Abstract
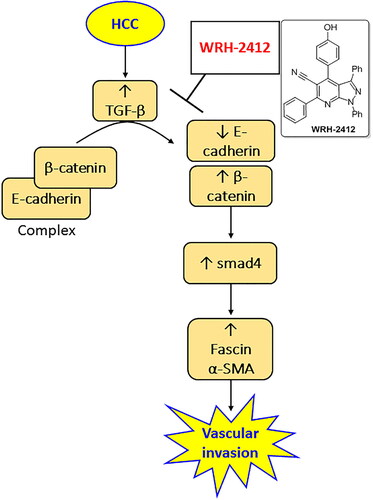
Hepatocellular carcinoma is considered one of the most lethal cancers, which is characterised by increasing prevalence associated with high level of invasion and metastasis. The novel synthetic pyrazolo[3,4-b]pyridine compound, WRH-2412, was reported to exhibit in vitro antitumor activity. This study was conducted to evaluate the antitumor activity of WRH-2412 in HCC induced in rats through affecting the TGF-β/β-catenin/α-SMA pathway. Antitumor activity of WRH-2412 was evaluated by calculating the rat’s survival rate and by assessment of serum α-fetoprotein. Protein expression of TGF-β, β-catenin, E-cadherin, fascin and gene expression of SMAD4 and α-SMA were determined in hepatic tissue of rats. WRH-2412 produced antitumor activity by significantly increasing the rats’ survival rate and decreasing serum α-fetoprotein. WRH-2412 significantly reduced an HCC-induced increase in hepatic TGF-β, β-catenin, SMAD4, fascin and α-SMA expression. In addition, WRH-2412 significantly increased hepatic E-cadherin expression.
Introduction
Hepatocellular carcinoma (HCC) is ranked as the sixth most frequent cancer in the world. It occupies the fourth leading cause of cancer-related death. Hepatocarcinogenesis process is considered as a complex multistep process that is affected by a group of risk factors leading to tumour progression and metastasis.Citation1 It is characterised by several molecular modifications such as alterations in the gene expression of some growth factors, proteolytic enzymes, extracellular matrix components and inflammatory cytokines.Citation2 Due to its rapid growth rate as well as the surrounding fibrotic tissue created by the chronic inflammation, HCC is featured as a highly hypoxic tumour.Citation2 The median five-year survival of HCC patients is below 20% due to tumour recurrence and resistance to chemotherapy.Citation3 Therefore, there is an urge to find new therapeutic agents to improve the overall survival in HCC patients.
One of the candidate therapeutic agents against HCC is pyrazolo[3,4-b] pyridine compounds, which are small molecules proved as antitumor,Citation4,Citation5 antibacterial,Citation6 anti-inflammatoryCitation7 and antioxidant.Citation8 Of these novel synthesised pyrazolo[3,4-b]pyridine compounds is 4–(4-hydroxyphenyl)-1,3,6-triphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile, WRH-2412 (. Recently, WRH-2412 has been proven to have promising in vitro anticancer action with broad spectrum anti-proliferative activities as disclosed through the US-NCI assays, with outstanding growth inhibition full panel GI50 (MG-MID) value equals 2.16 μM.Citation9 Interestingly, the antitumor activity of WRH-2412 against HCC hasn’t been investigated in vitro or in vivo.
TGF-β can regulate the different processes in HCC such as tumour proliferation, diffusion and metastasis through binding with both type I and II transmembrane receptor of the serine-threonine kinases leading to heterodimerization of SMAD3 with SMAD4.Citation10 In addition, β-catenin is another important player that has a pivotal biological role in tumour development, growth and regeneration especially those of the liver ranging from hepatitis to HCC.Citation11 Therefore, this study was conducted to evaluate the antitumor activity of WRH-2412 in experimentally induced HCC in rats through affecting the TGF-β and β -catenin and their subsequent effect on the tumour invasion markers, fascin and α-SMA.
Materials and methods
Animal experiments
The animal protocol was approved by Institutional Animal Care and Use Committee, Beni-Suef University (BSU-IACUC), Approval number: 022–347. Forty Sprague–Dawley adult male rats weighing 180–200 g were used. All animals were kept under standard conditions of temperature (25 °C) with a regular 12 h light/12 h dark cycle. Each animal was examined daily and weighed weekly throughout the 16 weeks experiential period. They were classified randomly into five groups, each consisted of 10 rats.
Control group: Rats recieved intraperitoneal (i.p.) injection of phosphate buffer saline (PBS, 10 mM, pH 7.4) twice weekly for 16 weeks.
WRH-2412-treated control group: Rats were injected with 5 mg/kg i.p. WRH-2412 twice weekly for 16 weeks.
HCC group: Rats were i.p. injected with 200 mg/kg thioacetamide (TAA; Tocris Bioscience) twice weekly for 16 weeks.
HCC treated with WRH-2412: Rats were i.p. injected with 5 mg/kg WRH-2412 twice weekly for 16 weeks accompanied by 200 mg/kg TAA i.p., twice weekly for 16 weeks.
Collection of rat samples
Blood samples were collected from retro-orbital plexus of each rat under brief thiopental sodium anaesthesia (40 mg/kg, i.p). Blood was centrifuged at 3000 rpm for 5 min and sera were separated and subsequently stored at −80 °C prior to further analysis. Whole rat livers were removed, dried, weighed, rinsed with normal saline and divided into two aliquots. One aliquot was fixed in 10% buffered formaldehyde for subsequent morphological analysis. The second portion was homogenised in 10 mM PBS, pH 7.4 and stored at −80 °C for biochemical analysis.
Morphologic analysis of liver tissue
Liver samples were cut and fixed in 10% buffered formalin and embedded in paraffin wax. Five micrometer sections were cut and stained with hematoxylin and eosin (H&E). Liver sections were anonymously coded and examined in a blinded manner using a digital camera-aided computer system (Nikon Digital Camera, Japan).
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed on 5-μm paraffin sections cut from a paraffin block of liver. Sections were deparaffinized by heating at 55 °C for 1 h and then rehydrated using xylene and descending concentrations of ethanol. Sections were then subjected to microwave treatment in Antigen retrieval 6. All non-specific binding sites were blocked by using blocking buffer (10% FCS in 0.5% Triton-PBS). Sections were incubated with monoclonal antibodies for TGF-β and β-catenin (Abcam) in 1:500 dilution at 4 °C overnight. Then, were incubated with secondary antibodies (Envision + Dual Link System-HRP, Dakocytomation) conjugated to HRP. The used chromagen was 2% DAB in 50 mM Tris-buffer, pH 7.6. Slides were subsequently counterstained with (H&E).
Evaluation of hepatoprotective effects
Hepatoprotective effects were assessed by measuring the serum activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (BioDiagnostic Co.) spectrophotometrically.
ELISA
α-Fetoprotein (AFP), β-catenin (Cat. Number. MBS700622 MyBioSource, Cat. Number. MBS843456 Mybiosource, respectively). E-cadherin, TGF-β (ab202413) Abcam, (ab119558) Abcam, respectively. Fascin (Cat. Number. MBS266620 Mybiosource) levels were analysed using commercially available ELISA kits.
Quantitative real-time polymerase chain reaction (RT-PCR)
Real-Time-Polymerase Chain Reaction (RT-PCR) was used for determination of SMAD4 and α-SMA gene expression. Total RNA was isolated from liver tissues by using the RNeasy Purification Reagent (Qiagen, Valencia, CA, USA). The list of used primers for SMAD4 and α-SMA were showed in . Real time quantitative PCR was used to determine gene expression according to the instructions for Applied Bio systems version 3.1 software and SYBR Green I (Step One™, USA). β-actin gene was used as a housekeeping gene and an internal reference control and all data are expressed relative to it.
Table 1. Primer sets used in the PCR.
Statistical analysis
The mean ± SEM was used to express data. Kolmogorov-Smirnov test was used to examine normality of sample distribution. Rat survival was assessed by using Kaplan–Meier method. One way ANOVA was used for determination of the differences among groups, followed by Bonferroni post hoc test. SPSS version 20 (IBM Corp.) was used to perform the statistical analysis. Statistical significance was predefined as p < 0.05.
Results
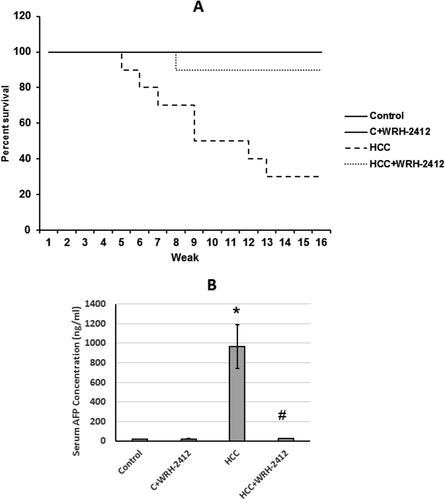
WRH-2412 effect on HCC-induced mortality and AFP elevation
The rat survival rate was increased from 30% in the HCC group to 90% in HCC rats treated with 5 mg/kg of WRH-2412. Control rats treated with WRH-2412 at 5 mg/kg exhibited 100% survival at the end of the experiment. In addition, the percent of rats’ survival was associated with a significant reduction in the serum level of AFP compared with the control group (966 ng/mL in HCC group and 26.48 ng/mL in HCC rats treated with WRH-2412) ().
Figure 2. Effect of 5 mg/kg WRH-2412 on survival rate and AFP serum levels in HCC rats. (A) Survival rate represented as Kaplan-Meier curve. (B) AFP serum levels in the experimental groups. Values are presented as the mean ± SEM, *p < 0.05 vs. control; #p ≤ 0.05 vs. HCC group; AFP: α-fetoprotein; HCC: hepatocellular carcinoma; C: control.
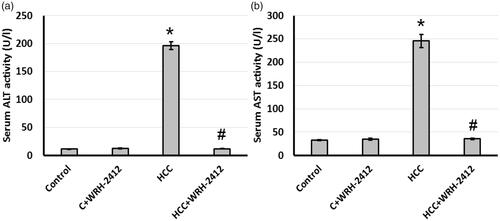
Effect of WRH-2412 on liver function tests
Compared with the control group, serum ALT and AST activities were significantly elevated in the HCC group. Treating HCC rats with 5 mg/kg WRH-2412 resulted in a significant reduction in serum ALT and AST activities compared with HCC group. These results suggested WRH-2412 had hepatoprotective effects against HCC rats (.
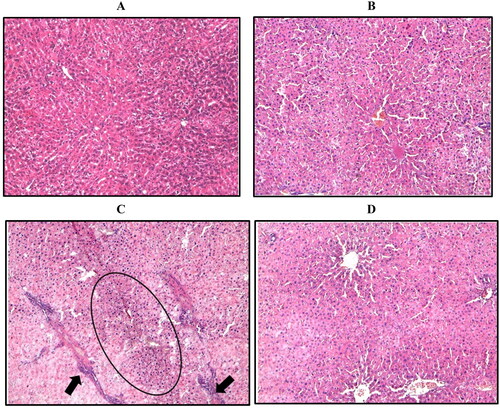
Effect of WRH-2412 on HCC-induced morphological changes
Control group revealed normal appearance of hepatic lobule and liver architecture. While, the hepatocyte architecture in HCC group showed massive breakdown of hepatic tissues, with hyperplastic nodules (Encircled) and apparent heteromorphism. The nuclei were prominent and occupied most of the cells. Prominent connective tissue septa with many blood vessels are also noticed (arrow). The liver of rats treated with WRH-2412 showed a significantly reduction in these histopathological features (.
Figure 4. Representative image of hepatic sections stained with H/E. (A) Control group. (B) Control group treated with 5 mg/kg WRH-2412. (C) The liver architecture of HCC group showed massive break down of hepatic tissue together with hyperplastic nodules (Encircled) and apparent heteromorphism. (D) WRH-2412 treated rats showed greatly reduction in these histopathological features in the liver.
Effect of WRH-2412 on HCC-induced expression of TGF-β
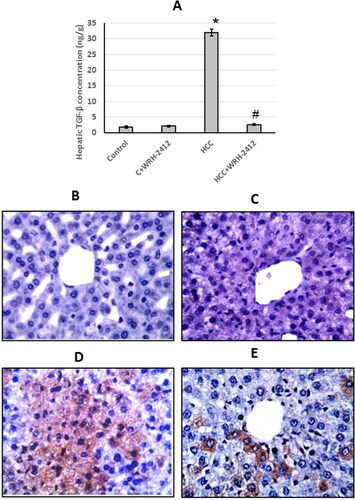
HCC rats showed a significant increase (17.29-fold) in TGF-β protein expression levels compared to the control groups. In addition, liver sections stained with anti-TGF-β antibodies showed increased immunostaining in sections from HCC group as compared to the control group. However, WRH-2412 administration resulted in a significant decrease in TGF-β protein expression levels as compared with the HCC group with no effect on the control group (.
Figure 5. Effect of 5 mg/kg WRH-2412 on TGF-β protein levels (A) as well as liver sections stained with anti- TGF-β antibody in control group (B), control group treated with WRH-2412 (C), HCC group (D) and HCC group treated with WRH-2412 (E). Values are expressed as the mean ± SEM, *p < 0.05 vs. control; #p ≤ 0.05 vs. HCC group. TGF-β: transforming growth factor-β; HCC: hepatocellular carcinoma; C: control.
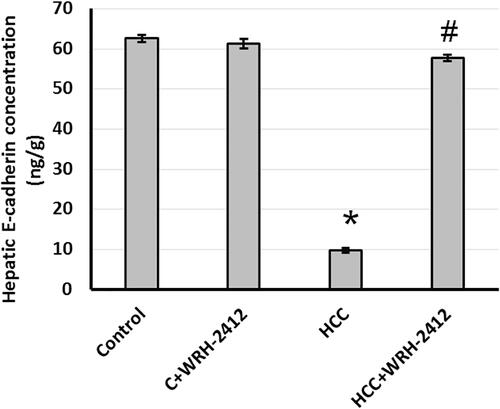
Effect of WRH-2412 on E-cadherin expression
HCC resulted with 83% reduction in E-cadherin expression level compared with the control groups. Nevertheless, HCC rats treated with WRH-2412 resulted in reversion of this effect (.
Effect of WRH-2412 on β-catenin expression
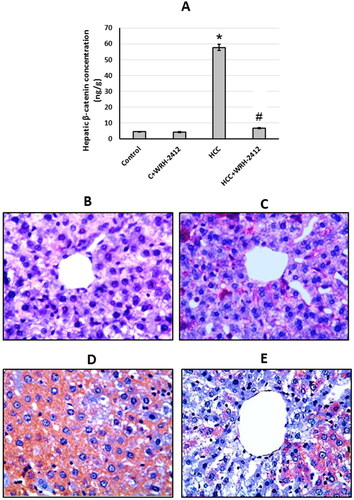
HCC resulted in elevation (12.95-fold) in β-catenin protein expression levels in comparison to the control groups. In addition, liver sections which stained with anti-β-catenin antibodies showed increased immunostaining in sections from HCC group as compared with the control group. Even so, HCC rats treated with WRH-2412 resulted in significant reduction in the expression of β-catenin in HCC rats with no effect on the control rats ().
Figure 7. Effect of 5 mg/kg WRH-2412 on β-catenin protein levels (A) as well as liver sections stained with anti- β-catenin antibody in control group (B), control group treated with WRH-2412 (C), HCC group (D) and HCC group treated with WRH-2412 (E). *p < 0.05 vs. control; #p ≤ 0.05 vs. HCC group; HCC: hepatocellular carcinoma; C: control.
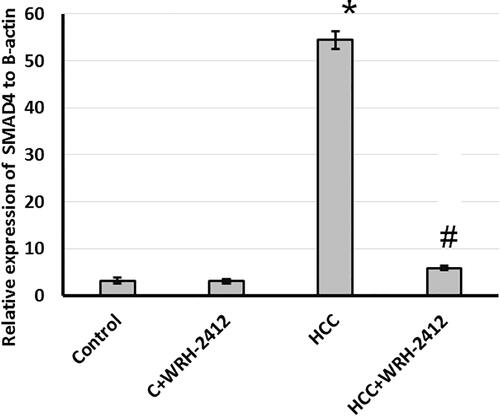
Effect of WRH-2412 on SMAD4 expression
Furthermore, evaluation of hepatic levels of SMAD4 showed significant increase in HCC rats as compared to the control rats. This elevation is blocked by treatment of rats with WRH-2412 with no effect on the control group (.
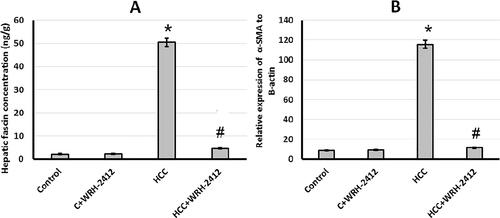
Effect of WRH-2412 on vascular invasion markers
HCC rats demonstrated 22.58- and 12.95-fold increase in fascin protein expression and α-SMA gene expression levels, respectively, compared with the control groups. Nevertheless, HCC group treated with 5 mg/kg WRH-2412 resulted in reduction of both fascin and α-SMA expression compared to HCC group with no effect on the control group ().
4. Discussion
HCC is considered the sixth widespread cancer and the fourth worldwide cause of cancer-related death. There are three systemic drugs approved by FDA for advanced HCC treatment. The microenvironment inside the liver tissues contains many compounds such as cytokines, growth factors and extracellular matrix (ECM) as well as, many other cells as immune cells, kupffer cells, endothelial cells and fibroblasts. All these supplemented the hepatic carcinogenic tissue microenvironment with many valuable resources that help the development of de-novo HCC tumours.Citation12
The first line therapy is represented by sorafenib and lenvatinib, while regorafenib is considered the second line of treatment.Citation13 Although the most effective treatment of patients is liver resection and transplantation, these techniques are not suitable for most patients. Therefore, HCC is placed as the second most lethal cancer, with about five-year survival of 18%. One of the candidate therapeutic agent against HCC are the small molecules, pyrazolo[3,4-b]pyridine compounds, such as WRH-2412 with many therapeutic activities as antiviral and anticancer. Treatment of HCC rats with WRH-2412 proved promising antitumor activity. This can be proved by WRH-2412 ability to reduce HCC-induced elevation in serum α-fetoprotein and number of nodules in the liver. Furthermore, the percent of HCC rats survival was doubled by using WRH-2412. In addition, WRH-2412 produced many improvements in the structure of hepatic histopathological features as indicated by attenuation of destroyed cells, reduction of hyperplastic nodules and heteromorphism. It is the first time to prove the ability of WRH-2412 to produce anticancer activity against experimentally induced HCC.
TGF-β is a pleiotropic, potent, regulatory cytokine and a major component that leads to EMT. Its signaling pathway has a great role in many cellular processes as cellular proliferation, differentiation, migration, apoptosis, adhesion, angiogenesis, immune surveillance and survival.Citation14 In normal cases or in early stages of tumour TGF-β has tumour suppressor effects, while in advanced tumours it has a tumour progression and metastasis effects. Activation of TGF-β receptor with subsequent enhancement of β-catenin/SMAD4 pathway leads to many processes inside the tumour cells as cell reprogramming induction, epithelial phenotyping of primary tumour cells to acquire interstitial cell characteristics and tumour cell invasion to ECM leading to tumour metastasis.Citation15 TGF- β has been recently considered as possible liver tumour marker.Citation16 However, β-catenin is involved in regulating EMT and upregulated in tumours. β-catenin is a complicated protein that plays an important role in regulating several physiological processes, such as differentiation, proliferation, and tissue homeostasis and directly linked to HCC.Citation17 It is associated with cell-to-cell adhesion and activation of the components of ECM. It is activated in about 30% of patients with HCC.Citation18 Finally, one of the downstream of TGF-β/β-catenin is SMAD4, which is overexpressed in many types of cancer. In addition, deletion of SMAD4 gene produced protective effects against pancreatic cancer.Citation19 However, we found that HCC results in overexpression of TGF-β, β-catenin and SMAD4 in rats. HCC rats treated with WRH-2412 reversed all of these effects in HCC rats without affecting the control rats. It it the first time to report that WRH-2412 protects against HCC through blocking TGF-β/β-catenin/SMAD4 axis.
Vascular invasion is present in about 25–50% of HCC patients and represents a great risk factor that enhances tumour recurrence and leads to poor overall survival among patients with HCC.Citation20 Blood flowing into the HCC is done via neovascularization from arterial vessels and taken out primarily by via portal vein. The spread of HCC cells using the portal vein is considered as the major mechanism of intrahepatic metastasis.Citation21 There is a cross talk between the HCC cells, microenvironment and ECM which might lead to vascular invasion.Citation22 One of the vascular invasion markers is fascin, which is an actin-binding protein. Fascin regulates cell motility. Fascin is not expressed in normal cells, while it is overexpression during tumour invasion and metastasis.Citation23 It helps in breaking intercellular junctions, enhancing cell movement and modifying ECM leading to tumour metastasis.Citation24 To enhance tumour invasion, fascin needs the help of many other factors. In addition, α-SMA activation is linked to activation of myofibroblasts. It is associated with progression of several types of malignancy as osteosarcomas, lung adenocarcinoma, head and neck squamous cell carcinoma.Citation25 Finally, E-cadherin is a transmembrane protein that plays a key role in establishing stable adherent junctions. It is associated with dedifferentiation, infiltration and metastasis in many cancers.Citation26 We found that HCC results in increased expression of fascin and α-SMA associated with reduction in E-cadherin expression. Treatment of HCC rats with WRH-2412 reversed these effects in HCC rats without affecting the control rats.
Conclusion
The present study indicated that WRH-2412 has an antitumor effects and may suppress HCC development via inhibition of the TGF-β/β-catenin/α-SMA pathway axis, which may suppress vascular invasion markers. Therefore, WRH-2412 stands out as a promising starting point and as a novel potential therapeutic drug for improving the outcome of patients with HCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/14756366.2023.2218746)
Additional information
Funding
References
- (a) Pisaturo M, Di Fraia A, Occhiello L, Minichini C, Starace M, Iodice V, et al. Genetic Variability in Patients with HCV-Related Hepatocellular Carcinoma. Infect Drug Resist. 2021; 14:5199–5208. (b) Kuchuk O, Tuccitto A, Citterio D, Huber V, Camisaschi C, Milione M, Vergani B, et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology. 2018;7(7):e1445452.
- Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015; 149(5):1226–1239 e1224.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1):7–30.
- (a) Sun Y, Tang H, Wang X, Feng F, Fan T, Zhao D, Xiong B, Xie H, Liu T. Identification of 1 H-pyrazolo [3, 4-b] pyridine derivatives as novel and potent TBK1 inhibitors: design, synthesis, biological evaluation, and molecular docking study. J Enzyme Inhib Med Chem. 2022;37(1):1411–1425. (b) Eissa IH, El-Naggar AM, El-Hashash MA. Design, synthesis, molecular modeling and biological evaluation of novel 1H-pyrazolo [3, 4-b] pyridine derivatives as potential anticancer agents. Bioorg Chem. 2016;67:43–56.
- (a) Liu N, Wang X, Fu Q, Qin Q, Wu T, Lv R, Zhao D, Cheng M. Design, synthesis and biological evaluation of pyrazolo [3, 4-b] pyridine derivatives as TRK inhibitors. RSC Med Chem. 2023;14:85–102; (b) Park A, Hwang J, Lee J-Y, Heo EJ, Na Y-J, Kang S, Jeong K-S, Kim KY, Shin SJ, Lee H. Synthesis of novel 1H-Pyrazolo [3, 4-b] pyridine derivatives as DYRK 1A/1B inhibitors. Bioorg Med Chem Lett. 2021;47:128226.
- El-Gohary NS, Gabr MT, Shaaban MI. Synthesis, molecular modeling and biological evaluation of new pyrazolo[3,4-b]pyridine analogs as potential antimicrobial, antiquorum-sensing and anticancer agents. Bioorg Chem. 2019; 89:102976.
- Mohamed LW, Shaaban MA, Zaher AF, Alhamaky SM, Elsahar AM. Synthesis of new pyrazoles and pyrozolo [3,4-b] pyridines as anti-inflammatory agents by inhibition of COX-2 enzyme. Bioorg Chem. 2019;83:47–54.
- Gouda MA. Synthesis and antioxidant evaluation of some new pyrazolopyridine derivatives. Arch Pharm. 2012;345(2):155–162.
- Barghash RF, Eldehna WM, Kovalova M, Vojackova V, Krystof V, Abdel-Aziz HA. One-pot three-component synthesis of novel pyrazolo[3,4-b]pyridines as potent antileukemic agents. Eur J Med Chem. 2022;227:113952.
- Zhang H-F, Gao X, Wang X, Chen X, Huang Y, Wang L, Xu Z-W. The mechanisms of renin-angiotensin system in hepatocellular carcinoma: From the perspective of liver fibrosis, HCC cell proliferation, metastasis and angiogenesis, and corresponding protection measures. Biomed Pharmacother. 2021;141:111868.
- Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45(5):1298–1305.
- El-Far YM, Khodir AE, Emarah ZA, Ebrahim MA, Al-Gayyar MMH. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Rep. 2022;27(1):9–20.
- Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8(1):e000317.
- Elsherbiny NM, Al-Gayyar MM. Anti-tumor activity of arjunolic acid against Ehrlich ascites carcinoma cells in vivo and in vitro through blocking TGF-beta type 1 receptor. Biomed Pharmacother. 2016;82:28–34.
- Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-beta/Smad signaling pathway. Onco Targets Ther. 2019;12:6897–6905.
- Tajul Arifin K, Sulaiman S, Md Saad S, Ahmad Damanhuri H, Wan Ngah WZ, Mohd Yusof YA. Elevation of tumour markers TGF-beta, M2-PK, OV-6 and AFP in hepatocellular carcinoma (HCC)-induced rats and their suppression by microalgae Chlorella vulgaris. BMC Cancer. 2017;17(1):879.
- Xu Y, Yu X, Sun Z, He Y, Guo W. Roles of lncRNAs mediating Wnt/beta-catenin signaling in HCC. Front Oncol. 2022;12:831366.
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907.
- Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A, Lord CJ, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2(53):53ra75.
- Huang DS, Liu TT, Lu WT, Wang CC, Lin CC, Yong CC, et al. Comparison of portal and capsular microscopic vascular invasion in the outcomes of early HCC after curative resection. Am J Cancer Res. 2022;12(6):2659–2672.
- Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28(4):376–381.
- Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347–354.
- Elewa MA, Al-Gayyar MM, Schaalan MF, Abd El Galil KH, Ebrahim MA, El-Shishtawy MM. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin Exp Metastasis. 2015;32(5):479–493.
- Alshehri MA, Alshehri MM, Albalawi NN, Al-Ghamdi MA, Al-Gayyar MMH. Heparan sulfate proteoglycans and their modification as promising anticancer targets in hepatocellular carcinoma. Oncol Lett. 2021;21(2):173.
- Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, Guo L, Cao L, Zhang X. Overexpression of B7-H3 in alpha-SMA-positive fibroblasts is associated with cancer progression and survival in gastric adenocarcinomas. Front Oncol. 2019;9:1466.
- Hashiguchi M, Ueno S, Sakoda M, Iino S, Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et al. Clinical implication of ZEB-1 and E-cadherin expression in hepatocellular carcinoma (HCC). BMC Cancer. 2013;13:572.