Abstract
Tumours reprogram their metabolism to acquire an evolutionary advantage over normal cells. However, not all such metabolic pathways support energy production. An example of these metabolic pathways is the Methylglyoxal (MG) one. This pathway helps maintain the redox state, and it might act as a phosphate sensor that monitors the intracellular phosphate levels. In this work, we discuss the biochemical step of the MG pathway and interrelate it with cancer.
Keywords:
Introduction
Reactive oxygen species (ROS) are highly reactive chemical species that target various biomolecules within the cell. ROS examples include superoxides, peroxides, singlet oxygen, hydroxyl radical, alpha-oxygen, and alkoxyl radicalsCitation1–3. The prevailing unifying scientific theory is that ROS, especially at lower levels, supports malignant transformation, carcinogenesis, and invasion, which supports metastatic transformationCitation4–8. However, ROS at a higher dosage inhibits tumour growth, with some anticancer agents' primary mode of action being ROS-induced cell injuryCitation8,Citation9. Such a paradox and biphasic or dual role depending on the dose is termed “hormesis”Citation10. The intracellular NADPH level is one of the key determinants that manipulates the ROS hormesis. Besides the pentose phosphate pathway (PPP), the MG pathway is an additional pathway that contributes to NADPH pooling of the cells.
Many tumour cells rely on anaerobic glycolysis even in presence of oxygen, an effect which is called “Warburg metabolism”. The methylglyoxal pathway (MG) is branching from the glycolysis pathway to manage the redox state of the cell rather than contributing to the production of energy in the form of ATP.
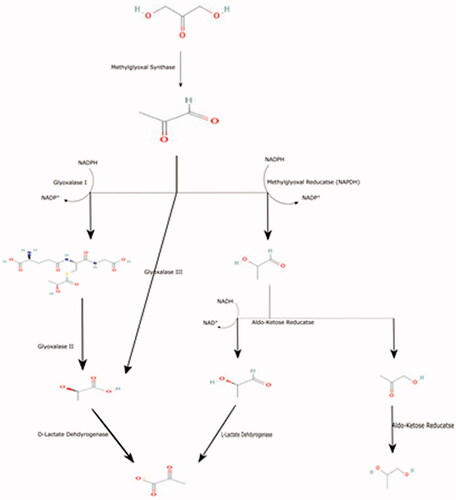
The MG pathway occurs in a series of steps that regulate the intracellular NADPH content, and it can also act as a phosphate sensor. MG is metabolised mainly either glutathione-dependent or glutathione-independent pathway, as follow (See ).
Branching of glycolysis
Glycolysis is composed of two parts: the first one is the preparatory phase, followed by the second part, called the pay-off phaseCitation11. The pay-off part starts with forming D-glyceraldehyde 3-phosphate, which it is a crossroad of many biochemical pathways, including glycolysisCitation11, pentose phosphate pathwayCitation12, as well as methylglyoxal metabolic pathway, as well as photosynthesisCitation13,Citation14.
D-glyceraldehyde 3-phosphate is isomerised to dihydroxyacetone phosphate (DHAP) by triosephosphate isomeraseCitation11. After that, DHAP is converted to MG (2-oxoaldehyde) and phosphate by the methylglyoxal synthase enzyme (MGS) activity.
MGS is also known as glycerone-phosphate phosphate-lyase (methylglyoxal-forming). Although MGS is a bacterial enzyme, early data showed that MGS was isolated from the goat liverCitation15.
The optimum pH for MGS activity is 7.5, i.e., alkaline pHCitation16,Citation17.
Phosphate acts as a competitive allosteric inhibitor of MGS. Some data concludes that the methylglyoxal pathway supports cells by phosphate and acts as a phosphate sensorCitation18,Citation19. ATP, 3-phosphoglycerate, and phosphoenolpyruvate inhibit MGSCitation15,Citation16. Therefore, it can be concluded that the MG pathway does not co-occur with the pay-off phase of the glycolysis pathwayCitation11. Other MGS inhibitors include: phosphoglycolohydroxamic acidCitation20.
MG can be formed via several biochemical pathwaysCitation5,Citation6. MG is involved in many disorders including, cancer, diabetes, CNS disorders, etc.Citation21. MG is a highly toxic compoundCitation22–24, and therefore, the body detoxifies the MG either through glutathione-dependent or glutathione-independent pathways.
Glutathione-Dependent pathway
Lactoylglutathione:
MG is isomerised to hemithioacetal adducts and then form (R)-S-lactoylglutathione spontaneously in the presence of glutathione. The reaction is catalysed by a lactoylglutathione lyase (glyoxalase I)Citation25–28.
The optimum pH for Glyoxalase I (GLO1) is broad, but generally, the optimum pH is alkaline around 8Citation29.
GLO1 is over-expressed in many cancer types, such as, lung, colon, prostate, etc.Citation30–32 GLO1 is also involved in their growth and progression, and resistance to the treatmentCitation33–37. GLO1 inhibition showed promising results as anti-tumour propertyCitation21, as well as re-sensitizes the resistant tumours to the treatmentCitation38.
GLO1 inhibitors include 4–(7-azaindole)-substituted 6-phenyl-N-hydroxypyridones, Flavonoids, S-bromobenzylglutathione cyclopentyl diester (BrBzGCp2), and CurcuminCitation21,Citation39–42. Other GLO1 inhibitors include Ionising radiation,Citation43, and nitric oxide (NO)Citation44.
One of the supported observations is that GLO-1 is highly associated with tumorigenesis and tumour invasionCitation45, where GLO-1 is GSH dependent and NADPH-dependent methylglyoxal reductase does not utilise GSH (see below).
D-Lactate
(R)-S-lactoylglutathione in the presence of water produced reduced glutathione and D-lactate via Hydroxyacylglutathione hydrolase (glyoxalase II)Citation46.
In cancer, the role of GLO2 might be more complex. Although, tumour suppressor genes, e.g., p63 and p73, up-regulate GLO2 expression by tumour genes, GLO2 supported pro-survival rate rather than apoptosis, which is paradoxical. Cytosolic GLO2, not mitochondrial, prevents the MG induced-apoptosisCitation47. Further contradiction is coming where GLO2 expression is lower in cancerous tissues than the normal parent tissue that might delve into other mysteriesCitation48. Therefore, it will be wisely to reveal that GLO2 expression is associated with growth arrest. One of the suggested answers that release this chain sinnet knot is that the correlation between (i) D-lactate (presence of GLO2 supports D-lactate production), (ii) reduced glutathione (absence of GLO2 prevent the reduced GSH recycle), and (iii) the state of the cell (phases of cell cycle, whether in growth phase, or proliferation, or even dormancy), in a way that solves the redox paradoxCitation31,Citation49–53. At the same time, the glutathione either supports the cell proliferation by diminishing the reactive oxygen species that initiate the programmed cell death or preventing the malignant transformationCitation12,Citation54,Citation55.
Although the optimum pH for GLO2 is broad from 6.8–7.5Citation46, it yet shifted towards alkalinity. Also, cytoplasmic acidification is accompanied by a subsequent decrease in its activityCitation56.
S-carbobenzoxyglutathione is one of many GLO2 inhibitorsCitation57 (and for further information ref Al-Shar’i et al.Citation58).
D-lactate is a toxic substance associated with many diseases, including short-bowel syndrome, D-Lactic acidosis, and neurotoxicityCitation59,Citation60. Potentially, D-Lactate might be metabolised to pyruvate via the putative human D-lactate dehydrogenaseCitation61–63, or excreted extracellularlyCitation63–66, or even recycled back to MG (MG-Shunt)Citation67–72. Some form of probiotics, e.g., lactobacillus sp. has D-lactate dehydrogenase activity, which might utilise the D-lactate, and therefore benefits during D—Lactic acidosisCitation73.
Glutathione-independent pathway
Due to the activity of NADPH dependent Aldose-ketose Reductase (AKR), MG can be metabolised into:
Lactaldehyde formation
In the presence of NADPH, AKR converts MG to lactaldehyde and produces NADP+. The NADP+ might be re-cycled to its reduced form (NADPH) using the pentose phosphate pathway (PPP)Citation12. Therefore, the possible crosstalk between the MG and PPP is likely in the cell's physiology to manage the cell's redox state. In other words, there is a possibility of MG-PPP shunt to restore the NADPH.
AKR (NADPH) is also called NADPH-dependent methylglyoxal reductase Gre2, lactaldehyde:NADP+ oxidoreductase, and lactaldehyde dehydrogenase (NADP+).
The optimum pH for AKR (NADPH) is 6.5Citation74, and the range is 5 to 7.5Citation75, which moves towards the acidic pH. Therefore, it would be wise to reveal if the AKR (NADPH) is associated with either (i) cellular arrest neurodegeneration and/or renal impairment in case of acidic pHiCitation76–79 or (ii) cellular senescence in case of alkaline pHi, and so the latter support the possibility of malignant transformation tooCitation80–83.
NADP+ inhibits NADPH-dependent MG-reductase; therefore it’s a negative feedback mechanismCitation75,Citation84. Calcium ion and 2-mercaptoethanol are examples of NADPH reductase inhibitorsCitation75,Citation84.
Formation of lactic acid
In the presence of NAD+, Lactaldehyde is converted to L-lactate by aldehyde dehydrogenase (ALDH) to produce – Lactate and NADH.
Aldehyde dehydrogenase is overexpressed in cancerCitation85 and associated with resistance to chemotherapy and radiotherapy, as wellCitation86.
Dyclonine, N,N‑diethylaminobenzaldehyde is an example of an ALDH inhibitorCitation86,Citation87. The optimum pH is around 7.4Citation88.
Acetol formation
MG is converted to hydroxyacetone (acetol) via Aldo–keto reductase (AKR)Citation89. AKR summarizes a broad family of oxidoreductase enzymes with varying capacities for the detoxification of MGCitation36.
The AKR metabolises the MG, and the product is 95% acetol and 5% D-lactaldehydeCitation90. Acetol is further metabolised to L-1,2-propanediolCitation90 by the same enzymeCitation90.
The optimum pH for AKR depends on the organism, tissue within the organism, etc. that might reflect enzymatic resilience in its activity to confers the organismal adaptability (evolutionary advantageous), e.g., the optimum pH of AKR in Helicobacter is in a range from 4–9, the optimum one is 5.5Citation91, however, in more complex organisms the optimum is more basic in the small intestineCitation92. Therefore, it will be challenging to detect or estimate the exact pH of AKR in cancer cells as these are characterised by their heterogeneityCitation93.
AKR is overexpressed in many types of cancer, such as lung, uterine, colorectal, etc.Citation92.
For AKR inhibitors, the Pharmacodiagnostics approach should be implemented for the rational use of selection for example, for
AKR1B1 is inhibited by epalrestatCitation94
AKR1C1 is inhibited by 3-bromo-5-phenylsalicylic acidCitation95.
AKR1C3 is inhibited by cinnamic acidCitation96,Citation97.
Notes on the MG metabolic pathway
Based on the reaction-diffusion kinetics, tumour neoplasm could be seen as multiple habitats. Tumour neoplasms show at least cline evolution from the macro-blood vessel (tumour cord). Therefore, tumour cells reprogram their metabolic machineries due to glucose, oxygen diffusion, and the lack of efficient removal of the metabolites (adaptive evolution)Citation93,Citation98,Citation99. Therefore, it will not be surprising if the multi-regional biopsy to diagnose the tumours will not find the expression of the enzymes that are involved in MG metabolic pathways to the same degree (see ), which is entirely predictable in the MG metabolic pathway as MG has a negative effect on the vasculatureCitation100.
Table 1. Shows the different set of enzymes involved in methylglyoxal metabolic pathway.
Also, due to the reaction-diffusion kinetics, the hypoxic, necrotic regions within the tumour due to accumulation of lactate, and decreasing oxygen supply -at farther area from the blood vessel- the production of ROS increasesCitation101–103, and this might result in increasing the activity of NADPH oxidase (primary cellular source of ROS production)Citation104–107. Therefore, the stimulation of oxidative stress-reducing agents is initiating the NADPH oxidase – MG metabolic pathway cross-talk, which has risen to come in a way that might confer the cancer cell survivalCitation12,Citation108–112.
Concluding remarks and future perspectives
MG is an intermediate product of many cross-roads’ biochemical pathways. The methylglyoxal products are toxic and must be detoxified consequently into many pathways based on various factors, e.g., the level of NADP+, GSH, pH, etc. many of the future perspectives in this issue include:
Detailed studying the interactions between the Pentose Phosphate Pathway (PPP) – as a primary source of NADPH – and MG Pathway, and their possible interrelation with cancerCitation12.
The scientific community should focus in determining the cellular level of MG as a critical determinant of many cellular biochemical pathways (causation) and a powerful tool that tracks the cellular dynamics trajectory (consequences).
Also, these pathways shed the light on importance of the stereochemistry of the cellular metabolites and their impact on carcinogenesis, besides the stereochemistry of the drugs
These biochemical pathways are involved in carcinogenesis, cancer resistance, and treatment regimes. Therefore, implementing the methylglyoxal pathway in tumour biology represents a promising strategy in the therapeutic approaches against cancer, which can add useful anticancer candidates to the community. These suggested candidates might not be the target of Achilles heels of cancer, but it contributes to rationale of the cancer management.
Author contributions
K.O.A. contributed to the conceptualisation, data curation, formal analysis, investigation, resources, software, and writing (original draft). S.J.R. and C.T.S. contributed to the supervision, conceptualisation, data curation, formal analysis, research, resources, software, and writing (review and editing). S.S.A., S.A., J.M., and C.T.S. contributed to the conceptualisation, data curation, methodology, resources, software, resources, and writing (original draft). All authors have read and agreed to the published version of the manuscript.
Disclosure statement
CT Supuran is Editor-in-Chief of Journal of Enzyme Inhibition and Medicinal Chemistry, and he was not involved in the assessment, peer review or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
References
- Jakubczyk K, Dec K, Kałduńska J, et al. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski 2020;48:124–7.
- Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr 1996;16:33–50.
- Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev 2016;116:3029–85.
- Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012;511:1–6.
- Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004;337:1–13.
- Irani K, Xia Y, Zweier JL, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science (80-) 1997;275:1649–52.
- Luanpitpong S, Talbott SJ, Rojanasakul Y, et al. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem 2010;285:38832–40.
- Yang H, Villani RM, Wang H, et al. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res 2018;37:266.
- Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010;44:479–96.
- Mattson MP. Hormesis defined. Ageing Res Rev 2008;7:1–7.
- Alfarouk KO, Verduzco D, Rauch C, et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 2014;1:777–802.
- Alfarouk KO, Ahmed SBM, Elliott RL, et al. The pentose phosphate pathway dynamics in cancer and its dependency on intracellular pH. Metab 2020;10:285.
- Li Z-G. Methylglyoxal. In: plant signaling molecules. Kidlington: Elsevier; 2019. p. 219–33.
- Bhagavan N, Ha C-E. Carbohydrate metabolism I. In: Essentials of medical biochemistry. 2nd ed. Waltham, MA: Elsevier; 2015. p. 165–85.
- Hopper DJ, Cooper RA. The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett 1971;13:213–6.
- Huang KX, Rudolph FB, Bennett GN. Characterization of methylglyoxal synthase from Clostridium acetobutylicum ATCC 824 and its use in the formation of 1, 2-propanediol. Appl Environ Microbiol 1999;65:3244–7.
- Hopper DJ, Cooper RA. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J 1972;128:321–9.
- Saadat D, Harrison DH. The crystal structure of methylglyoxal synthase from Escherichia coli. Structure 1999;7:309–17.
- Falahati H, Pazhang M, Zareian S, et al. Transmitting the allosteric signal in methylglyoxal synthase. Protein Eng Des Sel 2013;26:445–52.
- Marks GT, Harris TK, Massiah MA, et al. Mechanistic implications of methylglyoxal synthase complexed with phosphoglycolohydroxamic acid as observed by X-ray crystallography and NMR spectroscopy. Biochemistry 2001;40:6805–18.
- McMurray KMJ, Distler MG, Sidhu PS, et al. Glo1 inhibitors for neuropsychiatric and anti-epileptic drug development. Biochem Soc Trans 2014;42:461–7.
- Miyazawa N, Abe M, Souma T, et al. Methylglyoxal augments intracellular oxidative stress in human aortic endothelial cells. Free Radic Res 2010;44:101–7.
- Allaman I, Bélanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci 2015;9:23.
- Talukdar D, Chaudhuri BS, Ray M, Ray S. Critical evaluation of toxic versus beneficial effects of methylglyoxal. Biochemistry 2009;74:1059–69.
- Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 1990;269:1–11.
- Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med 1993;14:287–371.
- Thornalley PJ. Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 2003;31:1343–8.
- Mannervik B. Molecular enzymology of the glyoxalase system. Drug Metabol Drug Interact 2008;23:13–27.
- Uotila L, Koivusalo M. Purification and properties of glyoxalase I from sheep liver. Eur J Biochem 1975;52:493–503.
- Davidson SD, Milanesa DM, Mallouh C, Choudhury MS, et al. A possible regulatory role of glyoxalase I in cell viability of human prostate cancer. Urol Res 2002; May30:116–21.
- Davidson SD, Cherry JP, Choudhury MS, et al. Glyoxalase I activity in human prostate cancer: a potential marker and importance in chemotherapy. J Urol 1999;161:690–1.
- Ranganathan S, Walsh ES, Tew KD. Glyoxalase I in detoxification: studies using a glyoxalase I transfectant cell line. Biochem J 1995;309 ( Pt 1):127–31.
- Rulli A, Carli L, Romani R, et al. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res Treat 2001;66:67–72.
- Kreycy N, Gotzian C, Fleming T, et al. Glyoxalase 1 expression is associated with an unfavorable prognosis of oropharyngeal squamous cell carcinoma. BMC Cancer 2017;17:382.
- Antognelli C, Mezzasoma L, Fettucciari K, et al. Role of glyoxalase I in the proliferation and apoptosis control of human LNCaP and PC3 prostate cancer cells. Prostate 2013;73:121–32.
- Morgenstern J, Campos Campos M, Nawroth P, Fleming T. The glyoxalase system—new insights into an ancient metabolism. Antioxidants 2020;9:939.
- Sakamoto H, Mashima T, Kizaki A, et al. Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood 2000;95:3214–8.
- Sakamoto H, Mashima T, Sato S, et al. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res 2001;7:2513–8.
- Thornalley PJ, Edwards LG, Kang Y, et al. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol 1996;51:1365–72.
- Santel T, Pflug G, Hemdan NYA, et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS One 2008;3:e3508.
- Takasawa R, Takahashi S, Saeki K, et al. Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg Med Chem 2008;16:3969–75.
- Chiba T, Ohwada J, Sakamoto H, et al. Design and evaluation of azaindole-substituted N-hydroxypyridones as glyoxalase i inhibitors. Bioorg Med Chem Lett 2012;22:7486–9.
- Antognelli C, Palumbo I, Aristei C, Talesa VN. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-κB. Br J Cancer 2014;111:395–406.
- Miller AG, Smith DG, Bhat M, Nagaraj RH. Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem 2006;281:11864–71.
- Mearini E, Romani R, Mearini L, et al. Differing expression of enzymes of the glyoxalase system in superficial and invasive bladder carcinomas. Eur J Cancer 2002;38:1946–50.
- Cameron AD, Ridderström M, Olin B, Mannervik B. Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure 1999;7:1067–78.
- Xu Y, Chen X. Glyoxalase II, a detoxifying enzyme of glycolysis byproduct methylglyoxal and a target of p63 and p73, is a pro-survival factor of the p53 family. J Biol Chem 2006;281:26702–13.
- Antognelli C, Baldracchini F, Talesa VN, et al. Overexpression of glyoxalase system enzymes in human kidney tumor. Cancer J 2006;12:222–8.
- Chaiswing L, St Clair WH, St Clair DK. Redox paradox: a novel approach to therapeutics-resistant cancer. Antioxid Redox Signal 2018;29:1237–72.
- Frandsen JR, Narayanasamy P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol 2018;14:465–73.
- Chang T, Wang R, Olson DJH, et al. Modification of Akt1 by methylglyoxal promotes the proliferation of vascular smooth muscle cells. Faseb J 2011;25:1746–57.
- de Bari L, Scirè A, Minnelli C, et al. Interplay among oxidative stress, methylglyoxal pathway and s-glutathionylation. Antioxidants 2021;10:1–17.
- Braun JD, Pastene DO, Breedijk A, et al. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci Rep 2019;9:1152–14.
- Da Veiga Moreira J, Peres S, Steyaert J-MM, et al. Cell cycle progression is regulated by intertwined redox oscillators. Theor Biol Med Model 2015;12:10.
- Moreira J da V, Hamraz M, Abolhassani M, et al. The redox status of cancer cells supports mechanisms behind the Warburg effect. Metabolites 2016;6:33.
- Reiger M, Lassak J, Jung K. Deciphering the role of the type II glyoxalase isoenzyme YcbL (GlxII-2) in Escherichia coli. FEMS Microbiol Lett 2015;362:1–7.
- Hsu YR, Norton SJ. S-carbobenzoxyglutathione: a competitive inhibitor of mammalian glyoxalase II. J Med Chem 1983;26:1784–5.
- Al-Shar’i NA, Hassan M, Al-Balas Q, Almaaytah A. Identification of possible glyoxalase II inhibitors as anticancer agents by a customized 3D structure-based pharmacophore model. Jordan J Pharm Sci 2015;8:83–103.
- Puwanant M, Mo-Suwan L, Patrapinyokul S. Recurrent D-lactic acidosis in a child with short bowel syndrome. Asia Pac J Clin Nutr 2005;14:195–8.
- Thurn JR, Pierpont GL, Ludvigsen CW, Eckfeldt JH. D-lactate encephalopathy. Am J Med 1985;79:717–21.
- Flick MJ, Konieczny SF. Identification of putative mammalian D-lactate dehydrogenase enzymes. Biochem Biophys Res Commun 2002;295:910–6.
- Monroe GR, van Eerde AM, Tessadori F, et al. Identification of human D lactate dehydrogenase deficiency. Nat Commun 2019;10:1477.
- De Bari L, Atlante A, Guaragnella N, et al. D-lactate transport and metabolism in rat liver mitochondria. Biochem J 2002;365:391–403.
- Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol 2000;529(Pt 2):285–93.
- Halestrap AP. Monocarboxylic acid transport. Compr Physiol 2013;3:1611–43.
- Ullrich KJ, Rumrich G, Klöss S. Reabsorption of monocarboxylic acids in the proximal tubule of the rat kidney. I. Transport kinetics of D-lactate, Na+-dependence, pH-dependence and effect of inhibitors. Pflugers Arch 1982;395:212–9.
- Zhao Q, Su Y, Wang Z, et al. Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evol Biol 2014;14:86–18.
- Misra K, Banerjee AB, Ray S, Ray M. Glyoxalase III from Escherichia coli: a single novel enzyme for the conversion of methylglyoxal into D-lactate without reduced glutathione. Biochem J 1995;305(Pt 3):999–1003.
- Lee JY, Song J, Kwon K, et al. Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet 2012;21:3215–25.
- Subedi KP, Choi D, Kim I, et al. Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol Microbiol 2011;81:926–36.
- Ariga H, Takahashi-Niki K, Kato I, et al. Neuroprotective function of DJ-1 in Parkinson's disease. Oxid Med Cell Longev 2013;2013:683920.
- Pohanka M. D-lactic acid as a metabolite: toxicology, diagnosis, and detection. Biomed Res Int 2020;2020:3419034.
- Yilmaz B, Schibli S, Macpherson AJ, Sokollik C. D-lactic acidosis: successful suppression of D-lactate-producing lactobacillus by probiotics. Pediatrics 2018;142:e20180337.
- Saikusa T, Rhee HI, Watanabe K, et al. Metabolism of 2-oxoaldehydes in bacteria: Purification and characterization of methylglyoxal reductase from Escherichia coli. Agric Biol Chem 1987;51:1893–9.
- Schomburg D, Schomburg I, Chang A, editors. Methylglyoxal reductase (NADPH-dependent). In: Class 1·Oxidoreductases Vol S1. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. p. 32–7.
- Jung E, Kang WS, Jo K, Kim J. Ethyl pyruvate prevents renal damage induced by methylglyoxal-derived advanced glycation end products. J Diabetes Res 2019;2019:4058280.
- Ward RA, McLeish KR. Methylglyoxal: a stimulus to neutrophil oxygen radical production in chronic renal failure? Nephrol Dial Transplant 2004;19:1702–7.
- Jensen TM, Vistisen D, Fleming T, et al. Methylglyoxal is associated with changes in kidney function among individuals with screen-detected Type 2 diabetes mellitus. Diabet Med 2016;33:1625–31.
- Tezuka Y, Nakaya I, Nakayama K, et al. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology (Carlton) 2019;24:943–50.
- Beck J, Turnquist C, Horikawa I, Harris C. Targeting cellular senescence in cancer and aging: roles of p53 and its isoforms. Carcinogenesis 2020;41:1017–29.
- Lee S, Schmitt CA. The dynamic nature of senescence in cancer. Nat Cell Biol 2019;21:94–101.
- Kim YH, Park TJ. Cellular senescence in cancer. BMB Rep 2019;52:42–6.
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75:685–705.
- Inoue Y, Rhee H, Watanabe K, et al. Metabolism of 2-oxoaldehyde in mold. Purification and characterization of two methylglyoxal reductases from Aspergillus niger. Eur J Biochem 1988;171:213–8.
- Kang JH, Lee SH, Hong D, et al. Aldehyde dehydrogenase is used by cancer cells for energy metabolism. Exp Mol Med 2016;48:e272.
- Dinavahi SS, Bazewicz CG, Gowda R, Robertson GP. Aldehyde dehydrogenase inhibitors for cancer therapeutics. Trends Pharmacol Sci 2019;40:774–89.
- Wang W, Zheng S, He H, et al. N,N-diethylaminobenzaldehyde targets aldehyde dehydrogenase to eradicate human pancreatic cancer cells . Exp Ther Med 2020;20:662–70.
- Glatt H, Rost K, Frank H, et al. Detoxification of promutagenic aldehydes derived from methylpyrenes by human aldehyde dehydrogenases ALDH2 and ALDH3A1. Arch Biochem Biophys 2008;477:196–205.
- Ko J, Kim I, Yoo S, et al. Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases. J Bacteriol 2005;187:5782–9.
- Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem 1992;267:4364–9.
- Cornally D, Mee B, MacDonaill C, et al. Aldo-keto reductase from Helicobacter pylori-role in adaptation to growth at acid pH. Febs J 2008;275:3041–50.
- Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev 2008;40:553–624.
- Alfarouk KO, Ibrahim ME, Gatenby RA, Brown JS. Riparian ecosystems in human cancers. Evol Appl 2013;6:46–53.
- Ji J, Xu MX, Qian TY, et al. The AKR1B1 inhibitor epalrestat suppresses the progression of cervical cancer. Mol Biol Rep 2020;47:6091–103.
- Zeng C-M, Chang L-L, Ying M-D, et al. Aldo-Keto Reductase AKR1C1-AKR1C4: functions, regulation, and intervention for anti-cancer therapy. Front Pharmacol 2017;8:119.
- Brozic P, Golob B, Gomboc N, et al. Cinnamic acids as new inhibitors of 17beta-hydroxysteroid dehydrogenase type 5 (AKR1C3). Mol Cell Endocrinol 2006;248:233–5.
- Gobec S, Brožič P, Rižner TL. Nonsteroidal anti-inflammatory drugs and their analogues as inhibitors of aldo-keto reductase AKR1C3: New lead compounds for the development of anticancer agents. Bioorg Med Chem Lett 2005;15:5170–5.
- Alfarouk KO, Shayoub MEA, Muddathir AK, et al. Evolution of tumor metabolism might reflect carcinogenesis as a reverse evolution process (dismantling of multicellularity). Cancers (Basel) 2011;3:3002–17.
- Lloyd MC, Alfarouk KO, Verduzco D, et al. Vascular measurements correlate with estrogen receptor status. BMC Cancer 2014;14:279.
- Vulesevic B, McNeill B, Giacco F, et al. Methylglyoxal-induced endothelial cell loss and inflammation contribute to the development of diabetic cardiomyopathy. Diabetes 2016;65:1699–713.
- Tafani M, Sansone L, Limana F, et al. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid Med Cell Longev 2016;2016:3907147.
- Chen R, Lai UH, Zhu L, et al. Reactive oxygen species formation in the brain at different oxygen levels: the role of hypoxia inducible factors. Front Cell Dev Biol 2018;6:132.
- Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol (1985) 2007;102:2379–88.
- Rathore R, Zheng Y-M, Niu C-F, et al. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–31.
- Meitzler JL, Antony S, Wu Y, et al. NADPH oxidases: a perspective on reactive oxygen species production in tumor biology. Antioxid Redox Signal 2014;20:2873–89.
- Sedeek M, Nasrallah R, Touyz RM, Hébert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 2013;24:1512–8.
- Kleniewska P, Piechota A, Skibska B, Gorąca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp 2012;60:277–94.
- Sahoo S, Meijles DN, Pagano PJ. NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin Sci (Lond) 2016;130:317–35.
- Nigro C, Leone A, Raciti GA, et al. Methylglyoxal-glyoxalase 1 balance: the root of vascular damage. Int J Mol Sci 2017;18:188.
- Mukohda M, Morita T, Okada M, et al. Long-term methylglyoxal treatment causes endothelial dysfunction of rat isolated mesenteric artery. J Vet Med Sci 2013;75:151–7.
- Nass N, Sel S, Ignatov A, et al. Oxidative stress and glyoxalase i activity mediate dicarbonyl toxicity in MCF-7 mamma carcinoma cells and a tamoxifen resistant derivative. Biochim Biophys Acta – Gen Subj 2016;1860:1272–80.
- Wen X, Iwata K, Ikuta K, et al. NOX1/NADPH oxidase regulates the expression of multidrug resistance-associated protein 1 and maintains intracellular glutathione levels. Febs J 2019; 86:678–87.