?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
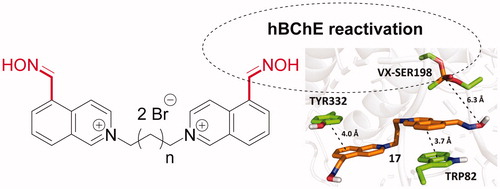
The series of symmetrical and unsymmetrical isoquinolinium-5-carbaldoximes was designed and prepared for cholinesterase reactivation purposes. The novel compounds were evaluated for intrinsic acetylcholinesterase (AChE) or butyrylcholinesterase (BChE) inhibition, when the majority of novel compounds resulted with high inhibition of both enzymes and only weak inhibitors were selected for reactivation experiments on human AChE or BChE inhibited by sarin, VX, or paraoxon. The AChE reactivation for all used organophosphates was found negligible if compared to the reactivation ability of obidoxime. Importantly, two compounds were found to reactivate BChE inhibited by sarin or VX better to obidoxime at human attainable concentration. One compound resulted as better reactivator of NEMP (VX surrogate)-inhibited BChE than obidoxime. The in vitro results were further rationalized by molecular docking studies showing future directions on designing potent BChE reactivators.
GRAPHICAL ABSTRACT
Introduction
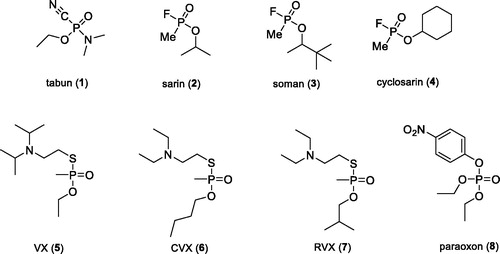
The function of the human nervous system is based on a complex interaction of nerves through neurotransmitters that maintain the activity of organs, glands, and other neurons in dynamic equilibriumCitation1,Citation2. This is done with the help of chemical synapses where the neurotransmitter released from the presynaptic terminus of the axon diffuses through a narrow synaptic cleft and postsynaptically binds to the respective receptors of the subsynaptic membrane of the neuron, gland, or muscle cellsCitation1. These very small and important molecules include, for example, acetylcholine (ACh), dopamine, histamine, serotonin etc. The processing of ACh may be seriously compromised when ACh receptors are blocked or the hydrolytic activity of acetylcholinesterase (AChE; EC 3.1.1.7) is impaired. The degradation of ACh is thus not proceeding and neurotransmitter overstimulates cholinergic receptorsCitation2,Citation3. The most known irreversible AChE inhibitors are organophosphates (OPs), which belong to the most dangerous and lethal substances developed by manCitation4,Citation5. OPs are a heterogeneous group of organic compounds that are a serious toxicological problem. These synthetic compounds have been discovered since the first half of 20th centuryCitation6. To date, they were misused in several military conflicts or terrorist attacks. They are also extensively used in agriculture as insecticidesCitation4,Citation7. Nerve agents can be divided into two groups. The first group labelled “G-series” includes e.g. tabun (1), sarin (2), soman (3), and cyclosarin (4) (). These agents are volatile, therefore, their solutions and vapours are very dangerous, even though their persistence in open terrain is low. The second group, referred to as the “V-series,” contains e.g. VX (5), CVX - the Chinese isomer (6) and RVX - the Russian isomer (7) ()Citation5. This group of agents is relatively non-volatile, has high persistence in the environment and enhanced toxicityCitation8.
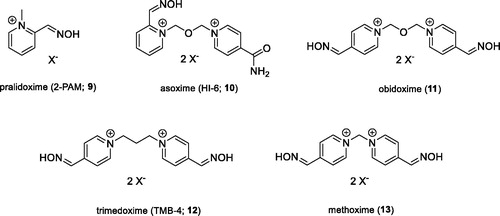
The second large group of OPs are insecticides, including e.g. chlorpyriphos, paraoxon (8), or methyl paraoxon, which might pose a threat to civilians due to their extensive agricultural useCitation9. The pesticides annually cause about 3 million of acute intoxications, with over 260,000 of them end fatallyCitation6. Some of these cases are deliberate suicidesCitation4. The main toxic mechanism of the OPs is based on the irreversible inhibition of the AChE enzyme, whose main biological function is the hydrolysis of the positively charged neurotransmitter AChCitation8. Inhibition thus leads to accumulation of ACh in cholinergic synapses and excessive stimulation of cholinergic receptors of muscarinic and nicotinic type. This pathological condition is referred to as “cholinergic crisis,” which is characterised by: sweating, saliva, diarrhoea, tremors, muscle twitching, or increased gastrointestinal effects. Death is caused by respiratory failureCitation6. The current antidotal treatment of OPs poisoning involves the administration of an anticholinergic agent atropine that blocks excessive stimulation of muscarinic receptors, and oximes that are capable of reactivating irreversibly inhibited AChE. The reactivators as pralidoxime (9), asoxime (syn. HI-6; 10), obidoxime (11), trimedoxime (12), and methoxime (13) () are approved as an antidotes against nerve agents, but unfortunately, their efficacy in suppressing acute toxic effects of all types of nerve agents is rather limitedCitation10. However, these are the most studied and commercially available reactivatorsCitation8.
In this work, the novel isoquinoline-5-carbaldoximes were designed, prepared and evaluated for the reactivation properties of phosphylated human acetylcholinesterase (hAChE) and butyrylcholinesterase (hBChE).
Experimental
Chemistry
All commercial reagents and solvents used were of the highest available purity from Sigma-Aldrich (Prague, Czech Republic). For flash column chromatography on silica gel, Kieselgel 60 (0.063–0.200 mm, 70–230 mesh, Fluka) was used. Solvents for flash column chromatography were purchased from Penta Chemicals Unlimited (Prague, Czech Republic). Thin-layer chromatography was run on Merck silica gel 60 F254 analytical plates; detection was carried out with ultraviolet light (254 nm). Melting points were recorded on a Melting Point Apparatus – Stuart SMP30 and are uncorrected. The 1H NMR and 13C NMR spectra were recorded in DMSO-d6 and CD3OD-d4 solution at ambient temperature on a Varian S500 spectrometer (499.87 MHz for 1H and 125.71 MHz for 13C). Chemical shifts, δ, are given in parts per million (ppm), and spin multiplicities are given as s (singlet), br s (broad singlet), d (doublet), t (triplet), or m (multiplet). Coupling constants, J, are expressed in hertz (Hz). For 1H δ relative to DMSO-d6 (δ = 2.50) or CD3OD-d4 (δ = 3.31) and for 13C relative to DMSO-d6 (δ = 39.43) or CD3OD-d4 (δ = 49.05). High Resolution Mass Spectrometry (HRMS) was determined by Q Exactive Plus hybrid quadrupole-orbitrap spectrometer.
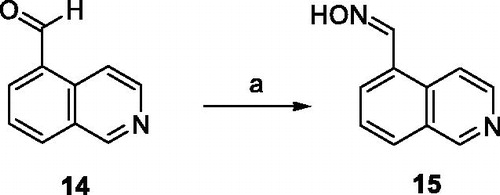
Synthesis of isoquinoline-5-carbaldehyde oxime (15)
To a solution of isoquinoline-5-carboxaldehyde (14) (5.00 g, 31.81 mM) in EtOH (500 ml) was added the 50% aq. sol. NH2OH (3.90 ml, 63.63mM). The resulting mixture was stirred for 24 h at room temperature. Subsequently, the mixture was concentrated under vacuum and the residue was chromatographed on silica gel (heptane-EtOAc, 1:1) to afford 3.85 g (70%) of compound 15 as a white solid, mp: 158.3–160.3 °C. 1H NMR (500 MHz, DMSO-d6): δ 7.69–7.72 (m, 1H, ArH), 8.03 (d, J= 7.2 Hz, 1H, ArH), 8.14 (d, J= 8.1 Hz, 1H, ArH), 8.52–8.58 (m, 2H, 2 × ArH), 8.76 (s, 1H, CH), 9.35 (s, 1H, ArH), 11.62 (s, 1H, OH). 13C NMR (126 MHz, DMSO-d6): δ 117.6, 127.0, 127.9, 128.5, 129.2, 130.7, 132.2, 143.8, 147.5, and 152.9. HRMS (HESI+): [M + H]+: calculated for C10H9N2O+ (m/z): 173.0709; found: 173.0707.
Synthesis of 5-((hydroxyimino)methyl)-2-methylisoquinolin-2-ium iodide (16)
Methyl iodide (CH3I) (0.36 ml, 5.80 mM) was added at room temperature to isoquinoline-5-carbaldehyde oxime (15) (500 mg, 2.90 mM) in EtOH (5 ml), and the resulting mixture was stirred under reflux for 24 h. Subsequently hot mixture was filtered, washed with hot EtOH and allowed to dry at room temperature. The compound 16 was isolated as a yellow solid in yield 650 mg (71%), mp: 220.0–221.5 °C. 1H NMR (500 MHz, DMSO-d6): δ 4.49 (s, 3H, CH3), 8.07–8.10 (m, 1H, ArH), 8.45–8.49 (m, 2H, 2 × ArH), 8.79 (d, J= 7.1 Hz, 1H, ArH), 8.88 (s, 1H, CH), 9.12 (d, J= 7.1 Hz, 1H, ArH), 10.08 (s, 1H, ArH), 11.94 (s, 1H, OH). 13C NMR (126 MHz, DMSO-d6): δ 47.7, 122.7, 127.7, 128.9, 130.8, 131.2, 133.5, 135.7, 136.4, 146.4, and 151.0. HRMS (HESI+): [M + H]+: calculated for C11H11N2O+ (m/z): 187.0866; found: 187.0863.
Synthesis of bisisoquinolinium salts 17–26
To a solution of isoquinoline-5-carbaldehyde oxime (15) (100 mg, 0.58 mM) in dimethylformamide (DMF; 0.5 ml) was added the dibromoalkane (0.26 mM). The resulting mixture was stirred for 48 h at 73 °C. The solvent was evaporated under reduced pressure, and the crude product purified by crystallisation from acetone, filtered, washed with acetone and allowed to dry at room temperature.
2,2′-(Propane-1,3-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (17)
Compound 17 was isolated as white solid, yield 100 mg (70%), mp: 249.0–251.0 °C. 1H NMR (500 MHz, DMSO-d6): δ 2.93–2.98 (m, 2H, CH2), 4.98 (t, J= 7.0 Hz, 4H, 2 × CH2), 8.09–8.12 (m, 2H, 2 × ArH), 8.47–8.52 (m, 4H, 4 × ArH), 8.89 (s, 2H, 2 × CH), 9.01 (d, J= 7.0 Hz, 2H, 2 × ArH), 9.17 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.35 (s, 2H, 2 ×ArH), 11.96 (s, 2H, 2 × OH). 13C NMR (126 MHz, DMSO-d6): δ 30.9, 57.3, 123.3, 128.0, 129.0, 130.9, 131.5, 133.9, 135.5, 136.0, 146.3, and 150.8. HRMS (HESI+): [M]2+: calculated for C23H22N4O22+ (m/z): 193.0866; found: 193.0865.
2,2′-(Butane-1,4-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (18)
Compound 18 was isolated as yellow solid, yield 124 mg (85%), mp: 246.5–248.5 °C. 1H NMR (500 MHz, CD3OD-d4): δ 2.27–2.33 (m, 4H, 2 × CH2), 4.89–4.92 (m, 4H, 2 × CH2), 8.05–8.10 (m, 2H, 2 ×ArH), 8.39–8.47 (m, 4H, 4 × ArH), 8.72–8.79 (m, 4H, 2 × ArH and 2 × CH), 9.36–9.39 (m, 2H, 2× ArH), 9.97–10.04 (m, 2H, 2 × ArH). HRMS (HESI+): [M]2+: calculated for C24H24N4O22+ (m/z): 200.0944; found: 200.0942.
2,2′-(Pentane-1,5-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (19)
Compound 19 was isolated as brown solid, yield 140 mg (94%), mp: 207.0–209.0 °C. 1H NMR (500 MHz, CD3OD-d4): δ 1.58–1.65 (m, 2H, CH2), 2.25–2.31 (m, 4H, 2 × CH2), 4.84 (t, J= 7.5 Hz, 4H, 2 × CH2), 8.05–8.08 (m, 2H, 2 × ArH), 8.38 (d, J = 7.2 Hz, 2H, 2 × ArH), 8.48 (d, J= 8.3 Hz, 2H, 2 × ArH), 8.71 (s, 2H, 2 × CH), 8.75 (d, J= 7.1 Hz, 2H, 2 × ArH), 9.33 (d, J= 7.0 Hz, 2H, 2 × ArH), 10.04 (s, 2H, 2 × ArH). 13C NMR (126 MHz, CD3OD-d4): δ 23.9, 31.5, 62.3, 125.9, 130.2, 131.3, 132.4, 132.6, 136.2, 136.4, 138.7, 148.2, and 151.3. HRMS (HESI+): [M]2+: calculated for C25H26N4O22+ (m/z): 207.1022; found: 207.1020.
2,2′-(Hexane-1,6-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (20)
Compound 20 was isolated as white solid, yield 69 mg (45%), mp: 249.5–250.5 °C. 1H NMR (500 MHz, CD3OD-d4): δ 1.57–1.60 (m, 4H, 2 × CH2), 2.15–2.21 (m, 4H, 2 × CH2), 4.79–4.82 (m, 4H, 2 × CH2), 8.06–8.09 (m, 2H, 2 × ArH), 8.40 (d, J= 7.2 Hz, 2H, 2 × ArH), 8.47 (d, J= 8.3 Hz, 2H, 2 ×ArH), 8.73–8.76 (m, 4H, 2 × ArH and 2 × CH), 9.37 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.02 (s, 2H, 2 × ArH). HRMS (HESI+): [M]2+: calculated for C26H28N4O22+ (m/z): 214.1101; found: 214.1098.
2,2′-(Heptane-1,7-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (21)
Compound 21 was isolated as white solid, yield 140 mg (89%), mp: 242.0–243.5 °C. 1H NMR (500 MHz, CD3OD-d4): δ 1.47–1.56 (m, 6H, 3 × CH2), 2.13–2.19 (m, 4H, 2 × CH2), 4.78–4.81 (m, 4H, 2 × CH2), 8.06–8.09 (m, 2H, 2 × ArH), 8.39 (d, J= 7.3 Hz, 2H, 2 × ArH), 8.47 (d, J= 8.4 Hz, 2H, 2 × ArH), 8.72 (s, 2H, 2 ×CH), 8.76 (d, J= 7.1 Hz, 2H, 2 × ArH), 9.36 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.02 (s, 2H, 2× ArH). 13C NMR (126 MHz, CD3OD-d4): δ 27.0, 29.5, 32.1, 62.7, 125.9, 130.2, 131.3, 132.4, 132.5, 136.1, 136.4, 138.6, 148.2, and 151.3. HRMS (HESI+): [M]2+: calculated for C27H30N4O22+ (m/z): 221.1179; found: 221.1177.
2,2′-(Octane-1,8-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (22)
Compound 22 was isolated as white solid, yield 71 mg (44%), mp: 246.0–247.1 °C. 1H NMR (500 MHz, CD3OD-d4): δ 1.44–1.49 (m, 8H, 4 × CH2), 2.11–2.18 (m, 4H, 2 ×CH2), 4.76–4.79 (m, 4H, 2 × CH2), 8.06–8.09 (m, 2H, 2 × ArH), 8.40 (d, J= 7.3 Hz, 2H, 2 × ArH), 8.47 (d, J= 8.2 Hz, 2H, 2 × ArH), 8.73–8.76 (m, 4H, 2 × ArH and 2 × CH), 9.37 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.00 (s, 2H, 2 × ArH). HRMS (HESI+): [M]2+: calculated for C28H32N4O22+ (m/z): 228.1257; found: 228.1255.
2,2′-(Nonane-1,9-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (23)
Compound 23 was isolated as white solid, yield 128 mg (78%), mp: 247.5–248.5 °C. 1H NMR (500MHz, CD3OD-d4): δ 1.40–1.50 (m, 10H, 5 × CH2), 2.11–2.17 (m, 4H, 2 × CH2), 4.78 (t, J= 7.6 Hz, 4H, 2 × CH2), 8.06–8.09 (m, 2H, 2 × ArH), 8.39 (d, J= 7.3 Hz, 2H, 2 × ArH), 8.47 (d, J= 8.3 Hz, 2H, 2 × ArH), 8.72 (s, 2H, 2 × CH), 8.75 (d, J= 6.9 Hz, 2H, 2 × ArH), 9.36 (d, J= 7.0 Hz, 2H, 2 × ArH), 10.01 (s, 2H, 2 × ArH). 13C NMR (126 MHz, CD3OD-d4): δ 27.3, 30.0, 30.2, 32.3, 62.9, 125.8, 130.2, 131.2, 132.4, 132.5, 136.1, 136.4, 138.6, 148.2, and 151.2. HRMS (HESI+): [M]2+: calculated for C29H34N4O22+ (m/z): 235.1335; found: 235.1333.
2,2′-(Decane-1,10-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (24)
Compound 24 was isolated as white solid, yield 84 mg (50%), mp: 240.5–241.5 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.25–1.31 (m, 12H, 6 × CH2), 1.98–2.07 (m, 2H, 2 × CH2), 4.74 (t, J= 6.8 Hz, 4H, 2 × CH2), 8.08–8.12 (m, 2H, 2 × ArH), 8.46–8.52 (m, 4H, 4 × ArH), 8.89 (s, 2H, 2 × CH), 8.95 (d, J= 6.7 Hz, 2H, 2 × ArH), 9.16 (d, J= 6.8 Hz, 2H, 2 × ArH), 10.27 (s, 2H, 2 × ArH), 11.94 (s, 2H, 2 × OH). 13C NMR (126 MHz, DMSO-d6): δ 25.4, 28.3, 28.6, 30.3, 60.5, 123.2, 127.9, 129.0, 130.8, 131.4, 133.8, 135.5, 135.8, 146.4, and 150.3. HRMS (HESI+): [M]2+: calculated for C30H36N4O22+ (m/z): 242.1414; found: 242.1409.
2,2′-(Undecane-1,11-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (25)
Compound 25 was isolated as yellow oil, yield 102 mg (60%). 1H NMR (500 MHz, CD3OD-d4): δ 1.28–1.48 (m, 14H, 7 × CH2), 2.11–2.17 (m, 4H, 2 × CH2), 4.78–4.81 (m, 4H, 2 × CH2), 8.04–8.08 (m, 2H, 2 × ArH), 8.37 (d, J= 7.2 Hz, 2H, 2 × ArH), 8.48 (d, J= 8.3 Hz, 2H, 2 × ArH), 8.70 (s, 2H, 2 × CH), 8.77 (d, J= 7.1 Hz, 2H, 2× ArH), 9.34 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.04 (s, 2H, 2 × ArH). 13 C NMR (126 MHz, CD3OD-d4): δ 27.3, 30.1, 30.4, 30.4, 32.3, 62.9, 125.8, 130.2, 131.1, 132.3, 132.5, 136.0, 136.5, 138.5, 148.2, and 151.2. HRMS (HESI+): [M]2+: calculated for C31H38N4O22+ (m/z): 249.1492; found: 249.1488.
2,2′-(Dodecane-1,12-diyl)bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) bromide (26)
Compound 26 was isolated as yellow solid, yield 58 mg (33%), mp: 70.5–72.5 °C. 1H NMR (500 MHz, CD3OD-d4): δ 1.27–1.48 (m, 16H, 8 ×CH2), 2.10–2.16 (m, 4H, 2 × CH2), 4.79 (t, J= 7.6 Hz, 4H, 2 ×CH2), 8.05–8.08 (m, 2H, 2 × ArH), 8.37 (d, J= 7.3 Hz, 2H, 2× ArH), 8.48 (d, J= 8.2 Hz, 2H, 2 × ArH), 8.70 (s, 2H, 2 × CH), 8.76 (d, J= 7.1 Hz, 2H, 2 × ArH), 9.35 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.03 (s, 2H, 2 × ArH). 13C NMR (126 MHz, CD3OD-d4): δ 27.3, 30.1, 30.5, 30.6, 32.4, 62.9, 125.8, 130.2, 131.2, 132.3, 132.5, 136.0, 136.5, 138.5, 148.2, and 151.2. HRMS (HESI+): [M]2+: calculated for C32H40N4O22+ (m/z): 256.1570; found: 256.1567.
Synthesis of unsymmetrical isoquinolinium-pyridiniumamide bisquaternary salts 30–32
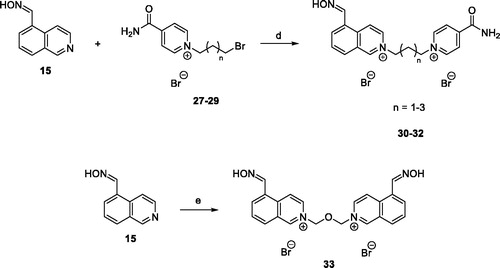
To a solution of monoquaternary isonicotinamide salts 27–29 (0.45 mM) in DMF (0.5 ml) was added isoquinoline-5-carbaldehyde oxime (15) (0.58 mM). The resulting mixture was stirred for 48 h at 73 °C. The solvent was evaporated under reduced pressure, and the crude product purified by crystallisation from EtOH, filtered, washed with EtOH and allowed to dry at room temperature.
2–(3-(4-carbamoylpyridinium-1-yl)propyl)-5-((hydroxyimino)methyl)isoquinolin-2-ium bromide (30)
Compound 30 was isolated as white solid, yield 171 mg (77%), mp: 261.0–262.5 °C. 1H NMR (500 MHz, DMSO-d6): δ 2.81–2.87 (m, 2H, CH2), 4.88–4.94 (m, 4H, 2 × CH2), 8.10–8.14 (m, 1H, ArH), 8.30 (br s, 1H, NH), 8.49–8.55 (m, 4H, 4× ArH), 8.74 (br s, 1H, NH), 8.91 (s, 1H, CH), 9.00 (d, J= 7.1 Hz, 1H, ArH), 9.20 (d, J= 7.1 Hz, 1H, ArH), 9.38 (d, J= 6.8 Hz, 2H, 2 × ArH), 10.34 (s, 1H, ArH), 11.95 (s, 1H, OH). 13C NMR (126 MHz, DMSO-d6): δ 31.3, 57.1, 57.4, 123.3, 125.9, 128.0, 129.0, 130.9, 131.5, 134.0, 135.6, 136.0, 145.9, 146.4, 148.1, 150.8, and 163.1. HRMS (HESI+): [M]2+: calculated for C19H20N4O22+ (m/z): 168.0788; found: 168.0785.
2–(4-(4-carbamoylpyridinium-1-yl)butyl)-5-((hydroxyimino)methyl)isoquinolin-2-ium bromide (31)
Compound 31 was isolated as white solid, yield 122 mg (53%), mp: 138.5–140.0 °C. 1H NMR (500 MHz, DMSO-d6): δ 2.05–2.14 (m, 4H, 2 × CH2), 4.77–4.85 (m, 4H, 2 × CH2), 8.09–8.12 (m, 1H, ArH), 8.28 (br s, 1H, NH), 8.46–8.48 (m, 3H, 3 × ArH), 8.52 (d, J= 8.2 Hz, 1H, ArH), 8.71 (br s, 1H, NH), 8.89 (s, 1H, CH), 8.97 (d, J= 7.1Hz, 1H, ArH), 9.17 (d, J= 7.1 Hz, 1H, ArH), 9.34 (d, J= 6.5 Hz, 2H, 2 × ArH), 10.32 (s, 1H, ArH), 11.95 (s, 1H, OH). 13C NMR (126 MHz, DMSO-d6): δ 26.7, 27.0, 59.6, 59.8, 123.2, 125.8, 128.0, 129.0, 130.8, 131.5, 133.9, 135.5, 135.9, 145.7, 146.4, 148.0, 150.5, and 163.2. HRMS (HESI+): [M]2+: calculated for C20H22N4O22+ (m/z): 175.0866; found: 175.0865.
2–(5-(4-carbamoylpyridinium-1-yl)pentyl)-5-((hydroxyimino)methyl)isoquinolin-2-ium bromide (32)
Compound 32 was isolated as yellow solid, yield 164 mg (70%), mp: 232.5–233.5 °C. 1H NMR (500 MHz, DMSO-d6): δ 1.35–1.41 (m, 2H, CH2), 2.01–2.15 (m, 4H, 2 × CH2), 4.70–4.79 (m, 4H, 2 × CH2), 8.09–8.13 (m, 1H, ArH), 8.29 (br s, 1H, NH), 8.47–8.48 (m, 3H, 3 × ArH), 8.54 (d, J= 8.3 Hz, 1H, ArH), 8.72 (br s, 1H, NH), 8.90 (s, 1H, CH), 8.99 (d, J= 7.1 Hz, 1H, ArH), 9.18 (d, J= 7.1 Hz, 1H, ArH), 9.37 (d, J= 6.7 Hz, 2H, 2 × ArH), 10.35 (s, 1H, ArH), 11.95 (s, 1H, OH). 13C NMR (126 MHz, DMSO-d6): δ 21.8, 29.4, 29.8, 59.9, 60.2, 123.2, 125.7, 127.9, 129.0, 130.8, 131.4, 133.8, 135.6, 135.9, 145.7, 146.4, 148.0, 150.4, and 163.2. HRMS (HESI+): [M]2+: calculated for C21H24N4O22+ (m/z): 182.0944; found: 182.0942.
Synthesis of 2,2′-(oxybis(methylene))bis(5-((hydroxyimino)methyl)isoquinolin-2-ium) chloride (33)
Bis(chloromethyl)ether (0.025 ml, 0.29 mM) was added at room temperature to isoquinoline-5-carbaldehyde oxime (15) (100 mg, 0.58 mM) in DMF (0.5 ml), and the resulting mixture was stirred for 48 h at 73 °C. The solvent was evaporated under reduced pressure, and the crude product purified by crystallisation from EtOH, filtered, washed with EtOH and allowed to dry at room temperature. The compound 33 was isolated as a yellow solid in yield 60 mg (45%), mp: 182.5–184.5 °C. 1H NMR (500 MHz, CD3OD-d4): δ 6.50 (s, 4H, 2 ×CH2), 8.11–8.14 (m, 2H, 2 × ArH), 8.46 (d, J = 6.8 Hz, 2H, 2 × ArH), 8.55 (d, J= 8.3 Hz, 2H, 2 × ArH), 8.72 (s, 2H, 2 × CH), 8.90 (d, J= 7.1 Hz, 2H, 2 × ArH), 9.41 (d, J= 7.1 Hz, 2H, 2 × ArH), 10.27 (s, 2H, 2 × ArH). 13C NMR (126 MHz, CD3OD-d4): δ 88.4, 126.0, 129.8, 131.5, 132.8, 133.3, 134.9, 137.1, 139.7, 148.0, 151.5. HRMS (HESI+): [M]+: calculated for C22H20N4O32+ (m/z): 194.0762; found: 194.0763.
Cholinesterase preparation
Human erythrocyte lysate containing AChE (3.1.1.7) and human plasma containing BChE (EC 3.1.1.8) were prepared at the Department of Toxicology and Military Pharmacy (University of Defence, Hradec Kralove, Czech Republic). The blood samples were collected from healthy volunteers from the vein into a disposable syringe containing 3.8% sodium citrate (the ration blood/citrate was 1:10 w/w). The citrated blood was centrifuged for 20 min at 2856 × g and the plasma was removed. The erythrocytes were washed three times with 100 mM phosphate buffer (pH 7.4) and then hemolysed in 100 mM phosphate buffer (pH 7.4) in a ratio 1:10 (w/w), frozen, and kept under −80 °CCitation11. Human plasma was used as a source of BChE and was prepared from heparinised human blood. Blood was centrifuged for 20 min (4 °C, 5000 RPM) by Hettich Universal 320 R centrifuge. The plasma was separated and stored at −80 °C.
The recombinant form of human AChE/BChE was prepared in Department of Chemistry (Faculty of Science, University of Hradec Kralove, Hradec Kralove, Czech Republic)Citation12 and purified using NGC Medium-Pressure Chromatography System (Bio-Rad, Hercules, CA). The total volume of 6–8 ml of medium containing secreted protein was desalted using 5 ml HiTrap Desalting column (GE Healthcare, Chicago, IL) equilibrated with buffer A (20 mM sodium phosphate buffer, 150 mM NaCl, 15 mM imidazole and 20% glycerol; pH 7.4). Acquired supernatant was loaded onto a 1 ml HisTrap FF column (GE Healthcare, Chicago, IL) equilibrated with buffer A. Captured proteins were eluted with buffer B (20 mM sodium phosphate buffer, 150 mM NaCl, 500 mM imidazole and 20% glycerol; pH 7.4). Imidazole was subsequently removed by repeated centrifugation in Amicon Ultra-4 (Ultracel-10K) tube (Merck Millipore, Burlington, MA). Protein concentration was determined by linearised Bradford method adapted for 96-well plate.
Inhibition assay
Inhibitory effect of the tested oximes on the AChE/BChE activity was determined using the Ellman’s method and is expressed as IC50 i.e. concentration that reduces the cholinesterase activity by 50%. 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman’s reagent, DTNB), phosphate buffer (PB, pH 7.4), acetylthicholine (ATCh) and butyrylthiocholine (BTCh), were purchased from Merck, Prague, Czech Republic. For measuring purposes – polystyrene Nunc 96-well microplates with flat bottom shape (ThermoFisher Scientific, Waltham, MA) were utilised. All assays were carried out in 0.1 M KH2PO4/K2HPO4 buffer, pH 7.4. Enzyme solutions were prepared at 2.0 units/ml in 2 ml aliquots. The assay medium (100 µl) consisted of 40 µl of 0.1 M phosphate buffer (pH 7.4), 20 µl of 0.25 mM DTNB, 10 µl of the enzyme, and 20 µl of 0.25 mM substrate (ATCh iodide solution).
Inhibitor solutions in concentration range 10−3 – 10−9 M were prepared. Tested compounds were 5 min preincubated. The reaction was started by the immediate addition of the substrate (20 µl). The activity was determined by measuring the increase in absorbance at 436 for AChE/412 nm for BChE at 37 °C at 2 min intervals – using a Multi-mode microplate reader Synergy 2 (Vermont, Perkinsville, VT). Each concentration was assayed in triplicate. Software GraphPad Prism version 5 (San Diego, CA) was used for the statistical data evaluation and IC50 values were calculated.
Reactivation assay
Reactivation ability of oxime 30 was evaluated on human erythrocyte AChE and reactivation ability of oximes 17–18 and 30–32 was evaluated on human plasmatic BChE. Enzyme was inhibited by the solution of appropriate cholinesterase inhibitor – sarin, VX and paraoxon in propan-2-ol at concentration 10−5 M for 60 min. Excess of OP inhibitor was subsequently removed using octadecylsilane-bonded silica gel SPE cartridge. The assay medium (100 µl) consisted of 40 µl of 0.1 M phosphate buffer (pH 7.4), 20 µl of 0.25 mM DTNB, 10 µl of the inhibited enzyme, 10 µl of oxime (concentration 100 µM or 10 µM) and it was incubated for 10 min at 37 °C. The reaction was started by addition of substrate ATCh/BTCh (0.25 mM, 20 µl). Activity of AChE/BChE was measured spectrophotometrically at 436 nm by the modified method according to EllmanCitation13 using Multi-mode microplate reader Synergy version 2 (Vermont, Perkinsville, VT). Each concentration of reactivator was assayed in triplicate. The obtained data were used to compute reactivation potency (R; EquationEquation (1)(1)
(1) . Results were corrected for oximolysis and intrinsic inhibition of by oxime reactivator.
(1)
(1)
ΔA0 indicates absorbance change caused by intact AChE/BChE (phosphate buffer was used instead of AChE/BChE inhibitor solution), ΔAi indicates absorbance change provided by cholinesterase exposed inhibitors and ΔAr indicates absorbance change caused by AChE/BChE incubated with solution of reactivator.
Reactivation kinetics
Selected compound (17) with best reactivation ability was further analysed in order to get reactivation kinetics parameters as follows. Human recombinant BChE was inhibited by 25 µM 4-nitrophenyl isopropyl methylphosphonate (NIMP-sarin surrogate) or 4-nitrophenyl ethyl methylphosphonate (NEMP-VX surrogate) for 30 min in order to obtain >99% inhibition. The excess of OP was removed by dialysis against 25 mM Na-phosphate buffer (pH 7.4) for 16 h (two buffer exchanges). Inhibited enzyme was incubated for 8 different times (0.5, 1, 1.5, 2, 5, 8, 10, and 15 min) with 7 different concentrations of tested oxime (5, 10, 30, 50, 80, 100, and 200 µM). The reaction mixture (100 µl final volume) consisted of 10 µl of inhibited enzyme (1.95 ng of total protein), 20 µl of 2.5 mM DTNB, 10 µl of corresponding oxime solution and 50 µl of 25 mM Na-phosphate buffer (pH 7.4). The reaction was started by addition of 10 µl of 10 mM substrate BTCh. The catalytic activity of reactivated enzyme was measured spectrophotometrically at 436 nm using Spark multimode microplate reader (Tecan, Männedorf, Switzerland). Acquired data were analysed by non-linear regression analysis according to Worek et al.Citation14–16 using GraphPad Prism version 8.2 (San Diego, CA).
Molecular docking procedure
From the online PDB database (rcsb.org), six various models of hAChE and hBChE (pdb id: 4ey7, 4bds, 5fpq, 6cqt, 5hf9, 2xqf) were downloaded and prepared for flexible molecular docking by MGL Tools utilities. The preparation of the receptors involved removal of the surplus copies of the enzyme chains, nonbonded inhibitors, addition of polar hydrogens, and merging of nonpolar ones. Default Gasteiger charges were assigned to all atoms. Flexible parts of the enzyme were determined by a spherical selection of residues (R = 11 Å) around the centre of the co-crystalised inhibitor in the enzyme active site. In the same points, the centres of the grid box of 33 × 33 × 33 Å were positioned. The rotatable bonds in the flexible residues were detected automatically by the AutoDock Tools version 1.5.4 programme (Scripps Research Institute, La Jolla, California, USA) . Given the limitation of the programme used for flexible molecular docking, water molecules had to be removed from the system. The flexible receptor parts contained 30–40 residues. The studied ligands were first drawn in HyperChem version 8.0 (Hypercube Inc., Gainesville, Florida, USA), then manually protonated as suggested by MarvinSketch version 6.2.0 software (http://www.chemaxon.com; ChemAxon Ltd., Budapest, Hungary), geometrically optimised by the semiempirical quantum-chemistry PM3 method, and stored as pdb files. The structures of the ligands were processed for docking in a way similar to the above-mentioned flexible parts of the receptor by AutoDock Tools version 1.5.4 programme. Molecular docking was carried out with the AutoDock Vina version 1.1.2 programme (Scripps Research Institute, La Jolla, California, USA) utilising computer resources of the Czech National Grid Infrastructure MetaCentrum. Each docking task was repeated 10 times with the exhaustiveness parameter set to 16, employing 16 CPU in parallel multithreading. From the obtained results, the solutions reaching the minimum predicted Gibbs binding energy were taken as the top-scoring modes. The graphic representations of the docked poses were rendered in PyMOL version 1.3 (the PyMOL Molecular Graphics System, version 1.5.0.4 Schrödinger, LLC New York, NY).
Results and discussion
Structural design and chemical synthesis
The isoquinolinium moiety represents structural template in the design of novel compounds. It is bulkier compared to pyridinium scaffold, ubiquitous feature in the commercial AChE reactivators. The isoquinolinium moiety was chosen for expected stronger binding via cation-π interactions with the cholinesterase binding sites which was previously confirmed for isoquinolinium AChE inhibitorsCitation17. Oxime moiety at position 5 of isoquinolinium scaffold was selected to plausibly effectively reactivate phosphylated AChE or BChE and, simultaneously, being able to penetrate to the close proximity of phosphylated serine within the active site. The peripheral binding ligands differ and include, for instance, bulky scaffolds like 5-carbaldoximequinolinium moiety attached via linker or medium size scaffolds like pyridinium amide attached via linker in order to define possible differences in further interactions with the AChE or BChE binding sites. Notably, pyridinium amides were formerly highlighted for binding within AChE peripheral siteCitation18.
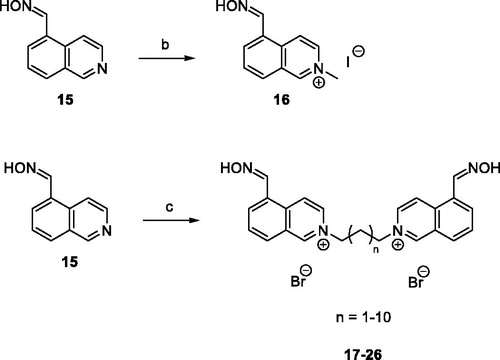
Regarding the synthesis, aldoxime precursor 15 was prepared by nucleophilic addition of a hydroxylamine (NH2OH) to the aldehyde 14 in EtOH in 70% yield (Scheme 1).
Subsequent nucleophilic bimolecular substitution (SN2) of 15 either with the methyl iodide (CH3I) or dibromoalkanes allowed formation of isoquinolinium salt 16 or bisisoquinolinium salts 17–26, respectively, with intermediate to quantitative yields (Scheme 2).
Scheme 2. Preparation of quaternary salts 16–26. Reagents and conditions: (b) CH3I, EtOH, reflux, 24 h, 71%; (c) dibromoalkanes, DMF, 73 °C, 48 h, 33–94%.
Further, the group of unsymmetrical isoquinoline-isonicotinamide bisquaternary salts was synthesized by SN2 reaction of aldoxime 15 with monoquaternary isonicotinamide salts 27–29. The reaction was carried out in DMF at 73 °C, and final products 30–32 were obtained in 53–77% yield (Scheme 3). Monoquaternary salts 27–29 were prepared according to the procedure described by Chennamaneni et al.Citation19. In this work, symmetrical bisisoquinolinium salt containing dimethylene ether chain was also synthesized by reacting aldoxime 15 with bis(chloromethyl)ether in DMF as a solvent. The product 33 was obtained in 45% yield (Scheme 3). Based on HPLC with UV detection (λ = 254 nm), the non-calibrated purity of all products was ≥95%.
In vitro inhibition
All developed compounds and standard obidoxime were initially tested for inhibition of hAChE or hBChE. The obidoxime resulted as poor AChE inhibitor (IC50 ∼197 µM) and insignificant BChE inhibitor (IC50 ∼5440 µM). The majority of novel compounds were found to inhibit hAChE in nanomolar or low micromolar range (). The exception was found for compound 30 that revealed inhibition in micromolar scale (IC50 ∼116 µM). As expected, the compounds with longer linkers (e.g. 23–26 with nine to twelve membered chains) were found powerful hAChE inhibitors. This phenomenon was previously described for similar quaternary isoquinolinium or quinolinium compounds and also correlates well with the topology of AChE cavityCitation20.
Table 1. Inhibition of hAChE and hBChE by prepared compounds.
Additionally, the majority of the compounds were found slightly less potent hBChE inhibitors with IC50 values at micromolar range. Besides these, five compounds displayed high micromolar inhibition (17–18) or low milimolar (30–32) inhibition. All of them bore shorter linkers (three to five-membered chains) that are probably not able to optimally interact with active site of hBChE. Compared to AChE, this discrepancy is obviously ascribed to different structural aspects between cholinesterasesCitation21. Interestingly, three of them were found in the subgroup containing pyridinium amide (30–32) that were also similarly ranked as weak inhibitors of hAChECitation18.
In vitro reactivation
For the purpose of reactivation screening, only slightly potent inhibitors of AChE or BChE should be considered in order to avoid cholinesterase inhibition increment already caused by OPs. From this point of view, the maximal attainable plasmatic concentration for animal/human should be considered. It is presumed that attainable plasmatic concentration is maximally 100 µM after i.m. administration of a high dose of reactivatorCitation22. Thus, two concentrations (100 and 10 µM, maximal attainable concentration and one order of magnitude lower concentration) were chosen for the screening and the prepared compounds with IC50 over 100 µM for AChE or BChE and residual enzyme activity over 50% were selected for reactivation experiments ().
For AChE, only compound 30 was found weak inhibitor and was tested for reactivation of sarin, VX or paraoxon inhibited hAChE and compared to obidoxime (). The obidoxime showed reactivation for all the tested OPs with less ability to counteract phosphylated VX. The isoquinolinium carbaldoxime with pyridinium amide moiety 30 resulted with minimal reactivation in case of all tested OPs and was expelled from further kinetic experiments.
Table 2. Reactivation of hAChE inhibited by sarin, VX and paraoxon by selected compounds.
For BChE, compounds 17–18 (inhibition of hBChE over 100 µM) and 30–32 (inhibition of hBChE over 1000 µM) were selected and tested for reactivation potential against sarin, VX or paraoxon inhibited hBChE and compared to obidoxime (). The obidoxime was found to have some reactivation ability for sarin and VX, but markedly lower for paraoxon. Apparently, some novel compounds (17–18) showed markedly improved reactivation than obidoxime for sarin (17), VX (17–18) and paraoxon (17) when tested at 100 µM. This finding seems to be important since obidoxime formerly resulted as the best reactivator of hBChE inhibited by tabunCitation23, although its reactivation was found not appropriate for constructing a pseudo-catalytic scavenger. More interestingly, these symmetrical isoquinolinium carbaldoximes with three or four-member linkers were found better hBChE reactivators than obidoxime at 10 µM for sarin and VX. On the other hand, the isoquinolinium carbaldoximes with pyridinium amide moiety (30–32) which were poor BChE inhibitors were endowed with minimal reactivation for all tested OPs.
Table 3. Reactivation of hBChE inhibited by sarin, VX and paraoxon by selected compounds.
Reactivation kinetics
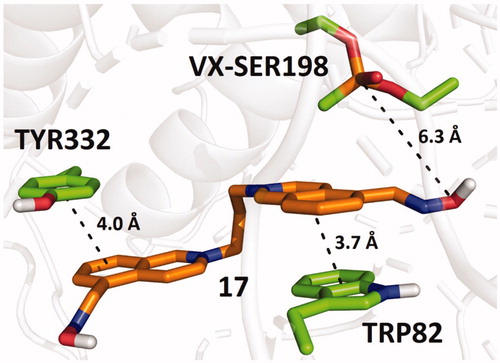
The best reactivator of OP-inhibited hBChE was selected for further kinetic experiments. The affinity of oxime 17 towards OP-inhibited human recombinant BChE (reflected by KD) and the ability to remove the phosphyl residue from the active site of the enzyme (reflected by the reactivity constant kr) were determined (). The specific overall second-order reactivation rate constants (kr2=kr/KD) were calculated. Although compound 17 showed a significantly higher affinity for the phosphylated enzyme, its ability to remove OP-moiety from hBChE was found lower compared to obidoxime for both used OPs. However, the overall reactivation rate indicated increased reactivation of NEMP (VX surrogate)-inhibited BChE by compound 17, when it resulted slightly lower for NIMP (sarin surrogate)-inhibited BChE if compared to obidoxime.
Table 4. Reactivation kinetics of human recombinant BChE inhibited by NIMP (sarin surrogate) and NEMP (VX surrogate) using selected compounds.
Molecular docking studies
In order to interpret obtained in vitro results, the molecular docking was conducted. Prediction of binding modes and binding affinities of the studied compounds in hAChE, hBChE, sarin-hAChE, VX-hAChE, POX-hAChE, and VX-hBChE models was performed with imposing torsional flexibility on several tens of the active site residues in AutoDock Vina version 1.1.2 software, employing high-performance computing grid networkCitation24.
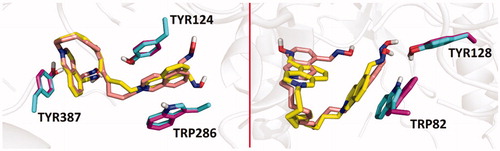
From the binding energy estimates of the top-scoring binding modes of the studied compounds and obidoxime in hAChE (pdb id: 4ey7), it seems probable that lengthening of the linker in bis-isoquinolinium aldoximes increases the binding affinity, which is also in a good correlation with decreasing experimental values of pIC50 for hAChE (R2=0.67, p= .0025). The strongest inhibition of hAChE in vitro was observed for compounds 25 and 26 that provided binding energy estimate of −13.9 and –13.7 kcal/mol, respectively (). A very similar trend for the IC50 values and the number of carbon atoms in the linker of bis-isoquinolinium aldoximes can be seen in the case of hBChE inhibition. Again, ligands 25 and 26, containing 11 and 12 carbons in the linker chain, showed the strongest experimental inhibition potency in hBChE. On the other hand, changes in the binding energy estimates of bis-isoquinolinium aldoximes and obidoxime in hBChE (pdb id: 4bds) correlate significantly worse with the values of pIC50 than it is observed in the case of hAChE inhibition (R2=0.35, p= 0.0176). In hBChE, compounds 25 and 26 provided binding energy estimates of –11.1 and –11.0 kcal/mol. In the case of hAChE simulation, compounds 25 and 26 occupy approximately the same region in the enzyme active site in which the compounds are stabilised by π–π stacking with TYR124, TRP286 and TYR337 (, left). These interactions are considerably limited in hBChE model, where the compounds 25 and 26 are fixed in the binding mode by π–π stacking only with TRP82 and by hydrogen bonding with TYR128 (, right).
Figure 3. Overlaid predicted binding modes of compounds 25 (yellow) and 26 (light pink) in hAChE (pdb id: 4ey7, left) and hBChE (pdb id: 4bds, right). The residues interacting with 25 and 26 are coloured in light green and purple, respectively.
Further molecular docking studies with hAChE inhibited by sarin, VX and POX revealed that the predicted binding energy of bis-isoquinolinium aldoximes reaches the optimal values if the linker contains 5 or 7 carbon atoms (i.e. 19 and 21). Nearly in all three cases of OP-inhibited hAChE, the estimated binding energy for bis-isoquinolinium aldoximes are higher than the estimated binding energy resulting from molecular docking of the compounds in intact hAChE model. Only the bis-isoquinolinium aldoxime 30 exhibited some reactivation potency for all types of OP-inhibited hAChE, whilst its estimated binding affinity in the inhibited enzymes reached relatively low levels (; i.e. sarin-hAChE –12.1 kcal/mol, VX-hAChE –11.6 kcal/mol, POX-hAChE –11.9 kcal/mol). Other isoquinolinium aldoximes exhibited mostly stronger in silico binding energy for OP-inhibited hAChEs. It can thus be hypothesized that high binding energy of the bis-isoquinolinium aldoximes decreases the reactivation ability. This may be a result of stronger inhibition ability, which is supported by stronger binding of the reactivator in the OP-inhibited active site.
Figure 4. Predicted binding mode of compound 30 (blue) in hAChE inhibited by sarin (A; pdb id: 5fpq), VX (B; pdb id: 6cqt) and paraoxon (C; pdb id: 5hf9).
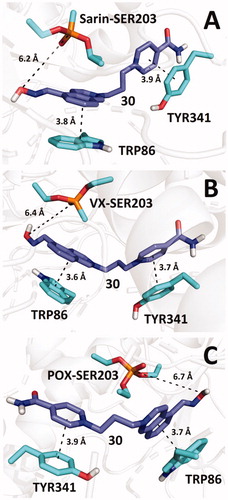
Compound 30 interacts with TRP86 and TYR341 by π–π stacking in such a way that the aldoxime group is not optimally pointed towards the phosphorus atom in OPs. Nonetheless, similar spatial arrangement of aldoxime reactivators can be found in various docking studies and even in X-ray models of OP-inhibited AChE, co-crystalised with aldoximes (e.g. HI-6 in model pdb id: 5fpq)Citation25,Citation26. Despite the fact that molecular mechanics cannot in principle predict the necessary orientation of the aldoxime group towards the phosphorus atom because this follows only from quantum chemistry calculations, such initial unfavourable geometric arrangements suggest that reactivation requires certain degree of conformational flexibility of the moiety bearing the aldoxime function to enable reactivation. High flexibility of the pyridinium aldoxime moiety was observed in computational simulations and confirmed in X-ray experiments for example in the case of sarin-inhibited hAChE reactivation by HI-6Citation25.
An opposite relationship was found when the predicted binding energies were compared with reactivation of hBChE inhibited by VX. The best reactivation ability in vitro was observed for compound 17 which also exhibited the highest binding energy estimate in hBChE-VX model (−12.3 kcal/mol). Comparing both trends in OP-hAChE/hBChE, it can be hypothesized that the binding energy of the reactivator in the inhibited enzyme active site has to reach an optimal value (i.e. approximately around −12.0 kcal/mol), otherwise, it loses its reactivation ability. Apparently, bis-isoquinolinium aldoximes exhibiting in silico binding energy far from the supposed optimum proved practically no reactivation potency in the studied OP-hAChE/hBChE complexes. According to several quantum-chemical simulations of similar reactivations processes in OP-hAChE/hBChE, it seems that binding of the aldoxime reactivators in the enzymes’ active sites stabilises the transitions states in the reactivation coordinateCitation27. Intermolecular interactions of aldoxime reactivators with residues in hAChE/hBChE influences also deprotonation of the oxime function of positively charged reactivators and contributes to release of serine anion and to its neutralisationCitation28. Nonetheless, more accurate studies, involving explicit water molecules, have to be performed in order to elucidate the relationships between the binding strength and the reactivation potencyCitation29.
The binding mode of 17 in hBChE-VX predicted by molecular docking, which exhibited the highest reactivation potency, is shown in . Compound 17 interacts with the enzyme mainly through π–π stacking with TRP82 and TYR332. The predicted binding energies of the studied compounds in the selected cholinesterase models are summarised in .
Table 5. The lowest predicted binding energies of the studied compounds in selected cholinesterase models by molecular docking.
Conclusion
In summary, the series of fifteen symmetrical and unsymmetrical isoquinolinium-5-carbaldoximes was designed and prepared. The novel compounds were evaluated for intrinsic hAChE and hBChE inhibition, when the majority of novel compounds resulted with high inhibition of both enzymes. The selected compounds with lower inhibitory ability were further tested for reactivation of hAChE or hBChE inhibited by sarin, VX or paraoxon. The AChE reactivation for all used OPs was found negligible if compared to the reactivation of obidoxime. On the contrary, two compounds were found to reactivate hBChE inhibited by sarin or VX in better way than obidoxime at human attainable concentration. One compound resulted as better reactivator to obidoxime for NEMP (VX-surrogate)-inhibited BChE. The in vitro results were further compared to in silico data determined by molecular docking studies and rationalized. The obtained data possess important structural insight and future directions on designing potent BChE reactivators.
Acknowledgement
Authors are grateful to Bc. Magdalena Herrmannova and Karolina Pisova for their skilful technical assistance.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol 1981;21:7–30.
- Dolezal R, Korabecny J, Malinak D, et al. Ligand-based 3D QSAR analysis of reactivation potency of mono-and bis-pyridinium aldoximes toward VX-inhibited rat acetylcholinesterase. J Mol Graph 2015;56:113–29.
- Moshiri M, Darchini-Maragheh E, Balali-Mood M. Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru 2012;20:81.
- Musilek K, Dolezal M, Gunn‐Moore F, et al. Design, evaluation and structure—Activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med Res Rev 2011;31:548–75.
- Watson A, Opresko D, Young RA, et al. Organophosphate nerve agents. Handbook of toxicology of chemical warfare agents. 2nd ed. London: Handbook of Toxicology of Chemical Warfare Agents; 2009.
- Žunec S, Radić B, Kuča K, et al. Comparative determination of the efficacy of bispyridinium oximes in paraoxon poisoning/Usporedno određivanje učinkovitosti bispiridinijevih oksima pri trovanju paraoksonom. Arh Hig Rada Toksikol 2015;66:129–34.
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002;40:803–16.
- Gorecki L, Korabecny J, Musilek K, et al. SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch Toxicol 2016;90:2831–59.
- Sharma R, Gupta B, Singh N, et al. Development and structural modifications of cholinesterase reactivators against chemical warfare agents in last decade: a review. Mini-Rev Med Chem 2015;15:58–72.
- Kassa J, Misik J, Karasova JZ. A comparison of the potency of a novel bispyridinium oxime K203 and currently available oximes (obidoxime, HI‐6) to counteract the acute neurotoxicity of sarin in rats. Basic Clin Pharmacol Toxicol 2012;111:333–8.
- Musilova L, Kuca K, Jung Y-S, et al. In vitro oxime-assisted reactivation of paraoxon-inhibited human acetylcholinesterase and butyrylcholinesterase. Clin Toxicol 2009;47:545–50.
- Gorecki L, Andrys R, Schmidt M, et al. Cysteine-targeted insecticides against A. gambiae acetyl-cholinesterase are neither selective nor reversible inhibitors. ACS Med Chem Lett in press. doi: 10.1021/acsmedchemlett.9b00477
- Ellman GL, Courtney KD, Jr, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Worek F, Thiermann H, Szinicz L, et al. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 2004;68:2237–48.
- Worek F, Wille T, Aurbek N, et al. Reactivation of organophosphate-inhibited human, Cynomolgus monkey, swine and guinea pig acetylcholinesterase by MMB-4: a modified kinetic approach. Toxicol Appl Pharmacol 2010;249:231–7.
- Worek F, von der Wellen J, Musilek K, et al. Reactivation kinetics of a homologous series of bispyridinium bis-oximes with nerve agent-inhibited human acetylcholinesterase. Arch Toxicol 2012;86:1379–86.
- Musilek K, Komloova M, Holas O, et al. Preparation and in vitro screening of symmetrical bis-isoquinolinium cholinesterase inhibitors bearing various connecting linkage–implications for early Myasthenia gravis treatment. Eur J Med Chem 2011;46:811–8.
- Zorbaz T, Malinak D, Maraković N, et al. Pyridinium oximes with ortho-positioned chlorine moiety exhibit improved physicochemical properties and efficient reactivation of human acetylcholinesterase inhibited by several nerve agents. J Med Chem 2018;61:10753–66.
- Chennamaneni SR, Vobalaboina V, Garlapati A. Quaternary salts of 4, 3′ and 4, 4′ bis-pyridinium monooximes: synthesis and biological activity. Bioorg Med Chem Lett 2005;15:3076–80.
- Sussman JL, Harel M, Frolow F, et al. Atomic-structure of acetylcholinesterase from torpedo-californica – A prototypic acetylcholine-binding protein. Science 1991;253:872–9.
- Bajda M, Więckowska A, Hebda M, et al. Structure-based search for new inhibitors of cholinesterases. Int J Mol Sci 2013;14:5608–32.
- Bajgar J, Fusek J, Kuca K, et al. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini-Rev Med Chem 2007;7:461–6.
- Kovarik Z, Katalinić M, Šinko G, et al. Pseudo-catalytic scavenging: searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem-Biol Interact 2010;187:167–71.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61.
- Allgardsson A, Berg L, Akfur C, et al. Structure of a prereaction complex between the nerve agent sarin, its biological target acetylcholinesterase, and the antidote HI-6. Proc Natl Acad Sci USA 2016;113:5514–9.
- Gorecki L, Soukup O, Kucera T, et al. Oxime K203: a drug candidate for the treatment of tabun intoxication. Arch Toxicol 2019;93:673–91.
- Zhang YK, Kua J, McCammon JA. Role of the catalytic triad and oxyanion hole in acetylcholinesterase catalysis: an ab initio QM/MM study. J Am Chem Soc 2002;124:10572–7.
- Driant T, Nachon F, Ollivier C, et al. On the influence of the protonation states of active site residues on AChE reactivation: a QM/MM approach. ChemBioChem 2017;18:666–75.
- Giacoppo JOS, Franca TCC, Kuca K, et al. Molecular modeling and in vitro reactivation study between the oxime BI-6 and acetylcholinesterase inhibited by different nerve agents. J Biomol Struct Dyn 2015;33:2048–58.