expression of TNF-α secretion (about 57%). After Schiff-base condensation reaction, 93 and 95–102 can inhibit the secretion of TNF-α and superior to PDTC group. Therein, BAPs 93, 96, 97, and 100 significantly inhibited the TNF-α secretion, which is 45% lower than PDTC. Especially, 96 exhibited the strongest inhibitory effect against TNF-α expression. Based on the analysis of cytotoxicity and anti-inflammatory activity, the most potent 96 () was selected for further biological assessment in this study.
Figure 2. (A) The cytotoxicity of all BAPs against RAW264.7 cells by MTT assay in vitro. (B) The levels of TNF-α induced by LPS stimulation in RAW 264.7 cells through ELISA analysis. PDTC was used as a positive control. The results were presented as the percent of LPS control. Each bar represents the mean ± SD of three independent experiments. Statistical significance relative to the LPS group is indicated: ∗p < 0.05; ∗∗p < 0.01.
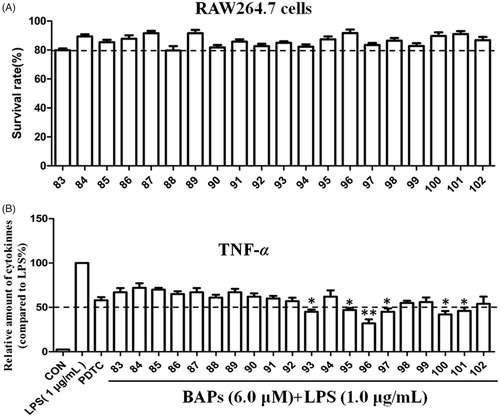
Figure 3. (A) The structures of 96. (B) 96 induces HepG2 cells apoptosis. **p < 0.01, ***p < 0.001 versus the negative control. (C) The effect of 96 on the expression of Bax, Bcl-2, and C-caspase-3 detected by western blot. The data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 versus the negative control (one-way ANOVA followed by Dunnett’s test).
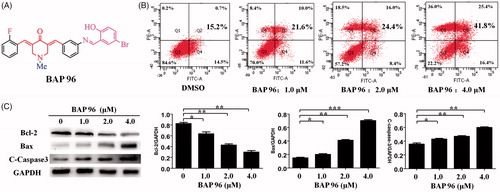
BAP 96 induced HepG2 cells apoptosis
To further investigate the underlying mechanism of decreased cell proliferation, the apoptosis mechanism of 96 on HepG2 cells was determined by flow cytometry. As shown in , the results showed that 96 (2.0 μM) can induce 24.4% apoptosis of HepG2 cells. Under the concentration 4.0 μM of 96, the apoptosis rate can reach about 41.8%. In addition, the results indicated that HepG2 cells showed a dose-dependent apoptosis after treatment with 96 for 24 h, including early and late apoptosis.
The effect of 96 on the expression levels of apoptosis-related proteins (Bcl-2, BAX, and C-Caspase-3) in HepG2 cells was determined by Western blot. The results are shown in . After treatment with 96 (1.0, 2.0, and 4.0 μM), the expression of anti-apoptotic protein Bcl-2 was decreased in a dose-dependent manner. Meanwhile, the expression of pro-apoptotic protein BAX and C-caspase-3 were dose-dependently increased. 96 (4.0 μM) showed the most effective effects, which is consistent with pattern of the apoptosis rate. In conclusion, 96 could induce HepG2 cells apoptosis through up-regulating the expression of C-caspase-3 and Bax proteins and down-regulating the expression of Bcl-2 protein.
BAP 96 inhibited NF-κB activation
It is known that NF-κB has been implicated in the crossroad of inflammation and cancerCitation25,Citation26. In our previous study, some BAPs (such as 23 and 67) could inhibit NF-κB activation by inhibiting the phosphorylation of NF-κB (P65) and IκBCitation13,Citation14. Herein, it is an urgent to resolve whether 96 has the same mechanism of action as 23 and 67. Therefore, the RAW264.7 cells were pretreated with LPS, and then treated with different concentrations (1.0, 2.0, 4.0 μM) of 96. As shown in , the expression levels of LPS-induced p-IκBα and p-p65 were effectively decreased in a dose-dependent manner. Similarly, the HepG2 cells were pretreated with TNF-α, then treated with different concentrations (1.0, 2.0, and 4.0 μM) of 96. As shown in , the expression levels of the phosphorylation of IκBα and p65 protein in HepG2 cells induced by TNF-α showed in a dose-dependent downward trend. These results suggest that 96 can markedly inhibit the phosphorylation of IκBα and p65 in RAW264.7 and HepG2 cells to exhibit anti-hepatoma and anti-inflammatory activity.
Figure 4. (A–D) Inhibitory effects of 96 on LPS-stimulated phosphorylation of IκBα (A) and p65 (B) in RAW264.7 cells and TNF-α-stimulated phosphorylation of IκBα (C) and p65 (D) in HepG2 cells, respectively. Data represent as the mean ± SD of triplicate tests. *p < 0.05, **p < 0.01 compared with the LPS or TNF-α alone group. (E, F) Effects of 96 on the nuclear translocation of NF-κB p65 protein in the LPS-stimulated RAW264.7 cells (E) or TNF-α-stimulated HepG2 cells (F) by immunofluorescence assay. These experiments were repeated three times with similar results.
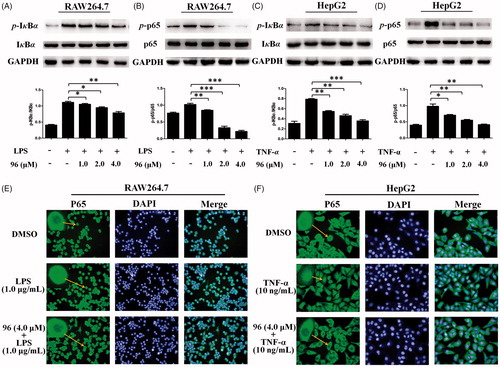
BAP 96 prevented the nuclear translocation of NF-κB induced by TNF-α or LPS
To evaluate the inhibitory effects of 96 against LPS or TNF-α-induced activation of NF-κB, the p65 nuclear translocation in RAW264.7 and HepG2 cells were detected using immunofluorescence. DMSO was taken as a control. As shown in , in control groups, majority of the p65 subunit were distributed in the cytoplasm of the RAW264.7 and HepG2 cells. After stimulated with 1.0 μg/mL of LPS or 10 ng/mL of TNF-α, many of p65 were transcribed into the cell nucleus of RAW264.7or HepG2 cells, but 96 (4.0 μM) prevented the p65 nuclear translocation and maintained p65 subunit mainly distributed in the cytoplasm of RAW264.7 cells or HepG2 cells, respectively. These results suggested that 96 could inhibit LPS or TNF-α-induced activation of NF-κB through preventing the P65 nuclear translocation to exhibit potential anti-hepatma and anti-inflammatory activities.
Molecular docking study
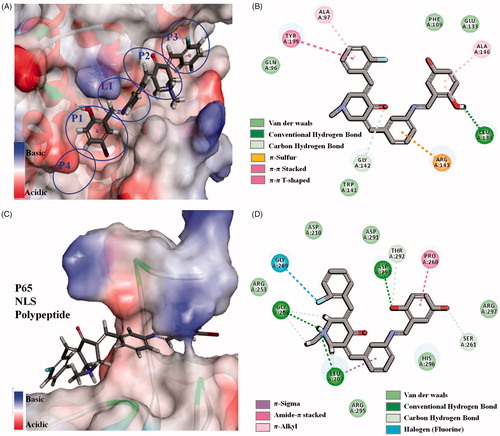
To further verify the interaction between 96 and Bcl-2, NF-κB/p65 proteins, C-DOCKER module in Discovery Studio 2017R2 was used as the molecular docking studyCitation27,Citation28. The crystal structures of Bcl-2 (PDB1YSW) and NF-κB/p65 (PDB 1MY5) protein were optimized. For Bcl-2 protein, 5-bromo-2-hydroxybenzylidene inserts into the biggest P1 hydrophobic pocket by hydrophobic interaction (). There is strong hydrogen bond between hydroxyl and hinge residue of LEU A134. The central benzylidene crosses the narrow and shallow L1 channel by π-sulphur from ARG A143. The central piperidone and 2-fluorobenzylidene of 96 inserts into P2 and P3 hydrophobic pocket in turn. It is different from reported 23 and 67 that longer linear molecule of 96 can stretch and insert into P3 hydrophobic pocket of Bcl-2 proteinCitation13,Citation14. For NF-κB/p65, Schiff-base bridged 5-bromo-2-hydroxybenzylidene of 96 crosses the channel of p65 protein, in which there are hydrogen bonds and π–π stacking interactions (). In addition, the central piperidone and 2-fluorobenzylidene attach to the surface of the protein by hydrogen bonds and Halogen from amino acid residue. For crystal structures of Bcl-2, NF-κB/p65 proteins, the linear molecule is more suitable for anastomosis with the tubular cavity of Bcl-2 protein, while flexible or semi-rigid linear molecules are easier to go through the channel of p65 protein. It happens that simulated 96 is a semi-rigid linear molecule, and the aforementioned discussion proved simulated 96 could reasonably bind to the active site of Bcl-2 and NF-κB/p65 protein. The results could verify the consistency between aforementioned western blot data and the data related to anti-hepatoma and anti-inflammatory activities of 96.
BAP 96 inhibited HepG2 xenografts growth in vivo
In order to detect the anti-hepatoma effect of 96 in vivo, the nude mice xenografts with HepG2 cells were established. The xenografts volume and body weight of nude mice were measured every 2 d. Twenty days after treatment with 96, all xenografts were obtained and measured. As shown in , 96 (0.2 mg/kg) and 96 (1.0 mg/kg) slightly decreased the growth of HepG2 xenografts; but 96 (5.0 mg/kg) significantly inhibited the growth of HepG2 xenografts as well as the positive drug DOX. However, the weight of all the mice increased until the end of the experiment, which showing that 96 is correspondingly nontoxic at the experimental doses. These results suggest that 96 could significantly inhibit the growth of HepG2 xenografts with nontoxic effect on the growth state of the mice.
Figure 6. The inhibitory effects of BAP 96 on inhibiting HepG2 xenografts growth in vivo. (A) The HepG2 xenografts growth curves very two days and mice body weight curves every 2 d. (B) The expression of Bcl-2, Bax, and C-caspase-3 in the HepG2 xenografts of five groups. (C) The morphology of the HepG2 xenografts of the five groups detected by HE staining. (D) The expression of Bcl-2 and (E) the expression of TNF-α in the HepG2 xenografts of the five groups investigated by immunohistochemistry staining. Scale bar = 60 μm (C, D, E).
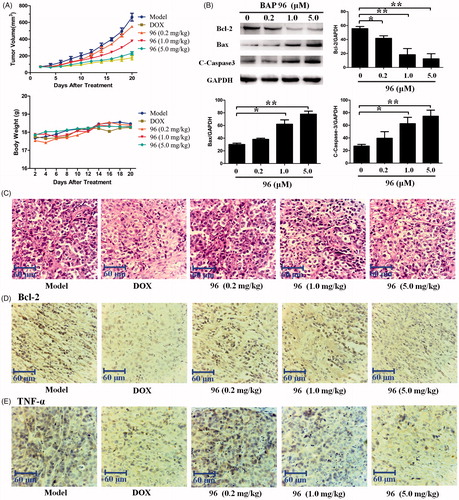
Western blotting was used to determine the expression levels of apoptosis-related proteins (Bcl-2, BAX, and C-Caspase-3) in HepG2 xenografts. As shown in , 96 (0.2, 1.0 and 5.0 mg/kg) dose-dependently decreased the expression of anti-apoptotic protein Bcl-2. Meanwhile, the expression of pro-apoptotic protein BAX and C-caspase-3 were significantly increased in a dose-dependent manner by 96. Collectively, 96 (5.0 mg/kg) showed the most effective effects in inducing the apoptosis of the HepG2 cells.
Haematoxylin and eosin (HE) staining was performed to investigate the histopathological changes. As shown in , 96 and DOX made the HepG2 cell nucleus wrinkled, which was completely different from that of smooth HepG2 cell nucleus in model group. Most obviously, a plenty of fracted cells were detected on 96 (5.0 mg/kg)- and DOX-treated groups. These results suggest that the anti-hepatoma effect of 96 (5.0 mg/kg) is considerable to the positive drug DOX in vivo.
Furthermore, immunohistochemistry staining of Bcl-2 and TNF-α in the HepG2 xenografts were carried out to evaluate the anti-hepatoma and anti-inflammatory activities. As shown in , both Bcl-2 and TNF-α were obviously expressed in the cytoplasm of HepG2 cells in model group, but were significantly reduced in DOX- and 96 (5.0 mg/kg)-treated groups. Therefore, we get the conclusion that 96 possess both the anti-hepatoma and anti-inflammation capabilities.
Conclusions
In this study, a series of dissymmetric BAPs 83–102 were synthesized and characterized, as well as evaluated their anti-chepatoma and anti-inflammatory activities. The 5-bromo-2-hydroxybenzylidene and 2-fluorobenzylidene substituted 96 showed the best bioactivities with IC50 values lower than 2.0 μM against experimental HepG2, A549, and THP-1 cells; The SI values can reach 12.4, 19.9, and 18.1, which proves lower toxicity toward LO2 cells. In addition, 96 could induce HepG2 cells apoptosis through up-regulating the expression of C-caspase-3 and Bax proteins, down-regulating the expression of Bcl-2 protein. Furthermore, 96 can markedly inhibit LPS or TNF-α-induced activation of NF-κB through both inhibiting the phosphorylation of IκBα and p65 and preventing the P65 nuclear translocation to exhibit anti-hepatoma and anti-inflammatory activities. This mechanism of action was also proved by the Molecular docking between simulated 96 and Bcl-2, NF-κB/p65 proteins. Moreover, 96 inhibited HepG2 xenografts growth in vivo. In conclusion, 96 possess both the anti-hepatoma and anti-inflammation capabilities, and could be developed as a potential multifunctional agent for treatment of inflammatory diseases and cancers.
Supplemental Material
Download PDF (372.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nelson KM, Dahlin JL, Bisson JJ, et al. The essential medicinal chemistry of curcumin. J Med Chem 2017;60:1620–37.
- Begum A, Jones MG, Morihara T, et al. Curcumin structure–function, bioavailability, and efficacy in models of neuroinflammation and alzheimer's disease. J Pharmacol Exp Ther 2008;326:196–208.
- Siviero A, Gallo E, Maggini V, et al. Curcumin, a golden spice with a low bioavailability. J Herb Med 2015;5:57–70.
- Dimmock JR, Raghavan SK, Logan BM, et al. Antileukemic evaluation of some Mannich bases derived from 2-arylidene-1,3-diketones. Eur J Med Chem 1983;18:248–54.
- Sun JF, Zhang SP, Yu C, et al. Design, synthesis and bioevaluation of novel N-substituted-3,5-bis(arylidene)-4-piperidone derivatives as cytotoxic and antitumor agents with fluorescent properties. Chem Biol Drug Des 2014;83:392–400.
- Selvendiran K, Tong L, Vishwanath S, et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem 2007;282:28609–18.
- Yin DL, Liang YJ, Zheng TS, et al. EF24 inhibits tumor growth and metastasis via suppressing NF-κB dependent pathways in human cholangiocarcinoma. Sci Rep 2016;6:32167.
- Lagisetty P, Vilekar P, Sahoo K, et al. CLEFMA – an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg Med Chem 2010;18:6109–20.
- Gundewar C, Ansari D, Tang L, et al. Antiproliferative effects of curcumin analog L49H37 in pancreatic stellate cells: a comparative study. Ann Gastro Enterol 2015;28:1–8.
- Chen L, Li Q, Weng B, et al. Design, synthesis, anti-lung cancer activity, and chemosensitization of tumor-selective MCACs based on ROS-mediated JNK pathway activation and NF-кB pathway inhibition. Eur J Med Chem 2018;151:508–19.
- Zhu HP, Xu TT, Qiu CY, et al. Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats. Eur J Med Chem 2016;121:181–93.
- Li N, Xin WY, Yao BR, et al. Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur J Med Chem 2018;147:21–33.
- Li N, Xin WY, Yao BR, et al. N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur J Med Chem 2018;155:531–44.
- Yao BR, Sun Y, Chen SL, et al. Dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones as anti-hepatoma agents by inhibiting NF-кB pathway activation. Eur J Med Chem 2019;167:187–99.
- Sun JF, Wang SW, Li HJ, et al. Synthesis, antitumor activity evaluation of some new N-aroyl-α,β-unsaturated piperidones with fluorescence. J Enzyme Inhib Med Chem 2016;31:495–502.
- Li HJ, Wang L, Zhao JJ, et al. Synthesis, structure and luminescence of novel co-crystals based on bispyridyl-substituted α,β-unsaturated ketones with coformers. J Mol Struct 2015;1079:414–22.
- Liu LD, Liu SL, Liu ZX, et al. Synthesis, structure, antitumor activity of novel pharmaceutical cocrystals based on bispyridyl-substituted α,β-unsaturated ketones with gallic acid. J Mol Struct 2016;1112:1–8.
- Zhang LS, Chen Q, Hou GG, et al. Hydroxyl-substituted double Schiff-base condensed 4-piperidone/cyclohexanones as potential anticancer agents with biological evaluation. J Enzyme Inhib Med Chem 2019;34:264–71.
- Yao BR, Li N, Wang CH, et al. Novel asymmetric 3,5-bis(arylidene)piperidin-4-one derivatives: synthesis, crystal structures and cytotoxicity. Acta Cryst 2018;C74:659–65.
- Chen Q, Hou Y, Hou GG, et al. Design, synthesis, anticancer activity and cytotoxicity of novel 4-piperidone/cyclohexanone derivatives. Res Chem Intermediat 2016;42:8119–30.
- Chen Q, Hou Y, Hou GG, et al. Synthesis, anticancer activity and cytotoxicity of novel double Schiff-base condensed α,β-unsaturated keto derivatives. J Chem Res 2016;40:400–3.
- Silva CMD, Silva DLD, Modolo LV, et al. Schiff bases: a short review of their antimicrobial activities. J Adv Res 2011;2:1–8.
- Makawana JA, Sangani CB, Lin L, et al. Schiff’s base derivatives bearing nitroimidazole and quinoline nuclei: new class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg Med Chem Lett 2014;24:1734–6.
- Singh P, Kaur S, Sharma A, et al. TNF-α and IL-6 inhibitors: conjugates of N-substituted indole and aminophenylmorpholin-3-one as anti-inflammatory agents. Eur J Med Chem 2017;140:92–103.
- Viatour P, Merville MP, Bours V, et al. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005;30:43–52.
- Zhang L, Shi L, Soars SM, et al. Discovery of novel small-molecule inhibitors of NF-кB signaling with antiinflammatory and anti-cancer properties. J Med Chem 2018;61:5881–99.
- Sathishkumar N, Sathiyamoorthy S, Ramya M, et al. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J Enzyme Inhib Med Chem 2012;27:685–92.
- Shishodia S, Majumdar S, Banerjee S, et al. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res 2003;63:4375–83.