ABSTRACT
Introduction
Before release, vaccine batches are assessed for quality to evaluate whether they meet the product specifications. Vaccine batch tests, in particular of inactivated and toxoid vaccines, still largely rely on in vivo methods. Improved vaccine production processes, ethical concerns, and suboptimal performance of some in vivo tests have led to the development of in vitro alternatives.
Areas covered
This review describes the scientific constraints that need to be overcome for replacement of in vivo batch tests, as well as potential solutions. Topics include the critical quality attributes of vaccines that require testing, the use of cell-based assays to mimic aspects of in vivo vaccine-induced immune responses, how difficulties with testing adjuvanted vaccines in vitro can be overcome, the use of altered batches to validate new in vitro test methods, and how cooperation between different stakeholders is key to moving the transition forward.
Expert opinion
For safety testing, many in vitro alternatives are already available or at an advanced level of development. For potency testing, in vitro alternatives largely comprise immunochemical methods that assess several, but not all critical vaccine properties. One-to-one replacement by in vitro alternatives is not always possible and a combination of methods may be required.
1. Background
Vaccination is a cost-effective strategy to prevent infectious diseases among humans and livestock. Vaccines are biologicals that are used to immunize large groups of healthy individuals and they may be subject to inherent batch-to-batch variation, which is why newly produced vaccine batches require quality assessment before being released to the market. Vaccines have traditionally been generated using trial and error approaches involving in vivo experiments [Citation1]. Subsequently produced batches of such established vaccines are often still tested using in vivo methods to confirm that the quality of the new vaccine batch meets the specifications as defined in the marketing authorization. These specifications concern purity (i.e. freedom from extraneous matter), potency (i.e. the capacity of a vaccine batch to exert its effect), safety (i.e. relative freedom of harmful effects) and efficacy (i.e. effect of vaccination on the target species/population under ideal circumstances) of the vaccine [Citation2]. Over one million animals were used for batch potency and safety testing of medicinal products in the EU in 2017, which corresponds to approximately 12% of total animal use for scientific purposes in the EU [Citation3]. Nowadays, there are several reasons to move away from the use of these in vivo potency and safety tests to assess the quality of vaccine batches. Firstly, significant improvements in and standardization of the vaccine production process, adherence to good manufacturing practice (GMP) standards and in-process controls have resulted in less batch-to-batch variation and a lower risk of producing unsafe or ineffective products [Citation4]. Secondly, the use of large numbers of animals in experiments that may inflict pain and distress is not in line with the ethics of contemporary research and the 3Rs principles of Replacement, Reduction and Refinement [Citation5–7]. Thirdly, the relevance of some in vivo tests is disputed because the test results show high variability and poor reproducibility [Citation7–12]. Fourthly, the use of animal models is expensive, time consuming and risky for personnel when models involve exposure to viable pathogenic organisms [Citation7].
The consistency approach was proposed as a strategy to enable the transition of in vivo to in vitro batch testing of vaccines [Citation13,Citation14]. This approach is based on the principle that quality is the consequence of consistent production of subsequent batches monitored by a GMP quality system [Citation15]. The evaluation of a number of pre-defined vaccine parameters using in vitro physicochemical, immunochemical and cell-based test methods should demonstrate that final batches are of consistent quality, making the use of in vivo tests unnecessary.
Major steps have been taken to promote the use of in vitro alternatives for vaccine batch testing, including the creation of a legal and logistic framework. For instance, Directive 2010/63/EU on the protection of animals used for scientific purposes, which includes regulatory testing of vaccines, states ‘The use of animals for scientific or educational purposes should only be considered where a non-animal alternative is unavailable’ and thus promotes the use of novel in vitro test methods [Citation16]. In addition, a general chapter on the ‘Substitution of in vivo methods by in vitro methods for the quality control of vaccines’ has been incorporated in the European Pharmacopoeia to provide guidance on the substitution of an in vivo test method with non-animal alternatives in cases where one-to-one test replacement cannot be achieved (5.2.14) [Citation17]. Bodies like the Biological Standardization Programme of the European Directorate for the Quality of Medicines & Healthcare (EDQM), which facilitates multi-center validation studies, and the European Center for Validation of Alternative Methods (ECVAM), which acts as a reference laboratory of the European Union, provide the logistic framework needed to validate novel in vitro methods [Citation16,Citation18]. Meanwhile, some in vivo tests of vaccines have been replaced by in vitro alternatives, whereas others are no longer used as will be discussed further below.
In September 2015, industrial, regulatory and scientific experts gathered for a workshop in the Netherlands to identify drivers and barriers for the implementation of the consistency approach, of which the results were published [Citation15]. Newer generation vaccines, including recombinant subunit vaccines (e.g. human papillomavirus and hepatitis B) or conjugate vaccines (e.g. Haemophilus influenzae type b, meningococcus and pneumococcus) are well defined and can be evaluated for quality using in vitro methods, which is why the workshop focused on established live attenuated, inactivated and toxoid vaccines that were in part still tested for quality using in vivo methods [Citation17,Citation19,Citation20]. Identification and development of in vitro methods that provide alternatives to currently used in vivo batch tests of vaccines is hampered by several scientific and other constraints including the following points [Citation15]:
It is difficult to mimic vaccine-induced immune responses using in vitro test methods.
Measuring vaccine properties of adjuvanted vaccines is complex.
There is a need to create subpotent formulations for method validation due to a lack of appropriate non-compliant batches to test (and validate) in vitro methods.
Research into in vitro test methods needs to be prioritized and financed based on number of test animals, level of severity, and performance of the in vivo test.
Knowledge about the critical quality attributes of vaccines and critical process parameters of vaccine production is often limited.
In the present review we will discuss the scientific barriers that have been overcome since the workshop or still need to be dealt with to enable a complete transition from in vivo to in vitro vaccine quality testing.
2. Current repertoire of in vitro vaccine quality tests
Despite the constraints described above, several human and veterinary vaccines already underwent the transition from in vivo to in vitro batch testing () [Citation15]. These vaccines could provide a roadmap to guide the transition to in vitro for the vaccines that are currently still tested for potency and safety using in vivo methods.
Figure 1. For several established inactivated and toxoid vaccines for human and veterinary use there has already been a transition from invivo to invitro batch testing in Europe [Citation5]. Advancements have been made for both potency (a.) as well as safety tests (b.). Conventional invivo tests are shown in red. Alternative invivo tests with reduced and refined use (i.e. less animals and less discomfort) of animals are shown in orange. Alternative invitro tests are shown in green
![Figure 1. For several established inactivated and toxoid vaccines for human and veterinary use there has already been a transition from invivo to invitro batch testing in Europe [Citation5]. Advancements have been made for both potency (a.) as well as safety tests (b.). Conventional invivo tests are shown in red. Alternative invivo tests with reduced and refined use (i.e. less animals and less discomfort) of animals are shown in orange. Alternative invitro tests are shown in green](/cms/asset/73715760-5073-4701-a138-a428971caf8d/ierv_a_1977628_f0001_oc.jpg)
For live attenuated vaccines, comprising bacteria or viruses, the use of in vivo batch tests is in general not demanded in the European Pharmacopoeia (Supplementary Table 1) [Citation17]. Due to their replicative nature, live attenuated vaccines can be tested for potency by viral or bacterial titration. Some live attenuated viral vaccines (e.g. influenza virus and viral poultry vaccines) are propagated and titrated on embryonated chicken eggs when suitable cell lines are not available. These chicken embryos are not regarded as laboratory animals in view of the European act on the protection of animals used of scientific purposes (Directive 2010/63/EU), which is not applicable to fetal stages of birds [Citation16]. However, the embryos may experience pain and cell-based alternatives to propagate and titrate these viruses are therefore desirable whenever available [Citation21]. Moreover, virus propagation through cell lines allows faster upscaling during epidemics and is better standardized than propagation through eggs [Citation22]. Finally, the transportation of chicken eggs may be prohibited by governments during avian influenza outbreaks.
Unlike live attenuated vaccines, inactivated vaccines and toxoid vaccines mostly rely on vaccination-challenge or vaccination-serology potency tests, unless antigen quantification methods are available as in vitro alternatives (Supplementary Table 1). These antigen quantification methods may be physiochemical or immunochemical assays that are specific for one or more dominant antigens of the vaccines. In particular, the development of enzyme-linked immunosorbent assays (ELISAs) for antigen quantification has been successful for the replacement of in vivo potency tests of several vaccines in Europe, including those against Newcastle disease in poultry, foot-and-mouth disease in cattle, leptospirosis in cattle and dogs (monographs 0447 and 1939), rabies in both animals and humans, and hepatitis A and B in humans () [Citation17,Citation20,Citation26–29]. Other ELISAs for antigen quantification have shown to be successful in determining the potency of some but not all inactivated vaccines and toxoid vaccines, but these are not yet fully validated and/or approved by the regulatory authorities in Europe. These include tests for infectious bronchitis virus and infectious bursal disease virus vaccines for poultry, furunculosis vaccines for salmonids, tetanus vaccines for human and veterinary use, and diphtheria vaccines for human use [Citation30–35]. In addition to the ELISA, another important antigen quantification method is the immunodiffusion test, which is used to determine the content of hemagglutinin antigens of inactivated or subunit influenza vaccines [Citation36]. For the whole-cell and acellular pertussis vaccines, there have been efforts to develop in vitro ELISA, Luminex, cell-based and proteomic assays as alternatives [Citation37–41].
With respect to safety, alternative in vitro methods have also become available in Europe (). Originally, vaccines were tested for pyrogenicity using the Rabbit Pyrogen Test (RPT), in which temperature changes in rabbits are evaluated after injection of a vaccine. To specifically quantify endotoxin pyrogens, comprising bacterial lipopolysaccharides (LPS), the Limulus Amebocyte Lysate (LAL) test is used. Although the LAL test is legally not regarded as an animal test, horseshoe crabs are captured and bled to obtain their blood cells as the main reagent of the LAL test. The horseshoe crabs do not always survive the bleeding procedure, which is estimated to result in an annual mortality rate of 100,000 horseshoe crabs in North America alone and impacts the survival of this threatened species [Citation42,Citation43]. Nowadays, the blood clotting factor C (rFC) from the horseshoe crab is commercially available as a recombinant protein, but the rFC test has been implemented to a limited extent despite its proven efficacy and its adoption in the European Pharmacopoeia as alternative reagent in endotoxin tests (general text 2.6.14, 2.6.32 and 5.1.10) [Citation17,Citation42]. The Monocyte Activation Test (MAT) was developed to detect both endotoxin and non-endotoxin pyrogens [Citation44–46]. In this test human whole blood or (cryopreserved) PBMCs are stimulated with the vaccine of interest after which the production of pro-inflammatory cytokines (TNF, IL-1β and IL-6) is assessed in ELISAs. The European Pharmacopoeia states that whenever possible and after product-specific validation, the RPT test should be replaced by the MAT (general text 2.6.8) [Citation17]. To justify the use of the LAL test or the rFC test as alternatives for the RPT test, a risk assessment using the MAT is recommended to rule out the presence of any non-endotoxin pyrogens in a vaccine (general text 5.1.10). These statements promote the use of the MAT as a safety test for pyrogenicity or bacterial endotoxins. The MAT test was recently optimized for pyrogenicity testing of the Encepur vaccine against tick-borne encephalitis [Citation44].
To test for residual toxicity of toxoid vaccines in vitro alternatives have been developed including the Chinese hamster ovary (CHO) cell clustering assay as an alternative to the in vivo Histamine Sensitization Test (HIST) for acellular pertussis vaccines [Citation47]. In addition, a VERO cell toxicity assay has been developed and implemented for specific toxicity testing of diphtheria toxoid vaccines as an alternative to the in vivo test in guinea pigs [Citation48]. Furthermore, a VERO cell toxicity assay to be used instead of the mouse toxicity test for veterinary Clostridium septicum vaccines is currently being validated [Citation49]. Finally, the binding and cleavage (BINACLE) assay evaluates residual tetanus toxicity of toxoid bulk that is produced for human and veterinary tetanus vaccines, as an alternative for the currently used test in guinea pigs [Citation50]. The BINACLE assay is currently being validated as part of the European Biological Standardization Programme. Unfortunately, the BINACLE assay cannot be used for vaccine final products that contain tetanus toxoid in an adjuvant-adsorbed state [Citation50].
In summary, although the quality of many inactivated vaccines and toxoid vaccines is still tested with in vivo potency tests, an increasing number of in vitro alternatives are being developed to achieve their replacement. These alternatives include immunochemical assays to quantify antigen, but also tests for other critical properties of vaccines, as will be discussed in the next section. For safety tests, many in vitro alternatives are already available in Europe and implementation of these methods has now become important to move the transition to in vitro safety testing forward.
3. Critical quality attributes of vaccines
Critical quality attributes of vaccines are physical, chemical or microbiological properties of vaccines that should be within certain limits to ensure vaccine quality [Citation51]. The in vitro methods that are currently in place for potency testing of vaccine batches are mainly used to evaluate antigen identity, quantity and integrity (). Some of these methods are antigen quantification methods that depend on the integrity of a single antigen [Citation52]. For example, the detection antibody that is used for antigen quantification of inactivated Newcastle disease vaccine is a monoclonal antibody raised against a linear epitope of Newcastle disease virus hemagglutinin-neuraminidase covering 20 amino acids [Citation53]. Similarly, monoclonal antibodies have been used for potency testing of non-adjuvanted veterinary rabies vaccines by quantifying G protein antigen [Citation54]. However, at the population level vaccination efficiency hardly ever depends on a single dominant epitope. Furthermore, the ability of immunochemical assays to measure the relevant epitopes of antigens inactivated with agents like formaldehyde and β-propiolactone should be validated. The inactivation may alter or hide specific epitopes of antigens and hence affect potency as measured in vitro, even when the potency as measured in vivo remains the same [Citation52,Citation55–57]. The use of a pool of multiple monoclonal, i.e. multiclonal, antibodies in ELISAs can result in more consistent and standardized quality testing of vaccines, without depending too much on single epitopes [Citation52]. Multiclonal antibodies are for example used for antigen quantification of hepatitis A vaccines [Citation58]. In addition to antigen identity, quantity and integrity characteristics, the potency of vaccines may depend on critical quality attributes like vaccine composition, susceptibility of the antigen to proteolytic degradation, the spatial organization (i.e. three-dimensional structure) of the antigen, or the presence of additional immunostimulatory molecules. A combination of assays addressing these different critical quality attributes may thus be required to sufficiently demonstrate batch-to-batch consistency for some vaccines.
The vaccine composition depends on production processes, and inconsistencies in these processes may affect the potency of the vaccine. For instance, for whole-cell pertussis vaccines it was shown that disturbances of the bacterial culture conditions may results in the downregulation of important immunogenic virulence proteins [Citation59]. Mass spectrometry-based proteome analysis was proposed as a method to evaluate the whole protein composition of vaccines to detect these disturbances [Citation41]. Another applicability of mass spectrometry is the assessment of the stability of antigens and their susceptibility to enzymatic degradation as an important part of antigen processing by antigen-presenting cells [Citation60]. Inactivation by formaldehyde and heat exposure were shown to affect the degradation kinetics of a model antigen and tetanus toxoid, respectively [Citation61,Citation62]. The importance of the spatial organization of antigens has been demonstrated for influenza vaccines by showing that during a priming vaccination whole-inactivated influenza vaccines induced higher antibody titers, both in mice and humans, and superior T cell responses in humans as compared to the less reactogenic split vaccines with spatially disrupted antigens [Citation63–67]. The antigens of the split vaccine lack the proper spatial organization to efficiently induce antibody production [Citation68]. Similar results have been found for detergent-treated inactivated vaccines against Newcastle disease virus for use in poultry [Citation69]. Finally, the presence of immunostimulatory molecules, which may include exogenous adjuvants or endogenous pathogen-associated molecular patterns (PAMPs), is important for vaccine potency. The contribution of PAMPs to vaccine potency has been demonstrated for both viral (e.g. influenza) and bacterial (e.g. pertussis) vaccines [Citation39,Citation64,Citation70]. Similar to the antigens, PAMPs can be destroyed or become less accessible by inactivating agents like formaldehyde or β-propiolactone [Citation55,Citation71]. The presence of immunostimulatory molecules can be evaluated using cell-based assays, as discussed in more detail below. The described quality attributes may require additional testing when antigen identity, quantity and integrity characteristics are insufficient to predict the potency of a vaccine.
The previous section demonstrated that many in vitro alternatives to test vaccines for pyrogenicity and to test toxoid vaccines for residual toxicity are already available. Furthermore, toxicity tests that were found to be unnecessary based on historical data have recently been deleted from the European Pharmacopoeia, including abnormal toxicity tests (to detect any unexpected hazards), some specific toxicity tests of human vaccines, and some residual toxicity tests of veterinary vaccines [Citation72,Citation73]. In contrast, the porcine actinobacillosis vaccine, porcine progressive atrophic rhinitis vaccine, and tetanus vaccines for human and veterinary use still require animal-based toxicity tests [Citation17,Citation72]. Moreover, safety tests of the Bacillus Calmette-Guérin vaccine, used to protect against tuberculosis, include the virulent mycobacteria test and the excessive dermal reactivity test, both in guinea pigs, to show absence of virulence and excessive reactogenicity, respectively [Citation17]. An alternative safety assay based on the proliferation of lymphocytes from sensitized guinea pigs has been proposed instead of the currently used excessive dermal reactivity test [Citation74]. A vaccine for rabbit hemorrhagic disease still requires a residual live virus safety test in rabbits [Citation17]. Some live attenuated viral vaccines (e.g. smallpox and poliomyelitis) require neurovirulence safety testing in monkeys or transgenic mice, although deep sequencing methods have been proposed as an alternative strategy to test these vaccines for genetic instability and to prevent the occurrence of neurovirulent viral mutants [Citation17,Citation75,Citation76]. Recently, a model based on brain cells in a transwell system, named the BBB-Minibrain culture device, was developed as a next step in search for an alternative neurovirulence test [Citation77]. Finally, batch release of pertussis vaccines still requires a test for residual dermonecrotic toxin in mice [Citation17]. Recently, the use of liquid chromatography mass spectrometry to quantify dermonecrotic toxin has been proposed as an alternative in vitro method [Citation78].
To summarize this section, antigen identity, quantity and integrity can be considered the most important critical quality attributes of inactivated and toxoid vaccines. However, additional quality attributes including vaccine composition, the spatial organization of the antigen, or the presence of additional immunostimulatory molecules may be critical for specific vaccines. Importantly, it is increasingly recognized that one-to-one replacement of an in vivo test for an in vitro test will be difficult and that a combination of assays to demonstrate batch-to-batch consistency may be needed for some vaccines [Citation11,Citation79].
4. Mimicking in vivo vaccine-induced immune responses using in vitro cell-based assays
The use of immunochemical and physicochemical methods for potency testing is based on evidence showing that consistency between new batches and batches of proven potency with regard to aspects like antigen identity, antigen quantity, antigen integrity, antigen structure, vaccine composition, and quantity of immunostimulatory molecules will also warrant consistent potency in vivo. For vaccines that are well defined (e.g. recombinant and subunit vaccines) the use of these methods may indeed be sufficient to guarantee the potency and safety of a vaccine. However, for less defined inactivated and perhaps even toxoid vaccines, additional information on the immunostimulatory capacity of vaccines may be needed to generate sufficient evidence about vaccine quality when in vitro methods are used. Cell-based assays can assess aspects of vaccine-induced immune reactivity and may identify critical quality attributes of vaccines.
An immune response is initiated by innate immune cells (; step 1), among which professional antigen-presenting cells, including dendritic cells, macrophages and B cells process and present antigen, and in addition express co-stimulatory molecules and release cytokines to activate T cells (; step 2). Several methods have been proposed to evaluate vaccine potency in cell-based assays with immune cells using either primary cells or immortalized cell lines [Citation40,Citation80–84]. Primary dendritic cell-based assays use monocyte-derived dendritic cells (moDCs) or bone marrow-derived dendritic cells (BMDCs) [Citation40,Citation82–84]. Whereas primary cells more closely represent the physiological nature of immune cells, they are collected from animals or human donors, often have a limited lifespan, are available in limited numbers, and may show variable responses due to genetic diversity of the individual donors. Cell lines do not have these disadvantages and are thus being explored for use in a vaccine quality control setting (), even though they may be less representative for the in vivo situation than primary cells. Most cell-based potency assays measure vaccine-induced activation of dendritic cell-, monocyte- and macrophage-like cells by expression of co-stimulatory molecules (e.g. CD40, CD80, CD83, CD86) or pro-inflammatory cytokines. Furthermore, dendritic cells have been used in in vitro antigen degradation assays, in which the susceptibility of antigens for proteolytic degradation is used as a biomarker for immunogenicity [Citation60]. Reporter cell lines like PRR-expressing human embryonic kidney 293 (HEK)-Blue cells have been used to evaluate the immunostimulatory properties of clinical isolates of Bordetella pertussis and may also be useful to test vaccines for potency [Citation85,Citation86].
Figure 2. Simplified overview of a vaccine-induced immune response. (1) Innate immune cells recognize pathogen-associated molecular patterns (PAMPs), e.g. bacterial cell wall components or double-stranded RNA that are part of vaccine antigens, through pattern recognition receptors (PRRs). (2) Upon recognition, vaccine antigens are taken up by innate immune cells, and processed for antigen presentation on major histocompatibility complexes (MHC) class I and II to (naive) antigen-specific T cells. Innate immune cells orchestrate the adaptive immune response by releasing cytokines that affect the differentiation of T cells. (3) As a result, CD4+ T cells differentiate into various types of CD4+ helper T cells (Th1, Th2, Th17, Treg etc.) or memory T cells, whereas CD8+ T cells differentiate into CD8+ cytotoxic T cells, or memory T cells. (4) Cytotoxic T cells recognize antigen-derived peptides presented on MHC class I molecules of infected host cells that are subsequently killed. (5) Antigen-specific naive B cells that bind antigens through their B cell receptor will endocytose and process the antigens, and present these on MHC class II molecules to helper T cells. Upon recognition helper T cells will produce cytokines that facilitate B cell proliferation and differentiation into plasma cells or memory B cells. (6) Plasma cells produce antigen-specific antibodies that can neutralize or opsonize pathogens. Opsonized pathogens can be bound by Fc receptors on innate immune cells, resulting in the uptake and destruction of the pathogens. Furthermore, antibodies can bind to antigens exposed on the surface of infected host cells and stimulate antibody-dependent cellular cytotoxicity (ADCC) by innate immune cells. The icons used in this figure are adaptations from icons retrieved from the Servier Medical Art collection, which are licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
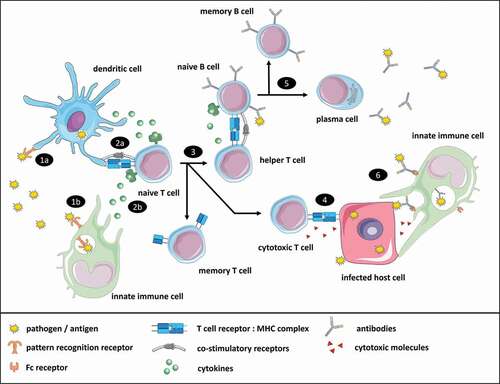
Table 1. Myeloid cell lines that have been described for use in potency testing of human and veterinary vaccines
Assays with innate immune cells have also been used to evaluate safety aspects of vaccines. Vaccine pyrogenicity can be evaluated by the MAT using monocytic cell lines like the MM6 cell line [Citation87]. Interestingly, the MM6 cell line has been explored for use in in vitro safety tests, as well as potency tests, both using secretion of the pleiotropic cytokines IL-1β and IL-6 as readouts [Citation40,Citation88]. The use of IL-1β and IL-6 as readouts for potency is in line with the role of these cytokines in T cell activation and differentiation, whereas their use as readouts for safety is in line with their ability to induce fever and other potential side effects of vaccination [Citation88–90]. Thus, potency and safety limits of pro-inflammatory cytokines need to be identified beforehand to discriminate between potent and potentially dangerous levels of vaccine-induced immune responses [Citation88].
After the innate immune response is initiated by a vaccine, antigen-presenting cells will activate the adaptive immune system (; step 3–5). An important aspect of the adaptive immune response for vaccination is the differentiation of B and T cells into effector and memory cells, of which the latter will quickly induce a secondary immune response upon future encounter with the same pathogen. Batches of whole cell pertussis vaccines and rabies vaccines of different potencies were found to differ in their ability to induce T cell responses in splenocytes from vaccinated mice, suggesting that T cell assays can be used to test for vaccine potency when these T cell responses are known correlates-of-protection [Citation91,Citation92]. The activation of naive T cells or the re-activation of memory T cells by vaccines has been mimicked in vitro using respectively autologous dendritic cell-T cell co-cultures or PBMCs to evaluate the potency of vaccines against yellow fever and influenza for use in humans, and for vaccines against blue tongue and rabies for use in cattle [Citation93–95]. However, T cells are highly heterogenous and T cell assays have only been explored for use in pre-clinical development of vaccines. In addition, T cell assays with primary cells require blood collection from vaccinated animals, similar to serological assays. The use of a combination of several epitope-specific T hybridoma cell lines might be more promising for potency testing of vaccine batches, but requires further development [Citation96].
The contribution of assays with innate immune cells to batch testing is product-specific and depends on the presence of PAMPs, adjuvants and other potentially immunostimulatory vaccine components. T cell assays may be useful when T cell responses are known correlates-of-protection. Due to their accessibility, infinite lifespan, high numbers and low variability, (reporter) cell lines are particularly suitable for cell-based assays to test vaccines. Furthermore, cell-based assays can be useful to investigate whether there are any functional synergistic or antagonistic interactions between the adjuvant and other components in the vaccine, as will be discussed further below. Finally, the need for cell-based assays depends on whether the vaccine will be applied for priming or boosting the immune response. For example, booster vaccines may stimulate the reactivation of memory T cells independently of dendritic cells through memory B cells and are therefore less dependent on stimulating innate immune cells than primer vaccines [Citation97].
5. Adjuvanted vaccines
Many inactivated, toxoid and subunit vaccines require adjuvants to support activation of the immune system. For a long period of time, only aluminum salts and mineral oil emulsions were used as adjuvants. Aluminum salts are used as adjuvants for human vaccines such as diphtheria and tetanus vaccines as well as veterinary vaccines such as the bluetongue vaccine for ruminants, some feline leukemia vaccines and the rabies vaccine for dogs, cats, ruminants and horses [Citation98,Citation99]. The first emulsion adjuvants were Freund’s incomplete adjuvant, based on mineral oil emulsified in a water-in-oil formulation, and Freund’s complete adjuvant, formulated with tubercle bacteria [Citation100]. Mineral oil-based water-in-oil adjuvants are too reactogenic for use in humans, but are widely used in poultry, cattle and fish [Citation101,Citation102]. The first emulsion adjuvant to be approved in humans was MF59, which is an oil-in-water adjuvant based on squalene [Citation103].
Before batch release, adjuvanted vaccines are tested for identity, concentration, physical properties like viscosity and stability of the adjuvant (monographs 0062 Vaccines for Veterinary Use and 0153 Vaccines for Human Use) [Citation17]. However, emulsion and aluminum salt adjuvants may interfere with in vitro methods to evaluate the antigens of final products [Citation104]. The following section describes how this problem can be circumvented by testing intermediary products before blending of antigens and adjuvants, by extracting the antigenic fraction from final products or by using in vitro methods that are not affected by adjuvants [Citation105].
Methods to extract antigens from final adjuvanted products have been developed for several vaccines. To enable the quantification of antigen by ELISAs, isopropyl myristate has been used to extract antigens from oil-adjuvanted poultry vaccines for Newcastle disease, infectious bronchitis and infectious bursal disease [Citation30,Citation106]. Similarly, antigens extracted from an oil-adjuvanted poultry vaccine against infectious coryza could be used to evaluate the presence of PAMPs by measuring their effects on nitric oxide production or cytokine expression in cell-based assays [Citation107]. Isopropyl myristate could not be used to extract antigens from vaccines for food-and-mouth disease, which are formulated with Montanide ISA201 oil adjuvant, but an extraction with benzyl alcohol could be used instead for this vaccine, showing that different emulsion adjuvants require different extraction methods [Citation108]. For aluminum salt-adjuvanted vaccines, electrophoretic or chemical competitive desorption methods can be used to retrieve the antigens, although the latter methods may result in denaturation of antigens and affect their integrity [Citation109,Citation110]. Such methods have been used by manufacturers for hepatitis A vaccines in order to enable antigen quantification by ELISA, but for commercial reasons the methods were not disclosed in the resulting publications [Citation58,Citation111].
Not all quantification methods require antigen extraction or desorption from adjuvanted vaccines. With respect to emulsion adjuvants, a multiplexed sandwich immunoassay for influenza vaccines against strains with pandemic potential was shown to be compatible with the squalene-based adjuvant MF59 [Citation112]. An in situ method based on the fluorescent nucleic acid-reactive dye SYBR Green II has been developed to determine the stability of an inactivated vaccine for foot-and-mouth disease and was found compatible with aluminum salt, water-in-oil and oil-in-water adjuvanted vaccines [Citation113]. However, to the best of our knowledge there are currently no in vitro antigen-specific methods to determine the quality of adjuvanted vaccines formulated as water-in-oil emulsions that do not require extraction of the antigen. Methods that nonspecifically determine protein content are compatible with aluminum salt adjuvants, including fluorescence spectroscopy, based on intrinsic fluorescence of tyrosine and tryptophan amino acids or fluorescent protein-reactive chemicals, and chemiluminescent nitrogen detection [Citation114–116]. These methods are not antigen-specific and therefore not suitable for vaccines that contain antigens in complex media with other proteins, e.g. allantoic fluid present in poultry vaccines [Citation117,Citation118]. Some antigen-specific immunoassays have shown compatibility with aluminum salt-adjuvanted vaccines, which include sandwich and competitive ELISAs, Luminex, and immunofluorescent assays analyzed with a fluorometer or flow cytometer [Citation38,Citation119–123]. Cell-based toxicity assays can be made compatible for aluminum salt-adjuvanted vaccines by using semi-permeable transwell inserts to prevent direct contact between the cells and aluminum salts, as demonstrated by the CHO cell-clustering assay used to test for residual toxicity of pertussis vaccines [Citation47].
In summary, adjuvanted vaccines create additional challenges for the development of in vitro potency and safety tests, but adsorption and extraction methods, and the development of adjuvant-compatible methods offer possibilities to overcome these.
6. Altered batches to validate in vitro test methods
Non-compliant vaccine batches of substandard quality are needed for the validation of new in vitro tests methods by confirming their capacity to discriminate between batches of different qualities [Citation124]. However, modern well-controlled vaccine production processes that adhere to GMP standards rarely result in failed batches anymore and there may be reluctance to share the remaining non-compliant batch material [Citation11,Citation15]. This has led to a paradox in which improved quality of vaccines makes it more difficult to validate new in vitro test methods. Another problem is the inherent variability of current in vivo release tests that are used as reference tests, which may limit the ability to which they can discriminate between batches of different quality [Citation7–12,Citation125]. In other words, in vivo tests may show variation to an extent that some batches fail the test, while they are in fact compliant, i.e. their capacity to induce protective immunity in the target species is sufficient [Citation125]. Such batches can be expected to pass appropriate in vitro tests. Likewise, batches may pass in vivo tests, while they are in fact non-compliant, i.e. their capacity to induce protective immunity in the target species is insufficient.
To solve these problems, vaccine batches could be altered intentionally, for example by exposure to stresses that may decrease the stability of a batch during the vaccine production process (e.g. decreased or increased pH, osmolality, temperature), by creating vaccines with reduced antigen content or a different composition, by deviating from the standard inactivation method, or by changing the degree of adsorption to adjuvants [Citation11,Citation34,Citation41,Citation57,Citation124,Citation126,Citation127]. Importantly, altered batches should be representative for non-compliant batches that may realistically be produced as a result of disturbances in vaccine production processes. In the European Pharmacopoeia, heat treatment is given as an example to create a proper altered batch of the inactivated poliomyelitis vaccines, which is used for product-specific validation of the in vitro D-antigen assay, in order to replace the former in vivo potency test in chickens, guinea pigs or rats (Ph. Eur method 2.7.20) [Citation17]. The altered batches need to be designed and produced already during the development phase of in vitro test methods to identify the sensitivity of these assays for detecting non-compliant batches.
7. Cooperation between academia, industry and regulatory institutions is crucial for the transition from in vivo to in vitro batch testing of vaccines
Two previously identified constraints for the transition to in vitro batch testing of vaccines were the lack of priority of research into in vitro test methods that are most needed, and the varying knowledge about vaccine production processes among scientists of different disciplines [Citation15]. International, multi-stakeholder (academia, industry and regulators) collaborations were described as key to move the transition to in vitro vaccine testing forward. Over the last five years, this necessity was met by the VAC2VAC consortium, funded by the Innovative Medicines Initiative (IMI2) programme [Citation128]. In this consortium, vaccines and their intermediary products (e.g. antigens, adjuvants, excipients and additives), knowledge about production processes, and new technologies for vaccine quality control were shared between the different stakeholders. The aim of the consortium was to develop new tests and approaches that support the use of the consistency approach in batch testing of established vaccines.
Assay development is only the first step of the transition from in vivo to in vitro batch testing, which has to be complemented by a validation process, first in small-scale feasibility studies to test the transferability of the assay to other labs, later in large-scale multi-center validation studies that are preferably conducted at an international or even global level to standardize novel methods [Citation18,Citation129]. The latter validation studies can be performed as part of the Biological Standardization Programme in Europe, in which Official Medicines Control Laboratories (OMCLs), manufacturers and other stakeholders can participate. Assays that have been successfully validated and are accepted by regulatory authorities can become incorporated into pharmacopoeias [Citation129]. After incorporation, the methods still need to be implemented by individual manufacturers and OMCLs, which have to conduct assay validation before the conventional animal-based test can be fully replaced and eventually eliminated from pharmacopoeias. Engagement between academia, industry and regulators at an early stage of the development of in vitro alternatives is essential for getting all stakeholders acquainted with new methods, to facilitate validation studies and to achieve wide implementation of the in vitro alternatives [Citation15,Citation18,Citation79].
8. Conclusions
This review describes scientific barriers that hampered the transition from in vivo to in vitro batch testing of vaccines, especially for those that include inactivated pathogens, toxoids or pathogen subunits, and the opportunities to overcome these. An increasing number of in vitro potency and safety tests have been developed to replace in vivo tests. Currently implemented in vitro potency tests largely comprise ELISAs or other immunochemical methods that assess antigen identity, quantity and integrity characteristics, while other properties may also be critical for vaccine quality. There is an increasing consensus that one-to-one replacement by in vitro alternatives is not always possible and that a combination of in vitro alternatives may be needed. Cell-based assays can provide functional information about the interaction between a vaccine and the immune system, which is impossible to capture with ELISAs only. Adjuvanted vaccines are notably hard to test for quality using in vitro alternatives due to their incompatibility with some of these methods. However, the current scientific literature provides many possibilities to overcome these difficulties, including desorption and extraction methods of adjuvants and strategies to study the potential interactions between different vaccine antigens. The use of purposely altered batches enables the validation of new in vitro test methods when there is a lack of non-compliant batches originating from disturbances in the routine vaccine production process due to quality systems and GMP that are in place at the manufacturer. Finally, it is recognized that cooperation between scientists in academia, industry and regulatory institutes is key to move the transition to in vitro vaccine testing forward by sharing of products, knowledge and technologies.
9. Expert opinion & five-year view
According to the most recent report on animal use for scientific purposes in the European Union [Citation3], more than a million animals were used in 2017 for regulatory batch testing of medicinal products, predominantly for potency assessment. Furthermore, over 264,000 animals were involved in batch potency tests that were classified as causing severe pain and distress. Replacement of in vivo potency and safety batch tests of vaccines by in vitro alternatives would reduce the number of animal tests considerably. In particular, batches of inactivated and toxoid vaccines are still assessed for potency and safety using in vivo methods (Supplementary Table 1). It is clear that many of these animal models have limitations including ethical issues, disputed relevance, high variability and high costs, and are therefore in need of replacement.
Improvement of vaccine potency assessment in animal models in terms of both animal welfare and test performance was initially made by introducing humane endpoints and potency tests based on serology rather than challenge. Moreover, some in vivo batch potency tests have nowadays been replaced by in vitro antigen quantification methods. Nevertheless, replacing in vivo batch potency tests for in vitro antigen quantification may not be sufficient to ensure all aspects of vaccine quality. With respect to safety testing, many in vitro alternatives are already available or at an advanced level of development. Furthermore, some toxicity tests have been or will be waived globally by regulatory authorities since these are no longer considered relevant [Citation72]. Product-specific validation and implementation of existing in vitro safety tests will be most important for the coming years to achieve safety testing without the need of animal tests.
As described in this review, increasing evidence shows that properties like vaccine composition, spatial organization of the antigen, and the presence of additional immunostimulatory molecules may be critical for vaccine quality and may thus require testing. One-to-one replacement of an in vivo test by an in vitro alternative will therefore often not be possible. A combination of physicochemical, immunochemical and cell-based assays may be required to demonstrate batch-to-batch consistency of multiple vaccine properties. In the past few years, studies have explored mass spectrometry to characterize vaccines, and cell-based assays as candidates to evaluate vaccine-induced immune reactivity. In the years ahead, these methods should receive further attention to ensure that replacement of in vivo potency tests will evolve beyond the development of suitable antigen quantification methods only. Obstacles for the development of in vitro vaccine potency tests, like the presence of emulsion or aluminum salt adjuvants, are being overcome. Several studies have now demonstrated that desorption and extraction methods can be applied to remove adjuvants to enable in vitro potency testing. However, a remaining objective is to investigate the extent to which desorption and extraction methods alter vaccine properties that require testing in comparison to the untreated final batches. Finally, regulatory guidelines for the development of altered batches that mimic non-compliant vaccine batches are needed to make sure that new in vitro methods are properly validated.
Article Highlights
There is a global intent to develop in vitro alternatives for animal-based batch potency and safety tests of vaccines. This review describes scientific barriers that have been overcome or still need to be overcome to move the transition from in vivo to in vitro batch testing forward.
For safety testing, many in vitro cell-based or immunochemical toxicity tests are already available.
Potency tests are often still performed in vivo, especially for vaccines based on inactivated pathogens, toxoids or pathogen subunits. An increasing number of immunochemical alternatives are being developed. These tests assess vaccines for antigen identity, quantity and integrity characteristics. However, some vaccines will require additional testing. For instance, cell-based assays can provide functional information about the interaction between a vaccine and the immune system.
Adjuvanted vaccines are notably hard to test for quality using in vitro alternatives. Fortunately, several desorption and extraction methods of adjuvants have been described.
Due to quality systems that are in place at the manufacturer there may be a lack of non-compliant vaccine batches, which are needed to validate new in vitro test methods. Altered batches, which are purposely made non-compliant batches, may provide a solution.
Cooperation between scientists in academia, industry and regulatory institutes is key to move the transition to in vitro vaccine testing forward by sharing of products, knowledge and technologies.
Author contributions
All authors have substantially contributed to the conception and design of the review article and interpreting the relevant literature, and have been involved in writing the review article or revised it for intellectual content.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are a member of the VAC2VAC consortium that this review refers to. Peer reviewers have no other relevant financial or other relationships to disclose.
Acknowledgments
The authors thank prof. dr. Anke Huckriede for providing her insights on the topics addressed in this review.
Additional information
Funding
References
- De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat Rev Immunol. 2014;14(7):505–514.
- Milstien J, Dellepiane N, Lambert S, et al. Vaccine quality—can a single standard be defined? Vaccine. 2002;20(7–8):1000–1003.
- Commission to the European Parliament and the Council. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015-2017.; 2020.
- Hendriksen C, Spieser JM, Akkermans A, et al. Validation of alternative methods for the potency testing of vaccines. Altern Lab Anim. 1998;26(6):747–761.
- Milne C, Buchheit KH. EDQM’s 3R activities in the field of quality control of vaccines. ALTEX. 2012;1/12.
- Walker A, Srinivas GB. Opportunities and strategies to further reduce animal use for Leptospira vaccine potency testing. Biologicals. 2013;41(5):332–337.
- Schiffelers MJ, Blaauboer B, Bakker W, et al., Replacing the NIH test for rabies vaccine potency testing: a synopsis of drivers and barriers. Biologicals. 2014;42(4): 205–217.
- Servat A, Kempff S, Labadie A, et al. In vivo potency tests of rabies inactivated vaccines for veterinary use. A 2-year retrospective analysis of data according to the criteria of the European Pharmacopoeia. Pharmeur Bio Sci Notes. 2008;20:655–664.
- Goris N, Merkelbach-Peters P, Diev VI, et al. European Pharmacopoeia foot-and-mouth disease vaccine potency testing in cattle: between test variability and its consequences. Vaccine. 2007;25(17):3373–3379.
- Reed NE, Varney WC, Goddard RD, et al. The maintenance of challenge strains used in the potency test for canine leptospira vaccines. Biologicals. 2000;28(1):25–28.
- EDQM. General chapter 5.2.14 Substitution of in vivo methods by in vitro methods for the quality control of vaccines. In: European Pharmacopoeia. 10th ed. (p. 679) Strasbourgh France: European Department for the Quality of Medicines; 2019.
- Xing D, Das RG, O’Neill T, et al. Laboratory testing of whole cell pertussis vaccine: a WHO proficiency study using the Kendrick test. Vaccine. 2001;20(3):342–351.
- Hendriksen C, Arciniega JL, Bruckner L, et al., The consistency approach for the quality control of vaccines. Biologicals. 2008;36(1):73–77.
- De Mattia F, Hendriksen C, Buchheit KH, et al. The vaccines consistency approach project: an EPAA initiative. Pharmeur Bio Sci Notes. 2015;(2015):30–56.
- Bruysters MWP, Schiffelers MJ, Hoonakker M, et al. Drivers and barriers in the consistency approach for vaccine batch release testing: report of an international workshop. Biologicals. 2017; 48:1–5.
- The European. Parliament and the Council of the European Union. DIRECTIVE 2010/63/EU on the protection of animals used for scientific purposes. Off J Eur Union. 2010;276:33–79.
- EDQM. European Pharmacopoeia. 10.5th ed. Strasbourgh France: European Department for the Quality of Medicines; 2020.
- Halder M, Depraetere H, Delannois F, et al. Recommendations of the VAC2VAC workshop on the design of multi-centre validation studies. Biologicals. 2018;52:78–82.
- Shank-Retzlaff M, Wang F, Morley T, et al., Correlation between Mouse Potency and In Vitro Relative Potency for Human Papillomavirus Type 16 Virus-Like Particles and Gardasil Vaccine Samples. Hum Vaccin. 2005;1(5):191–197.
- Descamps J, Giffroy D, Remy E, et al. A case study of development, validation, and acceptance of a non-animal method for assessing human vaccine potency. Procedia Vaccinol. 2011;5:184–191.
- Rosenbruch M. [The sensitivity of chicken embryos in incubated eggs]. ALTEX. 1997;14(3):111–113.
- Settembre EC, Dormitzer PR, Rappuoli R. Bringing influenza vaccines into the 21st century. Hum Vaccin Immunother. 2014;10(3):600–604.
- Zariri A, Beskers J, van de Waterbeemd B, et al. Meningococcal outer membrane vesicle composition-dependent activation of the innate immune response. Palmer GH, ed. Infect Immun. 2016;84(10): 3024LP - 3033.
- Vrieling H, Kooijman S, de Ridder JW, et al. Activation of human monocytes by colloidal aluminum salts. J Pharm Sci. 2020;109(1):750–760.
- Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, et al. Powerful complex immunoadjuvant based on synergistic effect of combined TLR4 and NOD2 activation significantly enhances magnitude of humoral and cellular adaptive immune responses. PLoS One. 2016;11(5):e0155650.
- Maas RA, Komen M, Van Diepen M, et al. Correlation of haemagglutinin-neuraminidase and fusion protein content with protective antibody response after immunisation with inactivated Newcastle disease vaccines. Vaccine. 2003;21(23):3137–3142.
- Pay TWF, Hingley PJ. Correlation of 140S antigen dose with the serum neutralizing antibody response and the level of protection induced in cattle by foot-and-mouth disease vaccines. Vaccine. 1987;5(1):60–64.
- Poston R, Hill R, Allen C, et al. Achieving scientific and regulatory success in implementing non-animal approaches to human and veterinary rabies vaccine testing: a NICEATM and IABS workshop report. Biologicals. 2019;60:8-14.
- Poirier B, Morgeaux S, Variot P, et al. In vitro potency assay for hepatitis A vaccines: development of a unique economical test. Biologicals. 2000;28(4):247–256.
- Maas PA, MPM DW, Venema S, et al. Antigen quantification as in vitro alternative for potency testing of inactivated viral poultry vaccines. Vet Q. 2000;22(4):223–227.
- Romstad AB, Reitan LJ, Midtlyng P, et al. Antibody responses correlate with antigen dose and in vivo protection for oil-adjuvanted, experimental furunculosis (Aeromonas salmonicida subsp. salmonicida) vaccines in Atlantic salmon (Salmo salar L.) and can be used for batch potency testing of vaccin. Vaccine. 2013;31(5):791–796.
- Coombes L, Tierney R, Rigsby P, et al. In vitro antigen ELISA for quality control of tetanus vaccines. Biologicals. 2012;40(6):466–472.
- Riches-Duit R, Hassall L, Rigsby P, et al. Evaluation of a capture antigen ELISA for the characterisation of tetanus vaccines for veterinary use. Biologicals. 2019;61:8–14.
- Coombes L, Stickings P, Tierney R, et al. Development and use of a novel in vitro assay for testing of diphtheria toxoid in combination vaccines. J Immunol Methods. 2009;350(1–2):142–149.
- Westdijk J, Metz B, Spruit N, et al. Antigenic fingerprinting of diphtheria toxoid adsorbed to aluminium phosphate. Biologicals. 2017;47:69–75.
- Minor PD. Assaying the potency of influenza vaccines. Vaccines (Basel). 2015;3(1):90–104.
- Westdijk J, van den Ijssel J, Thalen M, et al. Quantification of cell-associated and free antigens in Bordetella pertussis suspensions by antigen binding ELISA. J Immunoassay. 1997;18(3):267–284.
- Agnolon V, Bruno C, Galletti B, et al. Multiplex immunoassay for in vitro characterization of acellular pertussis antigens in combination vaccines. Vaccine. 2016;34(8):1040–1046.
- Hoonakker ME, Verhagen LM, Pupo E, et al., Vaccine-mediated activation of human TLR4 is affected by modulation of culture conditions during whole-cell pertussis vaccine preparation. PLoS One. 2016;11(8): e0161428.
- Hoonakker ME, Verhagen LM, Hendriksen CFM, et al. In vitro innate immune cell based models to assess whole cell Bordetella pertussis vaccine quality: a proof of principle. Biologicals. 2015;43(2):100–109.
- Metz B, Hoonakker M, Uittenbogaard JP, et al., Proteome analysis is a valuable tool to monitor antigen expression during upstream processing of whole-cell pertussis vaccines. J Proteome Res. 2017;16(2):528–537.
- Maloney T, Phelan R, Simmons N. Saving the horseshoe crab: a synthetic alternative to horseshoe crab blood for endotoxin detection. PLoS Biol. 2018;16(10):e2006607.
- Smith DR, Beekey MA, Brockmann HJ, et al. Limulus polyphemus. IUCN Red List Threatened Species. 2016;2016:e.T11987A80159830.
- Etna MP, Giacomini E, Rizzo F, et al. Optimization of the monocyte-activation-test for evaluating pyrogenicity of tick-borne encephalitis virus vaccine. ALTEX. 2020;37(4):532-544.
- Hasiwa N, Daneshian M, Bruegger P, et al. T4 Report evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX. 2013;30(2):169–208.
- Studholme L, Sutherland J, Desai T, et al. Evaluation of the monocyte activation test for the safety testing of meningococcal B vaccine Bexsero: a collaborative study. Vaccine. 2019;37(29):3761–3769.
- Isbrucker R, Daas A, Wagner L, et al. Transferability study of CHO cell clustering assays for monitoring of pertussis toxin activity in acellular pertussis vaccines. Pharmeur Bio Sci Notes. 2016;2015:97–114.
- Sesardic D, Prior C, Daas A, et al. Collaborative study for establishment of the European Pharmacopoeia BRP batch 1 for diphtheria toxin. Pharmeuropa bio. (2003);2003(1):5–21
- Daas A, Behr-Gross ME, Bruckner L, et al. Collaborative study for the validation of cell line assays for in-process toxicity and antigenicity testing of Clostridium septicum vaccine antigens - Part 1. Pharmeur Bio Sci Notes. 2020;2020:53–124.
- Behrensdorf-Nicol HA, Weisser K, Krämer B. “BINACLE” assay for in vitro detection of active tetanus neurotoxin in toxoids. ALTEX. 2015;32(2):137–142.
- Committee for Human Medicinal Products - European Medicines Agency. ICH guideline Q8 (R2) on pharmaceutical development. 2017:1–24
- Clough NEC, Hauer PJ. Using polyclonal and monoclonal antibodies in regulatory testing of biological products. ILAR J. 2005;46(3):300–306.
- Claassen I, Maas R, Daas A, et al. Feasibility study to evaluate the correlation between results of a candidate in vitro assay and established in vivo assays for potency determination of Newcastle disease vaccines. Pharmeuropa bio. 2003;2003(1):51–66
- Stokes W, McFarland R, Kulpa-Eddy J, et al. Report on the international workshop on alternative methods for human and veterinary rabies vaccine testing: state of the science and planning the way forward. Biologicals. 2012;40(5):369–381.
- Geeraedts F, Ter Veer W, Wilschut J. ter Veer W, Wilschut J, et al. Effect of viral membrane fusion activity on antibody induction by influenza H5N1 whole inactivated virus vaccine. Vaccine. 2012;30(45):6501–6507.
- Jagt HJM, Bekkers MLE, SAJT VB, et al., The influence of the inactivating agent on the antigen content of inactivated Newcastle disease vaccines assessed by the in vitro potency test. Biologicals;2010;38(1):128–134.
- Metz B, Tilstra W, van der Put R, et al. Physicochemical and immunochemical assays for monitoring consistent production of tetanus toxoid. Biologicals. 2013;41(4):231–237.
- Stalder J, Costanzo A, Daas A, et al. Establishment of a biological reference preparation for hepatitis A vaccine (inactivated, non-adsorbed). Pharmeur Bio Sci Notes. 2020;2010(1):15–29.
- van de Waterbeemd B, Streefland M, Pennings J, et al. Gene-expression-based quality scores indicate optimal harvest point in Bordetella pertussis cultivation for vaccine production. Biotechnol Bioeng. 2009;103(5):900–908.
- Egger M, Jürets A, Wallner M, et al. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS One. 2011;6(2):e17278.
- Michiels TJM, Meiring HD, Jiskoot W, et al. Formaldehyde treatment of proteins enhances proteolytic degradation by the endo-lysosomal protease cathepsin S. Sci Rep. 2020;10(1):11535.
- Michiels TJM, Tilstra W, Hamzink MRJ, et al. Degradomics-based analysis of tetanus toxoids as a quality control assay. Vaccines (Basel). 2020;8(4):712.
- Hagenaars N, Mastrobattista E, Glansbeek H, et al., Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model. Vaccine.2008;26(51):6555–6563.
- Geeraedts F, Goutagny N, Hornung V, et al. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by toll-like receptor signalling. Subbarao K, ed. PLoS Pathog. 2008;4(8):e1000138.
- Bernstein DI, Zahradnik JM, DeAngelis CJ, et al. Clinical reactions and serologic responses after vaccination with whole-virus or split-virus influenza vaccines in children aged 6 to 36 months. Pediatrics. 1982;69:4.
- Halbroth BR, Heil A, Distler E, et al. Superior in vitro stimulation of human CD8+ T-cells by whole virus versus split virus influenza vaccines. Stambas J, ed. PLoS One. 2014;9(7):e103392.
- Hampson AW. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann Acad Med Singapore. 2008;37(6):510–517.
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796.
- Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 2000;44(1):145–154.
- Higgins SC, Jarnicki AG, Lavelle EC, et al. TLR4 mediates vaccine-induced protective cellular immunity to bordetella pertussis : role of IL-17-producing T cells. J Immunol. 2006;177(11):7980–7989.
- Herrera-Rodriguez J, Signorazzi A, Holtrop M, et al. Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production. Vaccine. 2019;37(12):1630–1637.
- Viviani L, Halder M, Gruber M, et al. Global harmonization of vaccine testing requirements: making elimination of the ATT and TABST a concrete global achievement. Biologicals. 2020;63:101-105.
- Garbe JHO, Ausborn S, Beggs C, et al. Historical data analyses and scientific knowledge suggest complete removal of the abnormal toxicity test as a quality control test. J Pharm Sci. 2014;103(11):3349–3355.
- Spohr C, Kaufmann E, Battenfeld S, et al. A New Lymphocyte Proliferation Assay for Potency Determination of Bovine Tuberculin PPDs Supplementary Data. ALTEX. 2015;32(3):201–210.
- Neverov A, Chumakov K. Massively parallel sequencing for monitoring genetic consistency and quality control of live viral vaccines. Proc Natl Acad Sci. 2010;107(46): 20063LP - 20068.
- Charlton B, Hockley J, Laassri M, et al. The use of next-generation sequencing for the quality control of live-attenuated polio vaccines. J Infect Dis. 2020;222(11):1920–1927.
- Da Costa A, Prehaud C, Khou C, et al. Innovative in cellulo method as an alternative to in vivo neurovirulence test for the characterization and quality control of human live Yellow Fever virus vaccines: a pilot study. Biologicals. 2018;53:19–29.
- Szymkowicz L, Wilson DJ, James DA. Development of a targeted nanoLC-MS/MS method for quantitation of residual toxins from Bordetella pertussis. J Pharm Biomed Anal. 2020;188:113395.
- Akkermans A, Chapsal JM, Coccia EM, et al. Animal testing for vaccines. Implementing replacement, reduction and refinement: challenges and priorities. Biologicals. 2020;68:92–107.
- Vandebriel R, Hoefnagel MMN. Dendritic cell-based in vitro assays for vaccine immunogenicity. Hum Vaccin Immunother. 2012;8(9):1323–1325.
- Stoel M, Pool J, de Vries-Idema J, et al. Innate responses induced by whole inactivated virus or subunit influenza vaccines in cultured dendritic cells correlate with immune responses in vivo. PLoS One. 2015;10(5):e0125228.
- Tapia-Calle G, Stoel M, de Vries-Idema J, et al. Distinctive responses in an in vitro human dendritic cell-based system upon stimulation with different influenza vaccine formulations. Vaccines (Basel). 2017;5(3):21.
- Hoefnagel MHN, Vermeulen JP, Scheper RJ, et al. Response of MUTZ-3 dendritic cells to the different components of the Haemophilus influenzae type B conjugate vaccine: towards an in vitro assay for vaccine immunogenicity. Vaccine. 2011;29(32):5114–5121.
- Querec T, Bennouna S, Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–424.
- Brummelman J, Veerman RE, Hamstra HJ, et al.Bordetella pertussis naturally occurring isolates with altered lipooligosaccharide structure fail to fully mature human dendritic cells.Ba M,editor.Infect Immun. 2015;83(1):227–238
- Hovingh ES, Van Gent M, Hamstra H-J, et al. Emerging Bordetella pertussis strains induce enhanced signaling of human pattern recognition receptors TLR2, NOD2 and secretion of IL-10 by dendritic cells. Hozbor DF, ed. PLoS One. 2017;12(1):e0170027.
- Hoffmann S, Peterbauer A, Schindler S, et al. International validation of novel pyrogen tests based on human monocytoid cells. J Immunol Methods. 2005;298(1–2):161–173.
- Zaitseva M, Romantseva T, Blinova K, et al., Use of human MonoMac6 cells for development of in vitro assay predictive of adjuvant safety in vivo. Vaccine. 30(32): 4859–4865. 2012.
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4+ T cell populations. Annu Rev Immunol. 2010;28(1):445–489.
- Bent R, Moll L, Grabbe S, et al. Interleukin-1 beta—A friend or foe in malignancies. Int J Mol Sci. 2018;19(8):2155.
- Hoonakker ME, Verhagen LM, van der Maas L, et al., Adaptive immune response to whole cell pertussis vaccine reflects vaccine quality: a possible complementation to the pertussis serological potency test. Vaccine. 2016;34(37): 4429–4436.
- Joffret ML, Zanetti C, Morgeaux S, et al. Appraisal of rabies vaccine potency by determination of in vitro, specific interleukin-2 production. Biologicals. 1991;19(2):113–123.
- Moser JM, Sassano ER, Leistritz DC, et al. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J Immunol Methods. 2010;353(1–2):8–19.
- Tapia-Calle G, Born PA, Koutsoumpli G, et al. A PBMC-based system to assess human T cell responses to influenza vaccine candidates in vitro. Vaccines (Basel). 2019;7(4):181.
- Kangethe RT, Pichler R, Chuma FNJ, et al. Bovine monocyte derived dendritic cell based assay for measuring vaccine immunogenicity in vitro. Vet Immunol Immunopathol. 2018;197:39–48.
- Yu SCT, Nag B. Application of murine T-T hybridoma cells to in vitro potency assay of human synthetic peptide vaccines. Vaccine. 1996;14(14):1313–1321.
- Ise W, Inoue T, McLachlan JB, et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci U S A. 2014;111(32):11792–11797.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–293.
- Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J Vet Intern Med. 2003;17(3):273–281.
- Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Biol Med. 1937;37(3):509–513.
- Di Pasquale A, Preiss S, Da Silva FT, et al. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel). 2015;3(2):320–343.
- Van Doorn E, Liu H, Huckriede A, et al. Safety and tolerability evaluation of the use of Montanide ISA™51 as vaccine adjuvant: a systematic review. Hum Vaccines Immunother. 2016;12(1):159–169.
- O’Hagan DT, Ott GS, Van Nest G, et al. The history of MF59 ® adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12(1):13–30.
- Misquith A, Fung HWM, Dowling QM, et al. In vitro evaluation of TLR4 agonist activity: formulation effects. Colloids Surf B Biointerfaces. 2014;113:312–319.
- Stokes W, Srinivas G, McFarland R, et al. Report on the international workshop on alternative methods for Leptospira vaccine potency testing: state of the science and the way forward. Biologicals. 2013;41(5):279–294.
- Claassen I, Maas R, Oei H, et al. Validation study to evaluate the reproducibility of a candidate in vitro potency assay of newcastle disease vaccines and to establish the suitability of a candidate biological reference preparation. Pharmeuropa bio. 2004;2004(1):1–15
- van den Biggelaar RHGA, van Eden W, Rutten VPMG, et al. Macrophage activation assays to evaluate the immunostimulatory capacity of Avibacterium paragallinarum in a multivalent poultry vaccine. Vaccines (Basel). 2020;8(4):671.
- Saravanan P, Iqbal Z, Selvaraj DPR, et al., Comparison of chemical extraction methods for determination of 146S content in foot-and-mouth disease oil-adjuvanted vaccine. J Appl Microbiol. 2020;128(1): 65–73.
- Morgenroth A, Jakel V, Hanke-Robinson H, et al. A novel electrophoretic immunoblot as antigen desorption and quantification method for alum-adjuvanted veterinary rabies vaccines. Vaccine. 2020;38(27):4281–4287.
- Rinella JV, Workman RF, Hermodson MA, et al. Elutability of proteins from aluminum-containing vaccine adjuvants by treatment with surfactants. J Colloid Interface Sci. 1998;197(1):48–56.
- Wood D, Sands D, Heath A. Collaborative study for the establishment of three product specific European Pharmacopoeia biological reference preparations for inactivated adsorbed hepatitis A vaccines. Pharmeur Bio Sci Notes. 2000;2000(1):51–80.
- Byrne-Nash RT, Miller DF, Bueter KM, et al. VaxArray potency assay for rapid assessment of “pandemic” influenza vaccines. Npj Vaccines. 2018;3(1):43.
- Song Y, Yang Y, Lin X, et al. In-situ and sensitive stability study of emulsion and aluminum adjuvanted inactivated foot-and-mouth disease virus vaccine by differential scanning fluorimetry analysis. Vaccine. 2020;38(14):2904–2912.
- Nouchikian L, Roque C, Song JY, et al. An intrinsic fluorescence method for the determination of protein concentration in vaccines containing aluminum salt adjuvants. Vaccine. 2018;36(38):5738–5746.
- Zhu D, Saul A, Huang S, et al. Use of o-phthalaldehyde assay to determine protein contents of Alhydrogel-based vaccines. Vaccine. 2009;27(43):6054–6059.
- Amari JV, Levesque P, Lian Z, et al. Concentration determination of a recombinant vaccine antigen adsorbed onto an alum adjuvant by chemiluminescent nitrogen detection. Pharm Res. 2005;22(1):33–37.
- van den Biggelaar RHGA, van Eden W, Rutten VPMG, et al. Nitric oxide production and Fc receptor-mediated phagocytosis as functional readouts of macrophage activity upon stimulation with inactivated poultry vaccines in vitro. Vaccines (Basel). 2020;8(2):332.
- Da Silva M, Labas V, Nys Y, et al. Investigating proteins and proteases composing amniotic and allantoic fluids during chicken embryonic development. Poult Sci. 2017;96(8):2931–2941.
- Katz JB, Hanson SK, Patterson PA, et al. In vitro assessment of viral antigen content in inactivated aluminum hydroxide adjuvanted vaccines. J Virol Methods. 1989;25(1):101–108.
- Necchi F, Carducci M, Pisoni I, et al. Development of FAcE (Formulated Alhydrogel competitive ELISA) method for direct quantification of OAg present in Shigella sonnei GMMA-based vaccine and its optimization using Design of Experiments approach. J Immunol Methods. 2019;471:11–17.
- Zhu D, Huang S, Gebregeorgis E, et al. Development of a direct alhydrogel formulation immunoassay (DAFIA). J Immunol Methods. 2009;344(1):73–78.
- Li M, Wang X, Cao L, et al. Quantitative and epitope-specific antigenicity analysis of the human papillomavirus 6 capsid protein in aqueous solution or when adsorbed on particulate adjuvants. Vaccine. 2016;34(37):4422–4428.
- Ugozzoli M, Laera D, Nuti S, et al. Flow cytometry: an alternative method for direct quantification of antigens adsorbed to aluminum hydroxide adjuvant. Anal Biochem. 2011;418(2):224–230.
- Gibert R, Alberti M, Poirier B, et al. A relevant in vitro ELISA test in alternative to the in vivo NIH test for human rabies vaccine batch release. Vaccine. 2013;31(50):6022–6029.
- Stalpers CAL, Retmana IA, Pennings JLA, et al. Variability of in vivo potency tests of Diphtheria, Tetanus and acellular Pertussis (DTaP) vaccines. Vaccine. 2021;39(18):2506–2516.
- Chabaud-Riou M, Moreno N, Guinchard F, et al. G-protein based ELISA as a potency test for rabies vaccines. Biologicals. 2017;46:124–129.
- Toinon A, Moreno N, Chausse H, et al. Potency test to discriminate between differentially over-inactivated rabies vaccines: agreement between the NIH assay and a G-protein based ELISA. Biologicals. 2019;60:49–54.
- Vac2vac.eu [Internet]. London, UK: Manta Ray Media; [2021 August 03]. Available from: http://www.vac2vac.eu/
- Schiffelers MJWA, Blaauboer BJ, Bakker WE, et al. Regulatory acceptance and use of serology for inactivated veterinary rabies vaccines. ALTEX. 2015;32(3):211–221.
